Abstract
Following the original severe acute respiratory syndrome coronavirus 2 strain (Wuhan-Hu-1) in December 2019, the Delta variant in May 2021 and the Omicron variant in December 2021 were classified as variants of concern. The pandemic has been ongoing for more than two years, and the three-dose vaccination rate has reached approximately 50% in Korea. We analyzed anti-S antibodies (Abs) and neutralizing Abs (NAbs) in 32 healthcare workers at a university hospital, focusing on the first to third doses of ChAdOx1-ChAdOx1-BNT162b2, which is the most common vaccination regimen in Korea. Antibodies were analyzed at eight time points according to the vaccine regimen. The first to third doses of ChAdOx1-ChAdOx1-BNT162b2 produced high Ab concentrations; NAb concentrations after the third dose were predicted to remain high for a longer period than those after the first and second doses. The effectiveness of a second dose of ChAdOx1 in the real world was demonstrated by analyzing samples collected during an outbreak that occurred in the study period, 4–5 months after the second dose. The relative risk ratio was 88.0%, and the efficacy of the second ChAdOx1 dose was 12.0% (P<0.05). Therefore, maintaining appropriate Ab concentrations through regular vaccination will help protect against coronavirus disease-19.
Two years have passed since the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in December 2019. Korean Society for Laboratory Medicine and the Korea Centers for Disease Prevention and Control proposed guidelines to prevent the spread of the disease through rapid and accurate diagnosis of COVID-19 in Korea [1]. After that, vaccination has been used to in an attempt to achieve herd immunity for coronavirus disease (COVID-19). As the pandemic continued, antibodies (Abs) produced after the first and second doses were gradually exhausted. The neutralizing Abs (NAbs) induced by the vaccines targeting the original strain were less effective against newly emerging variants [2–4]. As the need for a third dose increased, studies to verify its safety and effectiveness were conducted [5–8]. In Korea, by 16 February 2022, 86.2% and 58.0% of the population had received second and third doses, respectively [9]. We evaluated the immunogenicity and side effects, focusing on the first to third doses of ChAdOx1-ChAdOx1-BNT162b2, the most common vaccination regimen in Korea [9]. For approximately one year, we measured Ab concentrations and evaluated side effects after each dose in 32 healthcare workers (HCWs) longitudinally. As an in-hospital outbreak occurred 4–5 months after the second dose was administered, we also analyzed the effectiveness of the vaccines in the “real world” of a university hospital in Korea.
The study was conducted between March 22, 2021 and May 31, 2022 and was approved by the Institutional Review Board of Soonchunhyang University Seoul hospital, Seoul, Korea (approval number: SCHUH-2021-03-015). The subjects were HCWs at a university hospital in Seoul; their baseline characteristics are listed in Table 1. The first (March 3–5, 2021) and second (May 25–27, 2021) doses were ChAdOx1 (AstraZeneca, Cambridge, UK), and the third (November 22–26, 2021) dose was BNT162b2 (Pfizer, New York, USA). Blood samples were collected at eight time points (immediately before the first dose; two weeks, one month, and three months after the first dose; one month, four months, and six months after the second dose; and one month after the third dose) and stored in serum separating tubes. Anti-S Abs and NAbs were quantified using widely available assays, which had shown good performance [10]. Total anti-S Abs (including IgG and IgM) against the receptor-binding domain (RBD) of SARS-CoV-2 were measured by an electrochemiluminescence immunoassay using the Elecsys Anti-SARS-CoV-2 S kit (Roche Diagnostics, Mannheim, Germany). It is a quantitative assay that detects anti-S Abs with a threshold value of 0.8 U/mL for positivity and an upper limit of detection of 250.0 U/mL (analyzed up to a 1/400 serum dilution). The signal inhibition rate (SIR) was used to assess the capacity of the Abs to neutralize a surrogate virus. The cPass ELISA-based SARS-CoV-2 Surrogate Virus Neutralization Ab Detection Kit (GenScript, Piscataway, NJ, USA) assesses the Ab-mediated inhibition of SARS-CoV-2 RBD binding to the human host receptor angiotensin-converting enzyme 2 (ACE2) as an indirect detection method. The SIR was calculated using the following formula:
