This article has been corrected. See "Corrigendum: Marginal bone loss around crestal or subcrestal dental implants: prospective clinical study" in Volume 48 on page 245.
Abstract
Objectives
The stability of crestal bone has been reported as a major factor in the success of dental implants. Implants can be placed in an equicrestal (crestal) or subcrestal position. The aim of this study was to evaluate the effect of implant depth placement on marginal bone loss.
Materials and Methods
The study was created in a split-mouth design. Immediately after implant surgery, digital parallel radiographs were prepared and levels of bone were measured where marginal bone loss and bone level changes occurred. These measurements were repeated at 3-month and 6-month follow-up periods.
Results
In this interventional study, 49 implants were evaluated in 18 patients. Primary bone height was not significant between the intervention and control groups in both mesial and distal aspects at 3 months and 6 months from the baseline. The mean marginal bone loss on the mesial side was 1.03 mm in the subcrestal group and 0.83 mm in the crestal group. In addition, mean marginal bone loss on the distal side was 0.88 mm and 0.81 mm in the subcrestal and crestal groups, respectively. Marginal bone loss was not significantly different between sexes, the maxilla or mandible, and in the anterior or posterior regions as well as between different lengths and diameters of implants.
Go to : 
The use of implants for full and partial edentulous reconstruction enhances masticatory performance and improves satisfaction as well as the quality of life compared to removable prosthetics. As a result, this treatment option has been adopted exponentially in recent decades. The success rate of dental implants is very high and has been reported to be up to 95% survival over 5 years1.
Stability depends on the amount of bone surrounding the implant. At the end of the remodeling phase, the implant is expected to cover approximately 60% to 70% of the bone, which is used as the degree of osseointegration criterion2. Early implant failure occurs in 1%-2% of patients in the first months due to failure of osseointegration. Secondary or late failure usually occurs in 5% of patients after a few years due to peri-implantitis3.
The average marginal bone loss of a functional implant in the first year is approximately 1 mm and the average bone loss in subsequent years is around 0.1 mm per year. Several factors can play a role in peri-implant marginal bone loss such as biological width establishment, vertical soft tissue thickness, microgaps at the level of the implant–abutment interface, the implant position relative to the alveolar crest, implant macro design (platform-switching and platform-matching implants), the implant-abutment connection, surface topography of the implant neck, history of smoking, and peri-implantitis4.
The vertical position of the implant is a factor affecting crestal bone loss. It is recommended that the implant be placed 2-3 mm below the cementoenamel junction of the adjacent tooth, especially in the aesthetic zone5. Through this method, the abutment is placed at the level where better remodeling can be achieved at the neck of the implant. In addition, the papillary form becomes better, which is very important in the esthetic area6, but in some studies it has been claimed that due to the subcrestal placement of the implant, bacterial colonization is more likely and the risk of peri-implantitis is increased7.
The main question of the study was “Does the depth of implant placement affect marginal bone loss?”
Go to : 
This study was performed as a split-mouth randomized clinical trial. The study was designed based on CONSORT (Consolidated Standards of Reporting Trials) criteria and was performed after approval by the Ethics Committee of the Mashhad University of Medical Sciences (No. IR.MUMS.DENTISTRY.REC.1400.036) and registered in the IRCT system (IRCT20180130038558N2).
