Abstract
Germline DDX41 lesions indicate a hereditary myelodysplastic syndrome and acute myeloid leukemia (MDS/AML). Canonical somatic mutations in this gene often coincide as a second hit with germline DDX41 mutations. We report a patient with inherited MDS/AML containing novel germline DDX41 mutations that harbor somatic mutations in the other DDX41 allele. The 72-year-old woman was diagnosed with myelodysplastic syndrome with excess blasts-2 (MDS-EB-2) and had gone through 16 rounds of chemotherapy. However, an increase in leukemic myeloblasts was observed in the bone marrow aspiration, resulting in transition to AML. Targeted gene panel sequencing revealed DDX41 c.308_309del (p.Glu103Valfs*31) with a variant allele frequency (VAF) of 49%, and DDX41 c.1589G>A (p.Gly530Asp) with a VAF of 6%. The c.308_309del variant was confirmed as a germline variant after analyzing buccal DNA. An identical germline DDX41 mutation was detected in her unaffected daughter and son. The patient was repeatedly hospitalized for neutropenic fever and eventually expired on account of sepsis. Genetic investigation is crucial for providing appropriate medical management to patients and determining the prognosis of a disease. In addition, it helps to provide appropriate counseling and raise awareness of inherited hematologic malignancies for family members.
초록
DDX41 유전자의 생식세포 변이는 유전성 골수형성이상증후군/급성골수성백혈 병(MDS/AML)에서 나타난다. DDX41 유전자는 생식세포 변이와 더불어 해당 유전자에서의 체세포 변이도 빈번하게 동반된다. 저자들은 이전에 보고되지 않았던 생식세포 DDX41 돌연변이를 가지면서 동일한 유전자의 체세포 돌연변이도 함께 가지고 있는 유전성 MDS/AML 환자 1예를 경험하였기에 이를 보고하고자 한다. 72세 여자환자는 MDS with excess blasts-2를 진단받고 항암치료를 진행하였으나, 골수검사상 진단 전보다 증가된 백혈병성 골수모구가 관찰되면서 AML로 재진단 받게 되었다. 추가로 시행한 표적 유전자 패널 염기서열분석법 결과 대립유전자빈도(VAF) 49%의 DDX41 c.308_309del (p.Glu103Valfs*31) 변이와 VAF 6%의 DDX41 c.1589G>A (p.Gly530Asp) 변이가 발견되었다. c.308_309del 변이는 구강 DNA 분석을 통해 생식세포 변이임이 확인되었으며, 환자의 딸과 아들에서도 동일한 생식세포 DDX41 돌연변이가 관찰되었다. 환자는 호중구감소성 발열로 인해 반복적인 입원치료를 하였으나 결국 패혈증으로 사망하였다. 유전자 검사는 질병의 예후를 결정하고 환자에게 적절한 치료를 제공하는데 있어서 매우 중요하다. 또한, 환자의 가족들에게 적절한 유전 상담을 시행하여 유전성 혈액암에 대한 인식을 높이는 것이 도움이 될 것이다.
Myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) typically present as sporadic diseases. However, some cases of MDS or AML are associated with germline mutations harboring specific genetic and clinical characteristics [1, 2]. To date, 10 genes have been identified in familial MDS or AML: RUNX1, CEBPA, TERC, TERT, GATA2, SRP72, ANKRD26, ACD, ETV6, and DDX41 [3].
MDS or AML with the germline DDX41 variant is characterized by a long latency, an advanced disease (high-risk MDS/AML), and a normal karyotype. [4]. The DDX41 gene, located on chromosome 5q35, is composed of 17 exons and encodes a DEAD (Aspartic acid-Glutamic acid-Alanine-Aspartic acid)-box helicase. As a tumor suppressor gene, it participates in pre-mRNA splicing and processing [4, 5]. The reported frequency of germline DDX41 variants is approximately 1–5% of all myeloid neoplasms [6-10]. In addition to germline DDX41 variants, somatic variants of this gene are often concomitant. Approximately 70% of MDS/AML cases that develop in germline carriers harbor a mutation on the other allele of DDX41 [9, 11]. To date, 177 different DDX41 gene mutations have been listed in the Bibliome Variant Database (https://bibliome.ai). However, a frameshift mutation of DDX41 c.308_309del, p.(Glu103Valfs*31) introduced in this case has never been reported earlier. In this report, we present a patient with inherited MDS/AML possessing a novel germline DDX41 variant that harbors a somatic mutation in the other allele.
A 72-year-old woman presented to the emergency department with fever and general weakness. Complete blood count (CBC) results revealed pancytopenia with a hemoglobin (Hb) value of 9.2 g/dL, white blood cell (WBC) count of 1.50×109/L, and platelet count of 31×109/L. Peripheral blood smear (PBS) results revealed a few blasts without any dysplastic cells. A bone marrow smear revealed slightly hypocellular marrow with 16.2% leukemic blasts of all nucleated cells. The patient was diagnosed with myelodysplastic syndrome with excess blasts-2 (MDS-EB-2) displaying a normal karyotype. She started chemotherapy with decitabine because of a high-risk score evaluated (risk score 6.5) according to the revised International Prognostic Scoring System (IPSS-R) category [12]. Following the 4th chemotherapy treatment, the PBS result showed no leukemic blasts with increased CBC parameters (Hb of 11.8 g/dL, WBC count of 2.70×109/L and platelet count of 143×109/L).