Samples were judged positive if they exhibited an SIR >30.0%.
The association between the NAb SIR and side effects following vaccination was analyzed by logistic regression using a generalized estimating equation (GEE) model [11]. A t-test was used to analyze the relationship between the NAb SIR and side effects according to the time point of each dose. Fisher’s exact test was used to determine whether the second dose was related to infection in vaccinated or unvaccinated patients in an in-hospital outbreak analysis. The relative infection risk ratio (RR) and vaccine effectiveness were calculated using the following formulas [12]:
All statistical analyses were performed using SPSS Statistics v.26 (IBM Corp., Armonk, NY, USA), and P<0.05 was considered significant.
The mean anti-S Ab concentration and NAb SIR two weeks after the first dose turned to be positive. One month after the first dose, the anti-S Ab concentration increased and NAb SIR decreased slightly. The mean anti-S Ab concentration three months after the first dose remained unchanged, and the NAb SIR was decreased. After the second dose, increased anti-S Ab and NAb SIR concentrations fell to the original concentrations at a rate slower than that after the first dose. After the third dose, anti-S Ab concentration increased 44.3-fold, and NAb SIR increased 1.8-fold, respectively. As for anti-S Ab concentrations, compared to right before the second dose (three months after the first dose), it increased 12.1-fold one month after the second dose, and 155.3-fold one month after the third dose. Accordingly, NAb concentrations increased 3.4- and 5.3-fold, respectively (Fig. 1).
The frequency of any side effects following the first to third doses were 93.8%, 63.3%, and 93.1% (Table 1). The most frequent side effects were myalgia, fever, and local pain after the first dose and local pain, fatigue, and myalgia after the second and third doses. The NAb SIR was 47.1-fold higher (P<0.05, GEE) in the group with systemic side effects than in that without. The differences in the NAb SIR according to local side effects, sex, and age were not significant. According to the time point of each dose, after the first dose, the NAb SIR was 18.2-fold higher in the group with systemic side effects than in that without (P=0.044, t-test). After the second and third doses, the NAb SIR was 4.7-fold and 1.9-fold higher in the group with systemic side effects than in the group without, respectively, but the differences were not significant.
A COVID-19 cluster outbreak among hospital HCWs occurred on September 17, 2021, 4–5 months after the second dose (Fig. 2). Among 2,185 HCWs, 1,682 (77.0%) had received the second dose of ChAdOx1, and 48 (2.9%) were infected. Of the 247 unvaccinated HCWs, eight (3.2%) were infected. The relative RR was 88.0%, and the effectiveness of the second ChAdOx1 dose was 12.0% (Fisher’s exact test, P<0.05). The mean anti-S Ab concentration and NAb SIR of HCWs who participated in this study were 293.1 U/mL and 52.6%, respectively. In a population-based cohort study of two-dose ChAdOx1 nCoV-19 vaccines in Scotland [13], the effectiveness decreased from 83.7% at 2–3 weeks to 63.7% at 18–19 weeks after the second dose. Like in our study, the Delta variant was dominant in Scotland from May 19, 2021 to Oct 25, 2021. It was confirmed that Ab concentrations decreased over time after vaccination, and vaccine effectiveness declined. Since we analyzed a hospital outbreak, the