Participants were selected from partially edentulous patients referred to the implant department of the Mashhad Dental School and Hekmat Clinic of Mashhad (Iran) from April to December 2021. Informed written consent forms were obtained from the participants. This study was performed as a double-blind study where both the patients and examiner were unaware of the intervention and control groups. The sample size was approximately 20 patients, but considering the 10% dropout in patients, this number was increased to 22 patients.
Inclusion criteria involved adult patients with no systemic diseases, healed alveolar ridges (at least 4 months after tooth extraction), adequate bone dimensions at the implant site, at least 2 implants in non-adjacent edentulous areas (if the patient needed two implants, one implant was positioned crestally, while the other was placed 2 mm subcrestally), and no history of bone regeneration at the implant site. Exclusion criteria included smoking, poor oral hygiene, bone defects in the alveolar ridge at the implant site, and the existence or history of periodontitis.
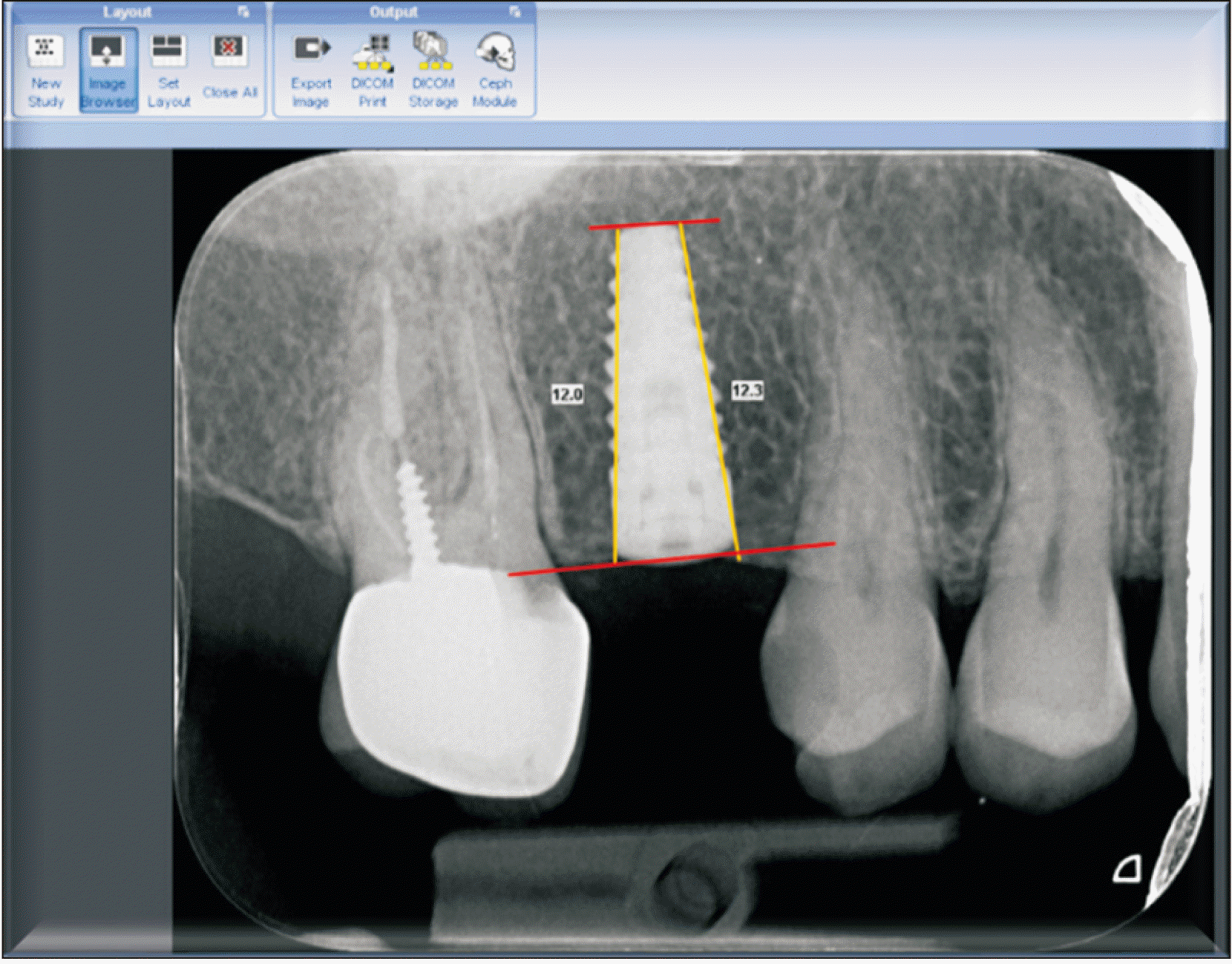
In order to select the correct dimensions of the implant, cone-beam computed tomography scans were evaluated for each patient. The surgical procedures were performed as follows: Local anesthesia 2% lidocaine with epinephrine 1:80,000 (New Stetic, Guarne, Colombia) was administered. A crestal incision was created followed by full thickness flap reflection. The implants were randomly divided into two groups as subcrestal and crestal. Platform switching bone level BioHorizon Implants (Birmingham, AL, USA) were placed. In the intervention group, the implant was placed 2 mm subcrestally, while the implant was positioned at the level of the bone crest in the control group.(Fig. 1) Two-stage surgical protocols were used for the implants. All the implants were placed by an expert surgeon. The following medications were prescribed: 500 mg amoxicillin three times a day with 400 mg gelofen every 6 hours a day for one week and chlorhexidine 0.2% mouthwash twice a day for two weeks.
Following surgery, digital periapical radiographs using the long-cone parallel technique were prepared. Periapical radiographs were prepared by dental digital No. 2 sensors (Durr, Bietigheim-Bissingen, Germany) (31 by 41 mm) and film holders (Kerr, Brea, CA, USA) with the radiographic system (ver. 2016; Xgenus, Olgiate Olona, Italy) set at 70 kVp and 8 mA. Follow-up periapical radiographs was obtained 3 and 6 months after surgery.
Mesial and distal bone heights relative to the fixture apex were evaluated in the three prepared radiographs. Likewise, bone level changes and marginal bone loss values were compared. Bone height was defined as a vertical distance from the crestal bone to the fixture apex. Marginal bone loss was defined as the distance from the implant platform to the alveolar crest. Radiograph analysis was measured by Romexis software (ver. 3.8.3) produced by Planmeca (Helsinki, Finland). All the measurements were performed by a trained examiner.
A 41-mm longitudinal line was drawn as a reference through the Software calibration tool in the radiograph images. The reason for selecting 41 mm as the calibrating scale was based on the length of the number 2 sensor. The length of the fixture was then measured from the platform to the end of the apex. This would be the basis of subsequent measurements. On both the mesial and distal sides, the bone height from the crestal bone to the end of the fixture apex (perpendicular to the tangent line at the end of the fixture) was measured and the numbers were recorded.
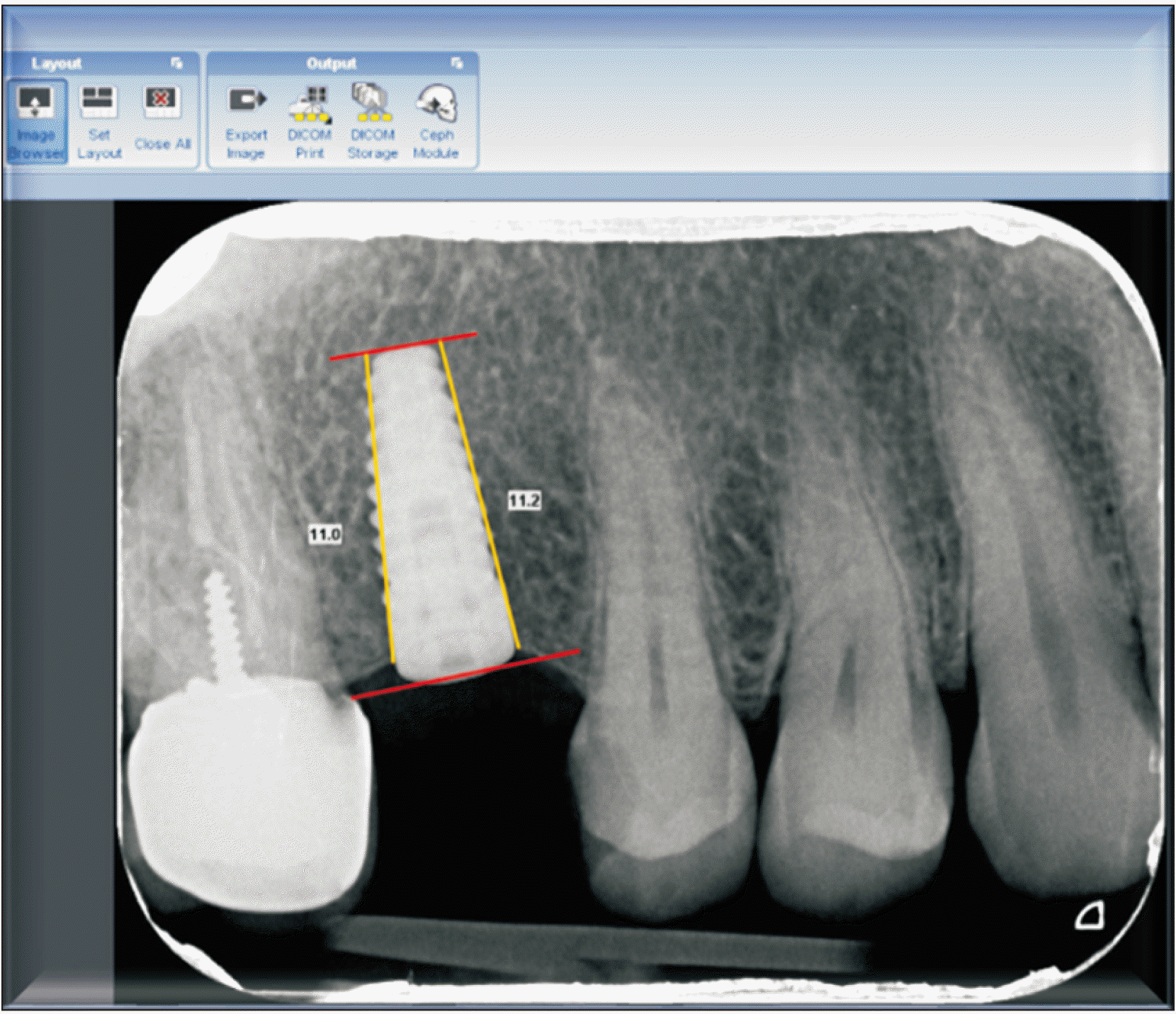
Second-stage patient radiographs were entered into the Romexis software. In order to rectify the possible radiographic angle changes based on a constant parameter (fixture), similar to the previous step, the lines at the coronal and apical parts of the fixture were drawn and the recorded numbers in the previous step were entered with the calibration tool. The measurements were then repeated.(Fig. 2) The third stage radiographs were performed as in the previous stage.