A follow-up bone marrow smear revealed normocellular marrow with 2.0% leukemic blasts of all nucleated cells, indicating complete remission. However, despite the 16th chemotherapy, pancytopenia persisted for 8 weeks. Moreover, leukemic myeloblasts (23.4% of all nucleated cells) were observed in the bone marrow sample, implying a transformation from MDS-EB-2 to AML. Chromosome analysis showed a normal karyotype in the follow-up studies. A multiplex nested reverse-transcription polymerase chain reaction analysis with HemaVision (DNA Diagnostic A/S, Risskov, Denmark), which enables the detection of 28 chromosomal rearrangements associated with acute leukemia, did not detect any rearrangement abnormalities. Targeted gene panel sequencing included AML-related 49 genes that were sequenced with MiSeq (Illumina, San Diego, CA, USA) according to manufacturer’s instructions. The sequencing results revealed the following: NM_000546.5:c.646G>A, p.(Val216Met), with an 8% variant allele frequency (VAF) of TP53, and NM_016222.2:c.1589G>A, p.(Gly530Asp), with 6% VAF of DDX41. These variants were confirmed as somatic variants because they were not detected in the buccal swab of the patient.
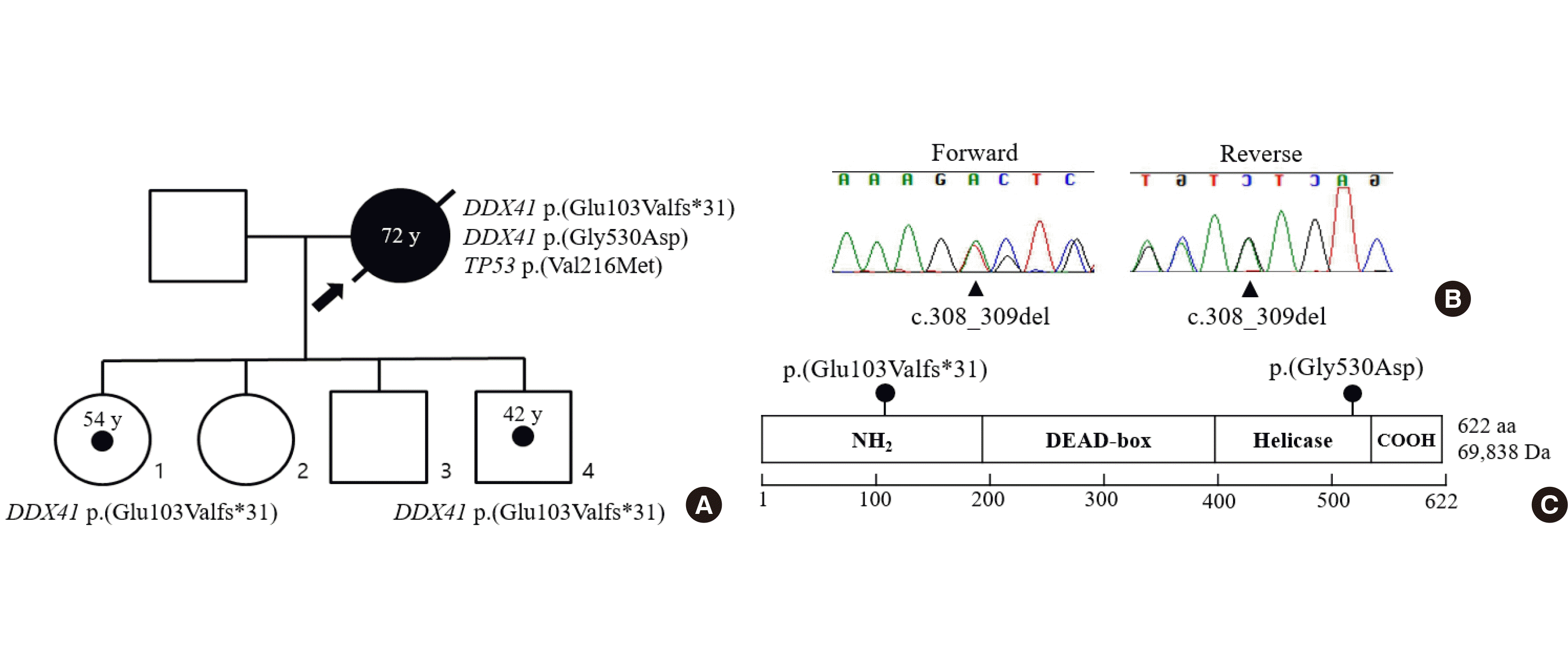
The panel also detected one suspected germline variant, NM_016222.2:c.308_309del, p.(Glu103Valfs*31) of DDX41, with a 49% VAF. This variant was confirmed to be germline in origin as it was detected in the buccal swab of the patient. The variant was also predicted to undergo non-mediated decay in exon 4 and form a frameshift change. In addition, the variant was not found in the gnomAD exome database or KRGDB (Korean Registry Gene Database). Consequently, it was interpreted as a likely pathogenic variant according to the 2015 American College of Medical Genetics and Genomics and the Association for Molecular Pathology Guidelines [13]. According to OMIM, the DDX41 gene is associated with familial myeloproliferative/lymphoproliferative neoplasms (OMIM 608170) in an autosomal dominant inheritance pattern. Upon clinical and genetic investigations of the patient’s family, an identical germline DDX41 gene variant was found in her 54-year-old unaffected daughter and 42-year-old unaffected son (Fig. 1). After being diagnosed with AML, the patient was repeatedly hospitalized owing to neutropenic fever, but eventually expired on account of sepsis.
DDX41 encodes an RNA helicase, and also acts as a tumor suppressor gene [6]. It contributes to specific processes within the cell, including pre-mRNA splicing, innate immunity, and ribosome biogenesis [14]. Specifically, mRNA splicing and ribosome biogenesis may be closely related to DDX41-driven leukemogenesis. DDX41 can be divided into four main domains: N-terminal, DEAD-box, helicase, and C-terminal [14]. The germline p.(Glu103Valfs*31) and somatic p.(Gly530Asp) variants of the DDX41 gene are located in the N-terminal and helicase domains, respectively. Defects in the helicase domain can lead to altered RNA splicing, such as exon skipping or exon retention. A specific mis-splicing pattern in°uences the altered expression of specific downstream genes, leading to leukemogenesis [4]. DDX41 also contributes to the processing of precursor ribosomal RNA to mature rRNAs in ribosome biogenesis [15, 16]. Kadono et al. [16] reported that an overexpression of the DDX41 R525H mutant located in the helicase domain leads to decreased hematopoietic stem cell growth. However, the effect of the DDX41 mutation on cell growth via ribosome biogenesis has yet to be elucidated [14]. Compared to the helicase domain, little information about the N-terminal domain has been revealed.
A DDX41 germline variant was first introduced by Polprasert et al. in 2015 [4]. Since then, novel variants of DDX41 have been continuously discovered. The germline p.(Glu103Valfs*31) frameshift variant found in this case is a novel variant, and the p.(Gly530Asp) missense somatic variant has been reported in four patients with AML, according to the literature [6, 14].