effectiveness was lower than that in the Scotland study, but the protective effect of vaccination was confirmed. Two months before the second dose, the mean NAb SIR decreased from 40.8% to 17.9% (a 22.9% reduction). The decrease in NAb SIR (17.9%) was lower than the cut-off concentration of 30.0% (Fig. 1). However, two months before the third dose, the mean NAb SIR decreased from 55.3% to 52.6% (a 2.7% reduction). This decrease in the NAb SIR was smaller than that before, and the NAb seropositivity rates and SIR remained higher than the highest concentrations following the first dose. Therefore, after the third dose, the NAb SIR is higher and remains high for a longer period than after previous doses.
By January 2022, the Omicron variant accounted for 50.3% of SARS-CoV-2 infections in Korea [14]. The effectiveness of existing vaccines for the Omicron variant is substantially reduced [15, 16]. According to the Korea Disease Control and Prevention Agency [14], among those who received ChAdOx1-ChAdOx1-BNT162b2, the mean geometric mean titers of 50% neutralizing dilution (GMT ND50) of the original strain and the Omicron variant are 4,632.0 and 260.0, respectively. However, compared to those right before the third dose, the GMT ND50 after the third dose increased by 21.0- and 28.9-fold, respectively. Memory T cells induced by existing vaccines can effectively act on the Omicron variants [17]. In heterologous ChAdOx1-BNT162b2 vaccination, both humoral and cellular responses were higher than those after homologous vaccination; NAb concentrations after ChAdOx1-BNT162b2 vaccination were 31.9- and 1.9-fold higher, respectively, than those after ChAdOx1-ChAdOx1 and BNT162b2-BNT162b2 vaccination [16], and cellular responses were 3.8- and 2.3-fold higher [19], respectively. According to the UK Health Security Agency [20], the effectiveness of a third dose of BNT162b2 following two doses of ChAdOx1 or BNT162b2 in terms of symptomatic disease, hospitalization for six months, and mortality up to three months against the Omicron variant was 40.0%–75.0%, 75.0%–95.0%, and 85.0%–99.0%, respectively.
This study had some limitations. First, the sex and age groups were not uniform owing to an insufficient number of subjects. Second, a control group for comparing Ab concentrations (other vaccinated or unvaccinated group) was not included. Third, Ab concentrations were followed up until one month after the third dose. However, our data are valuable for predicting Ab kinetics because ChAdOx1-ChAdOx1-BNT162b2 is the most common vaccination regimen in Korea. Moreover, we analyzed an in-hospital outbreak to assess the effectiveness of vaccines.
In conclusion, we showed that the first to third doses of ChAdOx1-ChAdOx1-BNT162b2 induced high Ab concentrations, and our results suggested that NAb concentrations remain high for a longer period after the third dose than after the first and second doses. Although the outbreak occurred as the NAb SIR decreased after the second dose, vaccine effectiveness was confirmed by the lower infection rate. Therefore, maintaining appropriate Ab concentrations through regular vaccination will help protect against COVID-19.
ACKNOWLEDGMENTS
We thank all the healthcare workers, especially our colleagues in the laboratory department of Soonchunhyang University Hospital, Seoul.
Notes
AUTHOR CONTRIBUTIONS
Kim JA: Data curation, formal analysis, and original draft writing. Bang HI: Conceptualization, project administration, formal analysis, funding acquisition, writing, review. Shin JW: Conceptualization and funding acquisition. Park Y: Methodology. Kim S: Methodology. Kim MY: Methodology. Jang EY: Methodology. Shin WY: Review. Kim J: Review. Park R: Review. Choi TY: Review.
REFERENCES
1. Hong KH, Lee SW, Kim TS, Huh HJ, Lee J, Kim SY, et al. 2020; Guidelines for Laboratory Diagnosis of Coronavirus Disease 2019 (COVID-19) in Korea. Ann Lab Med. 40:351–60. DOI: 10.3343/alm.2020.40.5.351. PMID: 32237288. PMCID: PMC7169629.