Statistical analyses were performed using SPSS software (ver. 12; SPSS, Chicago, IL, USA). Data were reported as mean±standard deviation. The normality of distribution was tested with Kolmogorov–Smirnov and Shapiro–Wilk tests. If the data were parametric, analysis of variance was used for analysis. The level of significance in all the tests was considered 5%.
Go to : 
Table 1 lists the statistical report of sex and age distribution by groups. In the intervention group, 53.8% of the patients were males and 46.2% were females. In the control group, 56.5% of the patients were males and 43.5% were females. The numbers of males and females in the study groups were not significantly different from each other. The maximum age was 70 years and the median age in both groups was 59 years. The groups did not differ significantly in terms of age.
Table 2 reveals that the primary bone height was not significant at the baseline and after three months plus six months between the intervention and control groups on both the mesial and distal aspects (P>0.05).
Measured bone height in the mesial and distal aspects at baseline, three months, and six months
Mesial and distal marginal bone loss was not significant in the first three months and the subsequent six months.(Table 3)
Marginal bone loss in the mesial and distal sides in three and six months
Table 4 lists the mesial and distal marginal bone loss values at intervals of three months and six months between different sexes, mandible or maxilla, as well as the anterior and posterior areas. No statistically significant difference was observed in any of these analyses.
Mesial and distal marginal bone loss at the third and sixth months between different sex groups
The correlation between implant length plus diameter and changes in mesial and distal bone height in the third and sixth months reported in Table 5 was not statistically significant.
Correlation between implant dimension with bone level changes in three- and six-month follow-ups
Marginal bone loss was inversely related to age in both the mesial and distal dimensions. This inverse correlation was observed at the sixth-month follow-up.(Table 6)
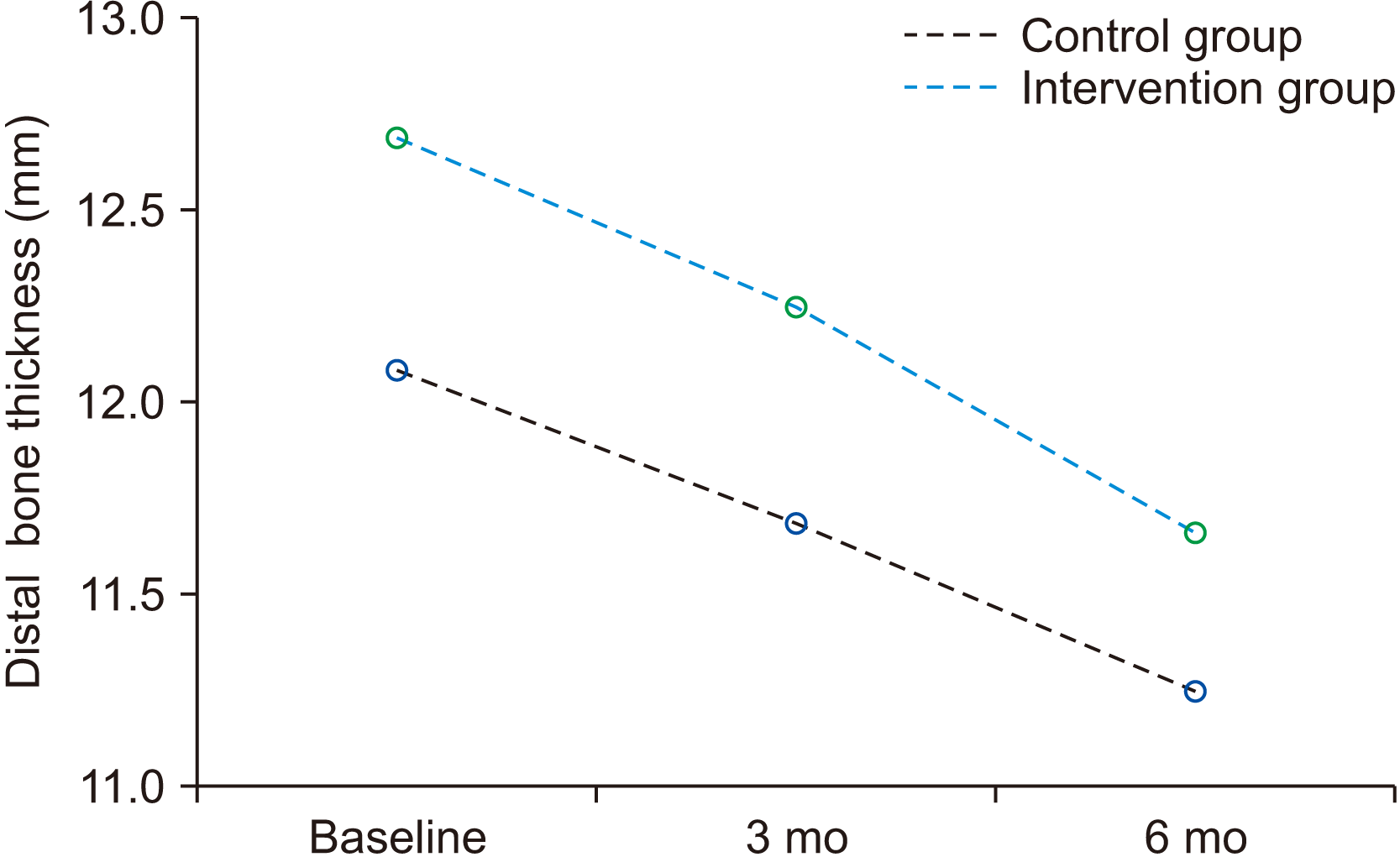
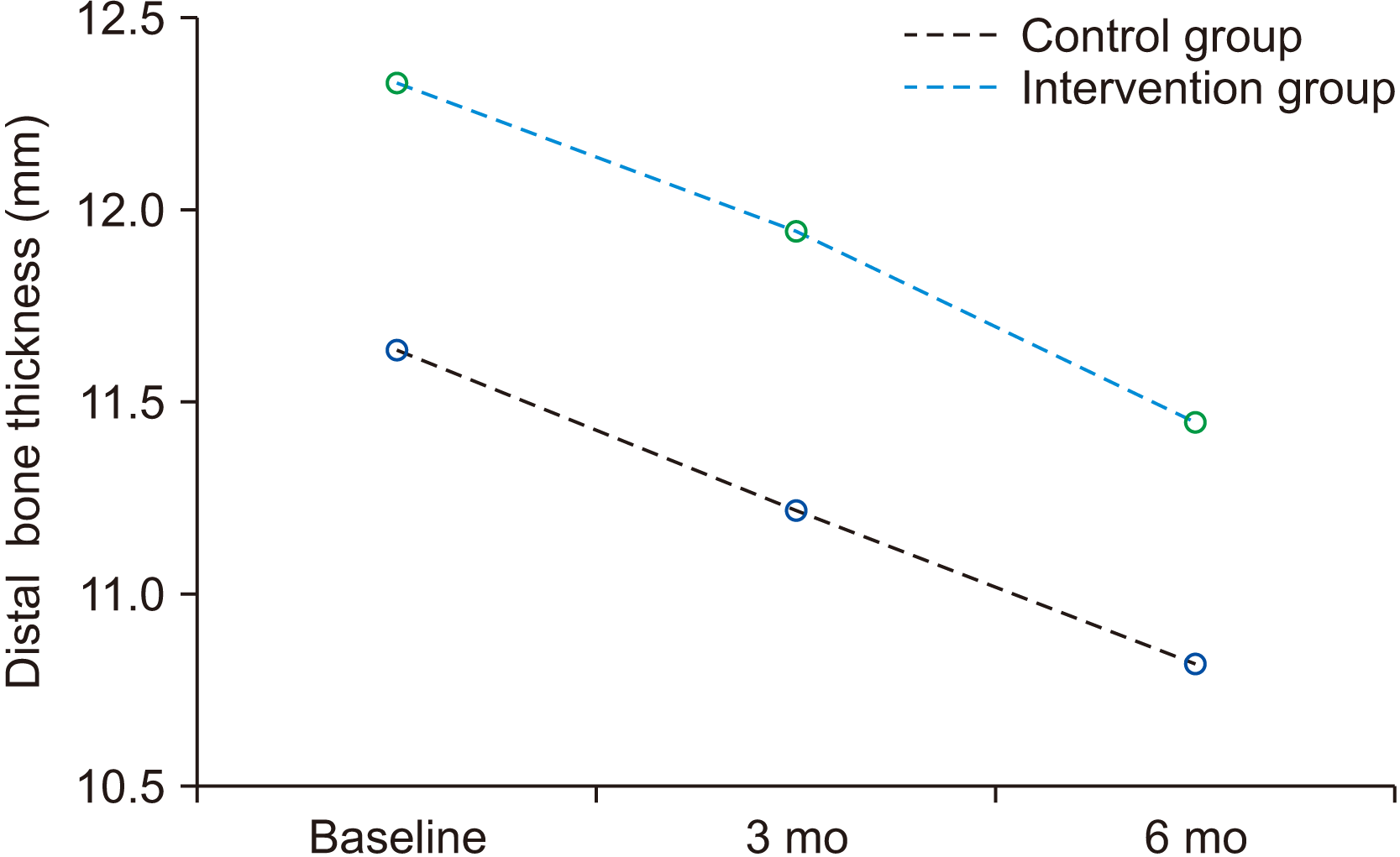
Mesial and distal aspect bone level changes between three and six months in the intervention and control groups are shown in Fig. 3 and 4, respectively. Bone height reduction during the three months and six months was significant for both groups on both the mesial and distal sides (P=0.000). However, the difference in bone level changes was not significant between the intervention and control groups (P>0.05).
Go to : 
The aim of this study was to evaluate the marginal bone loss around crestal and subcrestal implants in the six-month follow up period. In this interventional study, 49 implants were examined in 18 patients with a mean age of 57.84±6.73 years in the intervention group and 59.04±7.18 years in the control group. Primary bone height and marginal bone loss were not significantly different between the intervention and control groups in both the mesial and distal groups at three months and six months from the baseline. There was no significant difference either in marginal bone loss between the maxilla or mandible, implant site (anterior or posterior), and sexes. No significant correlation was observed between implant dimension and bone level changes in the mesial and distal dimensions during three and six months. Note that the mesial marginal bone loss was 1.03 mm in the subcrestal group and 0.83 mm in the crestal group. In addition, on the distal aspect in the subcrestal group, marginal bone loss was 0.88 mm and 0.81 mm in the crestal group. There was no significant difference in terms of marginal bone loss in the mesial and distal sides for both groups at three and six months.