Some genetic predisposing factors for myeloid malignancies (e.g., RUNX1, CEBPA, and GATA2) [17-19] are often associated with a younger age of malignancy onset. Compared with these predisposing genes, familial myeloid malignancies associated with DDX41 are late-onset at an age similar to those observed in de novo MDS or AML [6]. In the reported germline DDX41 mutation cases, the median age at which myeloid hematologic malignancies were diagnosed was 65 years, according to Cheah et al. [14], and 62 years, according to Lewinsohn et al. [6], outlining the long latency of this case. This feature of a long latency period makes it difficult for physicians to investigate the histories of other family members.
Germline DDX41 variants induce MDS/AML with substantial penetrance [4, 6]. In this case, both the unaffected 42-year-old son and the 54-year-old daughter were carriers of this variant. Currently, there are no consensus guidelines for family genetic counseling. However, Lewinsohn et al. reported germline variant carriers who develop MDS or AML that usually present with leukopenia (83.3%) with or without macrocytosis or other cytopenias [6]. In this case, the son and daughter had no abnormal findings on the CBC and PBS examination. Thus, CBC and PBS monitoring may be needed for clinical follow-up of asymptomatic carriers.
The prognostic significance of myeloid neoplasms with germline DDX41 variants remains unclear. Polprasert et al. reported a shortened overall survival in patients with DDX41-related MDS/AML than the wild-type cases [4]. In contrast, Sébert et al. reported that patients with DDX41-related inherited hematological malignancies had a longer median overall survival (5.2 years) than the wild-type matched controls (2.7 years) [9]. Li et al. also reported that all affected patients with germline DDX41 variants had complete remission following bone marrow transplant [11]. In this case, despite chemotherapy, the disease progressed rapidly, and the patient died two years after the diagnosis of MDS-EB-2, implying poor prognosis.
Germline DDX41 variants are also associated with hypocellular bone marrow, a higher risk of MDS/AML, and a normal karyotype [14]. In our case, the patient showed similar characteristics at the time of diagnosis. When these features are found in patients with late-onset MDS/AML, the possibility of harboring a DDX41 variant should be considered.
Germline alterations may cause predisposal to somatic mutations in the same gene [4]. Similar to CEBPA and JAK2 [17, 20], germline variants of DDX41 serve as a first hit that significantly enhances the likelihood of subsequent development of leukemia. Canonical somatic variants in the other allele of this gene coincide as a second hit with the germline DDX41 mutation, often at a low VAF during the initiation of leukemogenesis [4, 14]. Consequently, we hypothesize that our patient with the p.(Glu103Valfs*31) germline variant of the DDX41 had a long latency in an asymptomatic carrier state. However, owing to an unexplained cause, a somatic mutation p.(Gly530Asp) occurred in the same gene, as a second hit and caused a loss of function in the tumor suppressor gene, resulting in MDS/AML.
In conclusion, we have identified a novel germline DDX41 mutation in a 72-year-old female patient diagnosed with MDS/AML. Her clinical features were consistent with those of other patients with previously reported DDX41 variants. Genetic investigation is crucial for providing appropriate medical management to patients and determining the prognosis of the disease. In addition, it is helpful to provide appropriate genetic counseling and raise awareness of familial hematologic malignancies to family members. Guidelines for family genetic counseling and management of carriers in the family should be implemented.
REFERENCES
1. Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. 2016; The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 127:2391–405. DOI: 10.1182/blood-2016-03-643544. PMID: 27069254.