2. Yadegari I, Omidi M, Smith SR. 2021; The herd-immunity threshold must be updated for multi-vaccine strategies and multiple variants. Sci Rep. 11:22970. DOI: 10.1038/s41598-021-00083-2. PMID: 34836984. PMCID: PMC8626504.

3. Tré-Hardy M, Cupaiolo R, Wilmet A, Antoine-Moussiaux T, Della Vecchia A, Horeanga A, et al. 2021; Immunogenicity of mRNA-1273 COVID vaccine after 6 months surveillance in health care workers; a third dose is necessary. J Infect. 83:559–64. DOI: 10.1016/j.jinf.2021.08.031. PMID: 34437927. PMCID: PMC8380546.

4. Mishra SK, Pradhan SK, Pati S, Sahu S, Nanda RK. 2021; Waning of anti-spike antibodies in AZD1222 (ChAdOx1) vaccinated healthcare providers: a prospective longitudinal study. Cureus. 13:e19879. DOI: 10.7759/cureus.19879.

5. Patalon T, Gazit S, Pitzer VE, Prunas O, Warren JL, Weinberger DM. 2022; Odds of testing positive for SARS-CoV-2 following receipt of 3 vs 2 doses of the BNT162b2 mRNA vaccine. JAMA Intern Med. 182:179–84. DOI: 10.1001/jamainternmed.2021.7382. PMID: 34846533.

6. Barda N, Dagan N, Cohen C, Hernán MA, Lipsitch M, Kohane IS, et al. 2021; Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 398:2093–100. DOI: 10.1016/S0140-6736(21)02249-2.

7. Flaxman A, Marchevsky NG, Jenkin D, Aboagye J, Aley PK, Angus B, et al. 2021; Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: a substudy of two randomised controlled trials (COV001 and COV002). Lancet. 398:981–90. DOI: 10.1016/S0140-6736(21)01699-8.
8. Munro APS, Janani L, Cornelius V, Aley PK, Babbage G, Baxter D, et al. 2021; Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 398:2258–76. DOI: 10.1016/S0140-6736(21)02717-3.
9. Korea Disease Control and Prevention Agency (KDCA), Bureau of Infectious Disease Emergency Preparedness and Response. COVID-19 vaccination and domestic outbreak status (2.16). https://www.kdca.go.kr/board/board.es?mid=a20501010000&bid=0015&list_no=718680&cg_code=&act=view&nPage=1. Updated on March 24, 2022.
10. Yun S, Ryu JH, Jang JH, Bae H, Yoo SH, Choi AR, et al. 2021; Comparison of SARS-CoV-2 Antibody Responses and Seroconversion in COVID-19 Patients Using Twelve Commercial Immunoassays. Ann Lab Med. 41:577–87. DOI: 10.3343/alm.2021.41.6.577. PMID: 34108285. PMCID: PMC8203431.

11. Wang M, Kong L, Li Z, Zhang L. 2016; Covariance estimators for generalized estimating equations (GEE) in longitudinal analysis with small samples. Stat Med. 35:1706–21. DOI: 10.1002/sim.6817. PMID: 26585756. PMCID: PMC4826860.

12. Olliaro P, Torreele E, Vaillant M. 2021; COVID-19 vaccine efficacy and effectiveness-the elephant (not) in the room. Lancet Microbe. 2:E279–80. DOI: 10.1016/S2666-5247(21)00069-0.

13. Katikireddi SV, Cerqueira-Silva T, Vasileiou E, Robertson C, Amele S, Pan J, et al. 2022; Two-dose ChAdOx1 nCoV-19 vaccine protection against COVID-19 hospital admissions and deaths over time: a retrospective, population-based cohort study in Scotland and Brazil. Lancet. 399:25–35. DOI: 10.1016/S0140-6736(21)02754-9.

14. Korea Disease Control and Prevention Agency (KDCA), Bureau of Infectious Disease Emergency Preparedness and Response. Analysis of characteristics of omicron variation and preparedness of its diffusion (1.24). https://www.kdca.go.kr/board/board.es?mid=a20501010000&bid=0015&list_no=718415&cg_code=&act=view&nPage=1.
15. Collie S, Champion J, Moultrie H, Bekker LG, Gray G. 2022; Effectiveness of BNT162b2 vaccine against omicron variant in South Africa. N Engl J Med. 386:494–6. DOI: 10.1056/NEJMc2119270. PMID: 34965358. PMCID: PMC8757569.