Several authors have reported that implants placed approximately 2 mm subcrestally have significantly less marginal bone loss compared to implants placed crestally7,8. Conversely, other authors have observed more marginal bone loss with subcrestal implants9,10. Factors such study design, implant geometry, implant surface treatment, and implant placement surgical protocols may explain the discrepancies between the studies.
Note that factors such as smoking, poor oral hygiene, and history of periodontitis can increase marginal bone loss, and patients with these features were excluded from the study. Also in our study, the system of all the implants was consistent throughout, and the procedures were performed by an expert surgeon. Therefore, it can be concluded that differences in the surgical procedure could not affect the outcome of this study.
The association between implant dimensions, implant site, age, sex, and marginal bone loss was also evaluated in this study. Preparation of the implant site in the subcrestal group can cause stress on the crestal bone, leading to more marginal bone loss. Nevertheless, in the present study, tapered implants were used and the diameter of the implant was chosen correctly in order to have adequate bone in the buccal and lingual aspects surrounding the implant11.
Among similar human studies, Veis et al.12 placed implants 1-2 mm subcrestally. In a study by Romanos et al.13, the implants were placed 0.5 mm subcrestally. In addition, Kütan et al.6 placed their implants 1 mm subcrestally, Al Amri et al.14 2 mm subcrestally, and de Siqueira et al.15 between 1 to 3 mm subcrestally. All the studies employed standard periapical radiographs using parallel techniques to assess bone levels, except for two studies (Romanos et al.13 and Veis et al.12) which used panoramic radiography. Measurements on radiographic images have a limitation in that they may not be accurate and could potentially underestimate the level of the crestal bone around the implants.
Ercoli et al.16 evaluated 134 supracrestal, crestal, and subcrestal implants in terms of marginal bone loss. Although marginal bone loss was lower in the subcrestal group, the authors did not find any statistically significant differences between the groups in an eighteen-month follow-up period.
Valles et al.17 in a meta-analysis demonstrated that there was no significant difference in terms of marginal bone loss at different depths of implant positioning.
Cruz et al.18 in a meta-analysis examined 709 implants of which 351 and 358 were crestal and subcrestal implants, respectively. Cruz et al.18 concluded that crestal or subcrestal implant placement did not differ in terms of bone loss and soft tissue parameters. In addition, Cruz et al.18 found that marginal bone loss was independent of the surgical procedure.
A systematic review by Pellicer-Chover et al.4 found that there was no significant difference on marginal bone loss between crestal and subcrestal implants. Meanwhile, in four human studies of this systematic review, crestal implants exhibited higher marginal bone loss compared to sub-crestal implants with significant differences in only one study4. This result is consistent with the present study.
In the Gatti et al.11 study, the implants were randomly placed either 1 mm subcrestally or crestally. Radiographic examinations were performed using the digital periapical parallel technique at the time of implant placement (T0), at the time of prosthesis delivery (T1), and 12 months (T2) after prosthesis loading. A total of 54 implants were evaluated at the 12-month follow-up with radiographic images. After 1 year, mean bone loss was 0.721 mm in the subcrestal group and 0.418 mm in the crestal group. According to the results of the present study, although the subcrestal group exhibited more bone loss, it could be considered “physiological” compared to the other studies11.
In a histological study by Degidi et al.19, crestal implants exhibited 0.5 to 1.5 mm marginal bone loss, while subcrestal implants revealed bone growth on the implant platform.