2. West AH, Godley LA, Churpek JE. 2014; Familial myelodysplastic syndrome/acute leukemia syndromes: a review and utility for translational investigations. Ann N Y Acad Sci. 1310:111–8. DOI: 10.1111/nyas.12346. PMID: 24467820. PMCID: PMC3961519.

3. Cardoso SR, Ryan G, Walne AJ, Ellison A, Lowe R, Tummala H, et al. 2016; Germline heterozygous DDX41 variants in a subset of familial myelodysplasia and acute myeloid leukemia. Leukemia. 30:2083–6. DOI: 10.1038/leu.2016.124. PMID: 27133828. PMCID: PMC5008455.

4. Polprasert C, Schulze I, Sekeres MA, Makishima H, Przychodzen B, Hosono N, et al. 2015; Inherited and somatic defects in DDX41 in myeloid neoplasms. Cancer Cell. 27:658–70. DOI: 10.1016/j.ccell.2015.03.017. PMID: 25920683. PMCID: PMC8713504.

5. Linder P. 2006; Dead-box proteins: a family affair-active and passive players in RNP-remodeling. Nucleic Acids Res. 34:4168–80. DOI: 10.1093/nar/gkl468. PMID: 16936318. PMCID: PMC1616962.

6. Lewinsohn M, Brown AL, Weinel LM, Phung C, Rafidi G, Lee MK, et al. 2016; Novel germ line DDX41 mutations define families with a lower age of MDS/AML onset and lymphoid malignancies. Blood. 127:1017–23. DOI: 10.1182/blood-2015-10-676098. PMID: 26712909. PMCID: PMC4968341.
7. Polprasert C, Takeda J, Niparuck P, Rattanathammethee T, Pirunsarn A, Suksusut A, et al. 2020; Novel DDX41 variants in Thai patients with myeloid neoplasms. Int J Hematol. 111:241–6. DOI: 10.1007/s12185-019-02770-3. PMID: 31713024.