16. Lu L, Mok BW, Chen LL, Chan JM, Tsang OT, Lam BH, et al. Neutralization of SARS-CoV-2 omicron variant by sera from BNT162b2 or Coronavac vaccine recipients. Clin Infect Dis. 2021; ciab1041. DOI: 10.1101/2021.12.13.21267668.

17. Choi SJ, Kim DU, Noh JY, Kim S, Park SH, Jeong HW, et al. 2022; T cell epitopes in SARS-CoV-2 proteins are substantially conserved in the omicron variant. Cell Mol Immunol. 19:447–8. DOI: 10.1038/s41423-022-00838-5. PMID: 35043006. PMCID: PMC8764507.

18. Tenbusch M, Schumacher S, Vogel E, Priller A, Held J, Steininger P, et al. 2021; Heterologous prime-boost vaccination with ChAdOx1 nCoV-19 and BNT162b2. Lancet Infect Dis. 21:1212–3. DOI: 10.1016/S1473-3099(21)00420-5.

19. Liu X, Shaw RH, Stuart ASV, Greenland M, Aley PK, Andrews NJ, et al. 2021; Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 398:856–69. DOI: 10.1016/S0140-6736(21)01694-9.
20. UK health security agency (UKHSA). COVID-19 vaccine surveillance report-week 5. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1052353/Vaccine_surveillance_report_-_week_5.pdf. Updated on Feb 3, 2022.
Fig. 1