In animal studies, Jung et al.20 examined platform switching implants at different bone levels relative to the alveolar crest. Jung et al.20 placed a total of 62 implants at three different levels in the edentulous areas of five dog jaws. They reported that after 6 months, the highest amount of bone loss (1.32 mm) was in the group with subcrestal implants. Yi et al.21 also concluded that greater marginal bone loss was observed in subcrestal implants. Another study on dogs by Pontes et al.22 examined the effect of implant depth placement on marginal bone loss. However, these studies stated that crestal or subcrestal implant placement had no significant effect on marginal bone loss.
Fetner et al.23 explored the effect of subcrestal implants on marginal bone loss. Thirty-six two-piece implants were placed in the edentulous areas of six dogs. The implants were randomly placed crestal or 1.5 mm or 3 mm subcrestal. The authors concluded that subcrestal implant placement was not associated with significant marginal bone loss23.
In this study, marginal bone loss was not statistically significantly different between sex, anterior or posterior location, and the maxilla or mandible even though bone loss occurred more frequently in females. This can be justified due to menopause and osteoporosis status which can affect bone loss. The results of the studies in this field are contradictory24,25. On the other hand, greater marginal bone loss occurred in the posterior areas. Jang et al.26 and Güven et al.27 pointed out that the reason for further bone loss was due to unfavorable bone density and quality in the posterior areas. In addition, it should be considered that more occlusal forces are transmitted to posterior implants, increasing the marginal bone loss incidence26,27. In the present study, although it was not statistically significant, implants placed in the mandible exhibited greater marginal bone loss, which is consistent with the study by Raikar et al.28 and Güven et al.27. As mentioned, the reason for bone loss is more related to mandibular bone density28.
One of the study limitations was the use of periapical radiographs, which results in two-dimensional evaluations of bone, possibly leading to inaccuracies. In addition, our data were collected from few participants, potentially compromising the generalizability of the results. Further clinical studies with larger sample sizes and longer follow-up periods are required to improve the understanding of this issue. Meanwhile, factors affecting marginal bone loss such as the presence of keratinized tissue and soft tissue width were not considered. In addition, more research must be performed in order to investigate other factors affecting biological width establishment, identify effective factors, reduce bone loss, and provide more predictable implant treatment outcomes to patients.
Go to : 
Based on the findings of this study, it can be concluded that the marginal bone loss in the mesial and distal sides was not significant in both crestal or subcrestal implants at the three-month and the six-month follow-up. Also, there was no significant difference in marginal bone loss between different sexes, maxilla or mandible, implant area (anterior or posterior). Also no significant correlation was observed between implant dimension with changes in mesial and distal bone height during three-month and six-month follow-up.
Go to : 
Notes
Authors’ Contributions
N.S. participated in conceptualization and methodology. H.H.Z. and H.A. participated in methodology and resources. T.K. participated in investigation, original draft, and formal analysis. M.F.R. participated in original draft, writing, and review & editing. All authors read and approved the final manuscript.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of the Mashhad University of Medical Sciences (No. IR.MUMS.DENTISTRY.REC.1400.036). Informed written consent forms were obtained from the participants.
Go to : 
References
1. Borie E, Orsi IA, de Araujo CP. 2015; The influence of the connection, length and diameter of an implant on bone biomechanics. Acta Odontol Scand. 73:321–9. https://doi.org/10.3109/00016357.2014.961957. DOI: 10.3109/00016357.2014.961957. PMID: 25598357.