8. Qu S, Li B, Qin T, Xu Z, Pan L, Hu N, et al. 2021; Molecular and clinical features of myeloid neoplasms with somatic DDX41 mutations. Br J Haematol. 192:1006–10. DOI: 10.1111/bjh.16668. PMID: 32307695. PMCID: PMC9205684.

9. Sébert M, Passet M, Raimbault A, Rahmé R, Raffoux E, Sicre de Fontbrune F, et al. 2019; Germline DDX41 mutations define a significant entity within adult MDS/AML patients. Blood. 134:1441–4. DOI: 10.1182/blood.2019000909. PMID: 31484648.
10. Takeda J, Yoshida K, Makishima H, Yoshizato T, Shiozawa Y, Shiraishi Y, et al. 2015; Genetic predispositions to myeloid neoplasms caused by germline DDX41 mutations. Blood. 126:2843. DOI: 10.1182/blood.V126.23.2843.2843.

11. Li R, Sobreira N, Witmer PD, Pratz KW, Braunstein EM. 2016; Two novel germline DDX41 mutations in a family with inherited myelodysplasia/acute myeloid leukemia. Haematologica. 101:e228–31. DOI: 10.3324/haematol.2015.139790. PMID: 26944477. PMCID: PMC5013967.
12. Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F, et al. 2012; Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 120:2454–65. DOI: 10.1182/blood-2012-03-420489. PMID: 22740453. PMCID: PMC4425443.

13. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. 2015; Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 17:405–24. DOI: 10.1038/gim.2015.30. PMID: 25741868. PMCID: PMC4544753.

14. Cheah JJC, Hahn CN, Hiwase DK, Scott HS, Brown AL. 2017; Myeloid neoplasms with germline DDX41 mutation. Int J Hematol. 106:163–74. DOI: 10.1007/s12185-017-2260-y. PMID: 28547672.
15. Jiang Y, Zhu Y, Liu ZJ, Ouyang S. 2017; The emerging roles of the DDX41 protein in immunity and diseases. Protein Cell. 8:83–9. DOI: 10.1007/s13238-016-0303-4. PMID: 27502187. PMCID: PMC5291771.

16. Kadono M, Kanai A, Nagamachi A, Shinriki S, Kawata J, Iwato K, et al. 2016; Biological implications of somatic DDX41 p.R525H mutation in acute myeloid leukemia. Exp Hematol. 44:745–754.e4. DOI: 10.1016/j.exphem.2016.04.017. PMID: 27174803.
17. Smith ML, Cavenagh JD, Lister TA, Fitzgibbon J. 2004; Mutation of CEBPA in familial acute myeloid leukemia. N Engl J Med. 351:2403–7. DOI: 10.1056/NEJMoa041331. PMID: 15575056.

18. Hahn CN, Chong CE, Carmichael CL, Wilkins EJ, Brautigan PJ, Li XC, et al. 2011; Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat Genet. 43:1012–7. DOI: 10.1038/ng.913. PMID: 21892162. PMCID: PMC3184204.

19. Michaud J, Wu F, Osato M, Cottles GM, Yanagida M, Asou N, et al. 2002; In vitro analyses of known and novel RUNX1/AML1 mutations in dominant familial platelet disorder with predisposition to acute myelogenous leukemia: implications for mechanisms of pathogenesis. Blood. 99:1364–72. DOI: 10.1182/blood.V99.4.1364. PMID: 11830488.
20. Kilpivaara O, Mukherjee S, Schram AM, Wadleigh M, Mullally A, Ebert BL, et al. 2009; A germline JAK2 SNP is associated with predisposition to the development of JAK2V617F-positive myeloproliferative neoplasms. Nat Genet. 41:455–9. DOI: 10.1038/ng.342. PMID: 19287384. PMCID: PMC3676425.

Fig. 1
(A) Family pedigree of the patient. The arrow indicates the proband. Circled dots indicate heterozygous carriers of the germline DDX41 p.(Glu103Valfs*31) variant. Child 2 and 3 refused genetic testing. (B) Electropherogram showing the germline heterozygous c.308_309del variant detected in the peripheral blood sample of the patient. (C) DDX41 protein structure with germline DDX41 variants reported in this case.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download