Mean concentrations and seropositivity rates of (A) anti-S Abs and (B) NAbs after the first to third doses of ChAdOx1-ChAdOx1-BNT162b2. Horizontal bars at the top of the figures indicate fold-increases in Abs.
Abbreviations: Abs, antibodies; NAbs, neutralizing antibodies; SIR, signal inhibition rate.
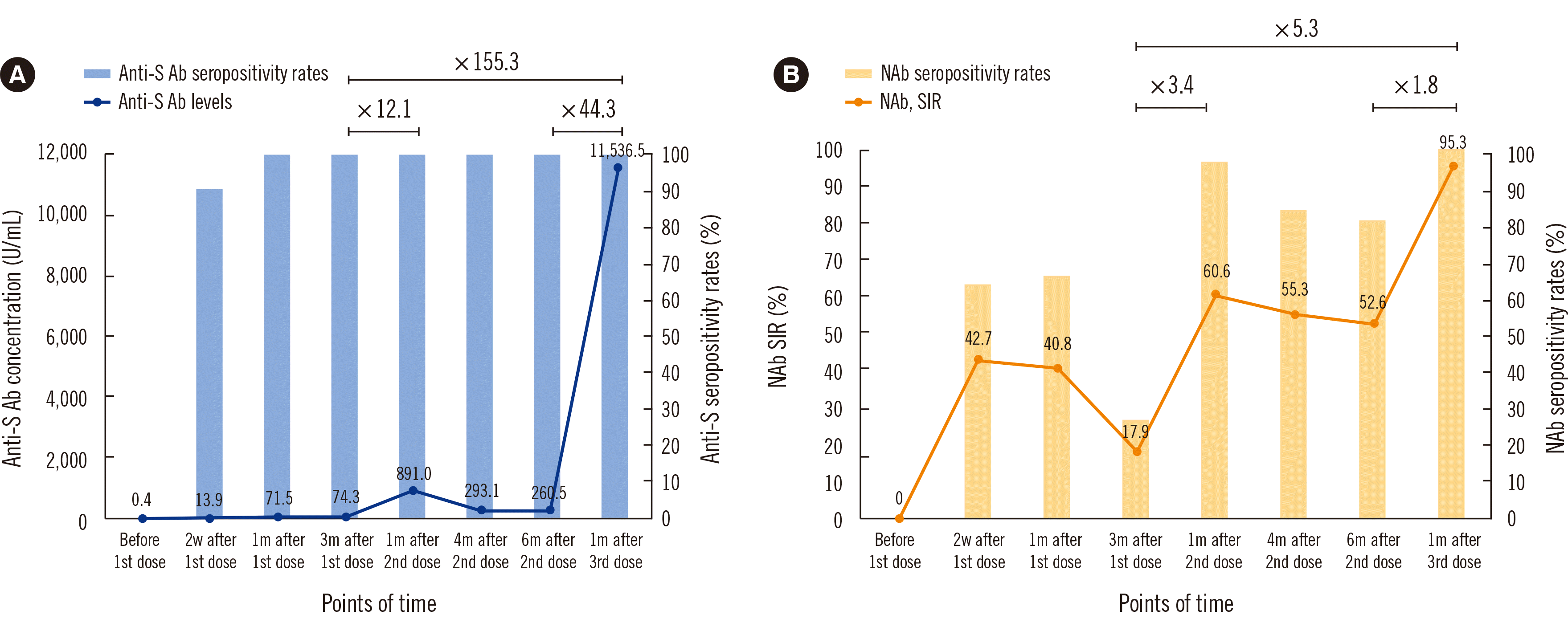
Fig. 2
Timeline of the COVID-19 outbreak among healthcare workers vaccinated with the first to second doses of ChAdOx1-ChAdOx1. The second dose was administered on May 25–27, 2021. Dates highlighted in green indicate the period in which the outbreak occurred.
Abbreviation: N, number.
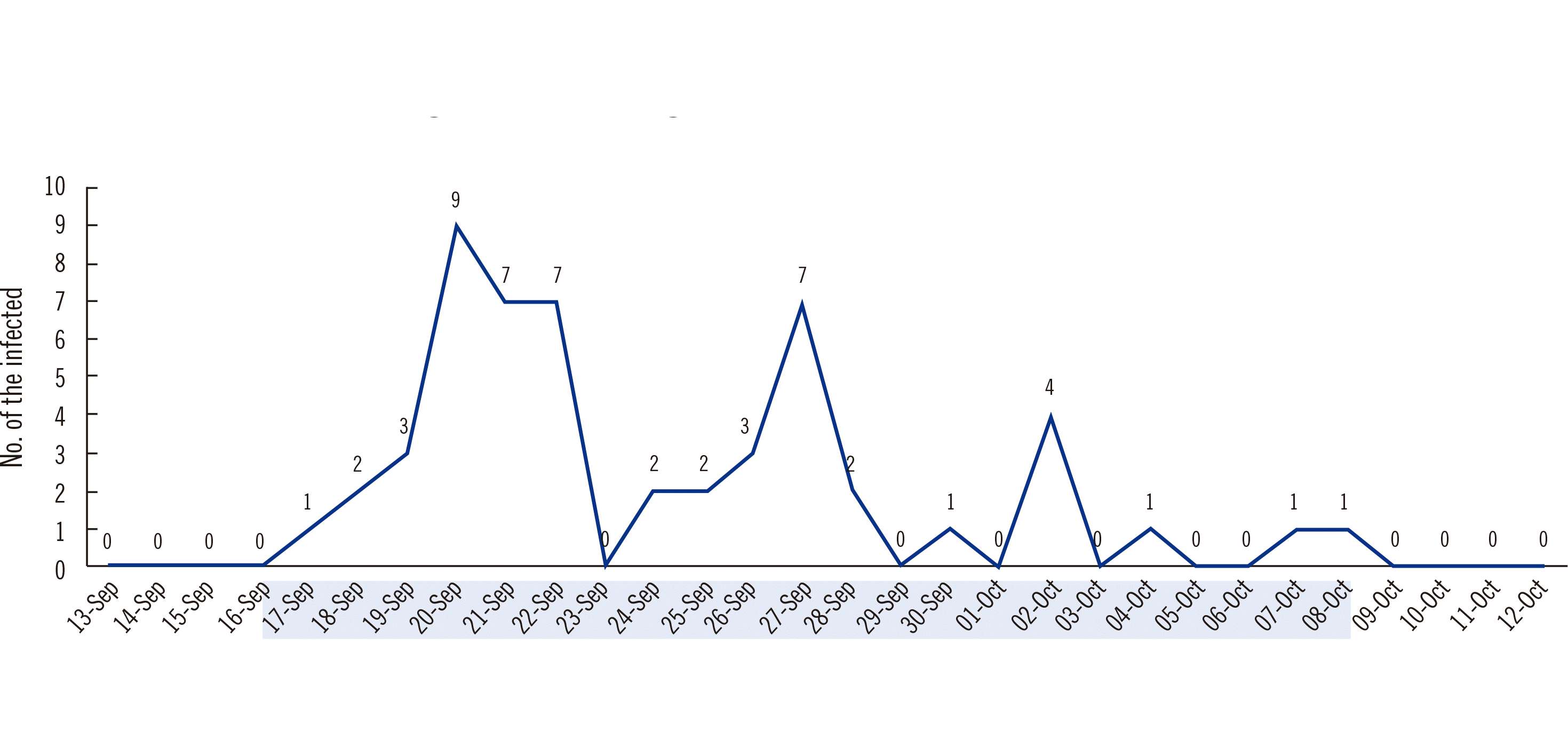
Table 1
Baseline characteristics of and side effects in healthcare workerHCWs in a Korean Uuniversity hospital following the first to third doses of ChAdOx1-ChAdOx1-BNT162b2
| Variable | First dose (N=32) | Second dose (N=30) | Third dose (N=29) |
|---|---|---|---|
| Age group (yr) | |||
| 20–29 | 7 (21.9%) | 5 (16.7%) | 4 (13.8%) |
| 30–39 | 14 (43.8%) | 14 (46.7%) | 13 (44.8%) |
| 40–49 | 1 (3.1%) | 1 (3.3%) | 1 (3.4%) |
| 50–59 | 9 (28.1%) | 9 (30.0%) | 10 (34.5%) |
| 60–69 | 1 (3.1%) | 1 (3.3%) | 1 (3.4%) |
| Sex | |||
| Female | 27 (84.4%) | 25 (83.3%) | 24 (82.8%) |
| Male | 5 (15.6%) | 5 (16.7%) | 5 (17.2%) |