2. von Wilmowsky C, Moest T, Nkenke E, Stelzle F, Schlegel KA. 2014; Implants in bone: part II. Research on implant osseointegration: material testing, mechanical testing, imaging and histoanalytical methods. Oral Maxillofac Surg. 18:355–72. https://doi.org/10.1007/s10006-013-0397-2. DOI: 10.1007/s10006-013-0397-2. PMID: 23430020.

3. Chrcanovic BR, Albrektsson T, Wennerberg A. 2014; Reasons for failures of oral implants. J Oral Rehabil. 41:443–76. https://doi.org/10.1111/joor.12157. DOI: 10.1111/joor.12157. PMID: 24612346.

4. Pellicer-Chover H, Díaz-Sanchez M, Soto-Peñaloza D, Peñarrocha-Diago MA, Canullo L, Peñarrocha-Oltra D. 2019; Impact of crestal and subcrestal implant placement upon changes in marginal peri-implant bone level. A systematic review. Med Oral Patol Oral Cir Bucal. 24:e673–83. https://doi.org/10.4317/medoral.23006. DOI: 10.4317/medoral.23006. PMID: 31433391. PMCID: PMC6764703.

5. Kowalski J, Lapinska B, Nissan J, Lukomska-Szymanska M. 2021; Factors influencing marginal bone loss around dental implants: a narrative review. Coatings. 11:865. https://doi.org/10.3390/coatings11070865. DOI: 10.3390/coatings11070865.

6. Kütan E, Bolukbasi N, Yildirim-Ondur E, Ozdemir T. 2015; Clinical and radiographic evaluation of marginal bone changes around platform-switching implants placed in crestal or subcrestal positions: a randomized controlled clinical trial. Clin Implant Dent Relat Res. 17 Suppl 2:e364–75. https://doi.org/10.1111/cid.12248. DOI: 10.1111/cid.12248. PMID: 25041252.

7. Palacios-Garzón N, Velasco-Ortega E, López-López J. 2019; Bone loss in implants placed at subcrestal and crestal level: a systematic review and meta-analysis. Materials (Basel). 12:154. https://doi.org/10.3390/ma12010154. DOI: 10.3390/ma12010154. PMID: 30621286. PMCID: PMC6337530.

8. Puisys A, Linkevicius T. 2015; The influence of mucosal tissue thickening on crestal bone stability around bone-level implants. A prospective controlled clinical trial. Clin Oral Implants Res. 26:123–9. https://doi.org/10.1111/clr.12301. DOI: 10.1111/clr.12301. PMID: 24313250.

9. Romanos GE. 2015; Wound healing in immediately loaded implants. Periodontol 2000. 68:153–67. https://doi.org/10.1111/prd.12058. DOI: 10.1111/prd.12058. PMID: 25867985.

10. Hämmerle CH, Brägger U, Bürgin W, Lang NP. 1996; The effect of subcrestal placement of the polished surface of ITI implants on marginal soft and hard tissues. Clin Oral Implants Res. 7:111–9. https://doi.org/10.1034/j.1600-0501.1996.070204.x. DOI: 10.1034/j.1600-0501.1996.070204.x. PMID: 9002829.

11. Gatti C, Gatti F, Silvestri M, Mintrone F, Rossi R, Tridondani G, et al. 2018; A prospective multicenter study on radiographic crestal bone changes around dental implants placed at crestal or subcrestal level: one-year findings. Int J Oral Maxillofac Implants. 33:913–8. https://doi.org/10.11607/jomi.6509. DOI: 10.11607/jomi.6509. PMID: 30025009.

12. Veis A, Parissis N, Tsirlis A, Papadeli C, Marinis G, Zogakis A. 2010; Evaluation of peri-implant marginal bone loss using modified abutment connections at various crestal level placements. Int J Periodontics Restorative Dent. 30:609–17. PMID: 20967307.
13. Romanos GE, Aydin E, Gaertner K, Nentwig GH. 2015; Long-term results after subcrestal or crestal placement of delayed loaded implants. Clin Implant Dent Relat Res. 17:133–41. https://doi.org/10.1111/cid.12084. DOI: 10.1111/cid.12084. PMID: 23675969.

14. Al Amri MD, Al-Johany SS, Al Baker AM, Al Rifaiy MQ, Abduljabbar TS, Al-Kheraif AA. 2017; Soft tissue changes and crestal bone loss around platform-switched implants placed at crestal and subcrestal levels: 36-month results from a prospective split-mouth clinical trial. Clin Oral Implants Res. 28:1342–7. https://doi.org/10.1111/clr.12990. DOI: 10.1111/clr.12990. PMID: 27743396.