| Medical history | |||
| Presence* | 2 (6.3%) | 2 (6.7%) | 2 (6.9%) |
| Absence | 30 (93.8%) | 28 (93.3%) | 27 (93.1%) |
| Adverse reactions | |||
| None | 2 (6.3%) | 11 (36.7%) | 3 (10.3%) |
| Any | 30 (93.8%) | 19 (63.3%) | 27 (93.1%) |
| Local | 25 (78.1%) | 10 (33.3%) | 18 (62.1%) |
| Local pain | 25 (78.1%) | 10 (33.3%) | 18 (62.1%) |
| Systemic | 29 (90.6%) | 13 (43.3%) | 23 (79.3%) |
| Myalgia | 26 (81.3%) | 7 (23.3%) | 20 (69.0%) |
| Fever | 25 (78.1%) | 5 (16.7%) | 10 (34.5%) |
| Fatigue | 24 (75.0%) | 9 (30.0%) | 16 (55.2%) |
| Headache | 19 (59.4%) | 6 (20.0%) | 9 (31.0%) |
| Vomiting | 5 (15.6%) | 1 (3.3%) | 1 (3.5%) |
| Mental change | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Dyspnea | 1 (3.1%) | 0 (0.0%) | 0 (0.0%) |
| Anaphylaxis | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Other | 3 (9.4%) | 2 (6.7%) | 4 (13.8%) |
| Sputum | 2 (6.3%) | 0 (0.0%) | 0 (0.0%) |
| Sore throat | 0 (0.0%) | 1 (3.3%) | 0 (0.0%) |
| Neck lymphadenopathy | 0 (0.0%) | 1 (3.3%) | 2 (6.9%) |
| Diarrhea | 1 (3.1%) | 0 (0.0%) | 0 (0.0%) |
| Nausea | 0 (0.0%) | 0 (0.0%) | 1 (3.5%) |
| Dizziness | 0 (0.0%) | 0 (0.0%) | 2 (6.9%) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download