15. de Siqueira RAC, Fontão FNGK, Sartori IAM, Santos PGF, Bernardes SR, Tiossi R. 2017; Effect of different implant placement depths on crestal bone levels and soft tissue behavior: a randomized clinical trial. Clin Oral Implants Res. 28:1227–33. https://doi.org/10.1111/clr.12946. DOI: 10.1111/clr.12946. PMID: 27480573.

16. Ercoli C, Jammal G, Buyers M, Tsigarida AA, Chochlidakis KM, Feng C, et al. 2017; Influence of apico-coronal implant placement on post-surgical crestal bone loss in humans. J Periodontol. 88:762–70. https://doi.org/10.1902/jop.2017.160802. DOI: 10.1902/jop.2017.160802. PMID: 28387610.

17. Valles C, Rodríguez-Ciurana X, Clementini M, Baglivo M, Paniagua B, Nart J. 2018; Influence of subcrestal implant placement compared with equicrestal position on the peri-implant hard and soft tissues around platform-switched implants: a systematic review and meta-analysis. Clin Oral Investig. 22:555–70. https://doi.org/10.1007/s00784-017-2301-1. DOI: 10.1007/s00784-017-2301-1. PMID: 29313133.

18. Cruz RS, Lemos CAA, de Luna Gomes JM, Pellizzer EP, Verri FR. Fernandes e Oliveira HF. 2022; Clinical comparison between crestal and subcrestal dental implants: a systematic review and meta-analysis. J Prosthet Dent. 127:408–17. https://doi.org/10.1016/j.prosdent.2020.11.003. DOI: 10.1016/j.prosdent.2020.11.003. PMID: 33358610.

19. Degidi M, Perrotti V, Shibli JA, Novaes AB, Piattelli A, Iezzi G. 2011; Equicrestal and subcrestal dental implants: a histologic and histomorphometric evaluation of nine retrieved human implants. J Periodontol. 82:708–15. https://doi.org/10.1902/jop.2010.100450. DOI: 10.1902/jop.2010.100450. PMID: 21138355.

20. Jung RE, Jones AA, Higginbottom FL, Wilson TG, Schoolfield J, Buser D, et al. 2008; The influence of non-matching implant and abutment diameters on radiographic crestal bone levels in dogs. J Periodontol. 79:260–70. https://doi.org/10.1902/jop.2008.070132. DOI: 10.1902/jop.2008.070132. PMID: 18251640.

21. Yi JM, Lee JK, Um HS, Chang BS, Lee MK. 2010; Marginal bony changes in relation to different vertical positions of dental implants. J Periodontal Implant Sci. 40:244–8. https://doi.org/10.5051/jpis.2010.40.5.244. DOI: 10.5051/jpis.2010.40.5.244. PMID: 21072222. PMCID: PMC2967813.

22. Pontes AE, Ribeiro FS, da Silva VC, Margonar R, Piattelli A, Cirelli JA, et al. 2008; Clinical and radiographic changes around dental implants inserted in different levels in relation to the crestal bone, under different restoration protocols, in the dog model. J Periodontol. 79:486–94. https://doi.org/10.1902/jop.2008.070145. DOI: 10.1902/jop.2008.070145. PMID: 18315431.

23. Fetner M, Fetner A, Koutouzis T, Clozza E, Tovar N, Sarendranath A, et al. 2015; The effects of subcrestal implant placement on crestal bone levels and bone-to-abutment contact: a microcomputed tomographic and histologic study in dogs. Int J Oral Maxillofac Implants. 30:1068–75. https://doi.org/10.11607/jomi.4043. DOI: 10.11607/jomi.4043. PMID: 26394343.

24. Pedro RE, De Carli JP, Linden MS, Lima IF, Paranhos LR, Costa MD, et al. 2017; Influence of age on factors associated with peri-implant bone loss after prosthetic rehabilitation over osseointegrated implants. J Contemp Dent Pract. 18:3–10. https://doi.org/10.5005/jp-journals-10024-1979. DOI: 10.5005/jp-journals-10024-1979. PMID: 28050977.

25. Wagenberg B, Froum SJ. 2006; A retrospective study of 1925 consecutively placed immediate implants from 1988 to 2004. Int J Oral Maxillofac Implants. 21:71–80. PMID: 16519184.
26. Jang HW, Kang JK, Lee K, Lee YS, Park PK. 2011; A retrospective study on related factors affecting the survival rate of dental implants. J Adv Prosthodont. 3:204–15. https://doi.org/10.4047/jap.2011.3.4.204. DOI: 10.4047/jap.2011.3.4.204. PMID: 22259704. PMCID: PMC3259446.

27. Güven SŞ, Cabbar F, Güler N. 2020; Local and systemic factors associated with marginal bone loss around dental implants: a retrospective clinical study. Quintessence Int. 51:128–41. https://doi.org/10.3290/j.qi.a42950. DOI: 10.3290/j.qi.a42950. PMID: 31942574.

28. Raikar S, Talukdar P, Kumari S, Panda SK, Oommen VM, Prasad A. 2017; Factors affecting the survival rate of dental implants: a retrospective study. J Int Soc Prev Community Dent. 7:351–5. https://doi.org/10.4103/jispcd.JISPCD_380_17. DOI: 10.4103/jispcd.JISPCD_380_17. PMID: 29387619. PMCID: PMC5774056.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download