Abstract
Human adenoviruses (HAdVs) are a major cause of epidemic keratoconjunctivitis. We investigated the types of adenoviruses responsible for the recent epidemic of keratoconjunctivitis in Korea. From January to November 2019, 218 conjunctival swab samples were collected from patients clinically suspected as having adenoviral keratoconjunctivitis. Genotyping targeting of adenovirus capsid hexon genes was performed using PCR and sequencing. Of the 218 samples collected, 128 (58.7%) were positive for the adenovirus genes by PCR, and 126 samples were successfully genotyped. Adenovirus type 8 (HAdV-D8) was the most common type (67.5%), followed by HAdV-D64 (11.1%), HAdV-D37 (9.5%), HAdV-B3 (5.6%), HAdV-D53 (4.0%), HAdV-E4 (1.6%), and HAdV-D56 (0.8%). Adenoviral keratoconjunctivitis cases were the most frequent in July and August 2019, which were mainly caused by type 8. Phylogenetic analyses revealed little genetic distance among adenoviruses of the same type detected in our study. Our results provide basic data for further studies of adenoviral keratoconjunctivitis.
Human adenoviruses (HAdVs) are associated with a wide range of clinical manifestations, including acute respiratory infections, gastroenteritis, conjunctivitis, hemorrhagic cystitis, and meningoencephalitis [1]. Adenoviral conjunctivitis accounts for up to 75% of all conjunctivitis cases worldwide, with an estimated 20–30 million people suffering from HAdV-associated conjunctivitis annually [2]. HAdVs, belonging to the family Adenoviridae and genus Mastadenovirus, are non-enveloped viruses that are 70–100 nm in diameter and have linear, double-stranded DNA 26–48 Kbp in length surrounded by capsid proteins. Three of the 11 HAdV structural proteins are capsid proteins composed of a hexon, a penton base, and fibers. Group- and type-specific epitopes are present on both the hexons and fibers. HAdVs exhibit more than 100 genotypes (http://hadvwg.gmu.edu) and are classified into seven species (A–G) based on the antigenic variants of the capsid protein [3, 4]. Infections with HAdV species result in varying clinical symptoms: species A, B, C, and E cause respiratory infection; species F and G cause gastroenteritis; and species B, D, and E cause keratoconjunctivitis [2-4].
Viruses, including HAdVs, continuously mutate, and viral genotypes can differ according to the geographic region and time period [4-7]. The most common cause of adenoviral keratoconjunctivitis has been reported to be type 8, followed by types 19 and 37 [2, 3, 8]. Type 8 accounts for only approximately 5% of adenoviral keratoconjunctivitis cases in the United States, Italy, and the United Kingdom but accounts for up to 30% and 60% of such cases in Japan and Taiwan, respectively [8]. Because of their diversity and complexity, differences in HAdV genotypes have not been widely examined. Owing to the difficulty of ocular sample collection, there are even fewer studies on the HAdV genotypes that cause keratoconjunctivitis. We investigated the genotypes of HAdVs responsible for epidemic keratoconjunctivitis in Korea in 2019.
Conjunctival swab samples were collected from patients suspected as having acute adenoviral keratoconjunctivitis from January to November 2019, based on signs of conjunctival injection, follicles, and discharge. The samples were scraped from the lower palpebral conjunctiva with a cotton swab to collect epithelial cells, transferred into Universal Transport Medium (Copan Diagnostics, Inc., Brescia, Italy), and stored at −70°C until use. This study was approved by the Institutional Review Boards of Hallym University Dongtan Sacred Heart Hospital (IRB No. 2018-10-003) and Hallym University Sacred Heart Hospital (IRB No. 2018-10-014). Informed consent was obtained from all participants.
Viral DNA was extracted from the transport media containing conjunctival samples for PCR and genotyping using QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) and the QIAcube platform (Qiagen). PCR and sequencing of the HAdV hexon gene were performed using specific primers (ADHEX1F/AD2) as described previously with slight modifications [4, 9, 10]. A different primer set (AD1/AD2) was used for another round of PCR if the first round showed amplification failure [4]. The 845-bp PCR products were visualized by electrophoresis on an agarose gel and analyzed by DNA sequencing using ABI PRISM BigDye Terminator version 3.1 (Applied Biosystems, Foster City, CA, USA). The obtained sequences were compared with reported HAdV genotypes obtained using the Basic Local Alignment Search Tool (https://blast.ncbi.nlm.nih.gov/Blast.cgi) in the National Center for Biotechnology Information GenBank database to identify HAdV strains according to their genetic similarity.
Phylogenetic analyses were performed to evaluate the genetic relationships between the HAdV sequences obtained in this study and the genetically closest sequences. Phylogenetic trees were constructed based on sequence alignment using the MEGA program (version 11) [11].
Among the 218 conjunctival swab samples collected from January to November 2019, 128 were HAdV-positive by PCR, with a positivity rate of 58.7%. The largest number of samples was collected in August, and the highest positivity rate (>70%) was observed during July–September 2019 (Table 1). Among the 128 positive samples, most were type 8 group D (HAdV-D8, 65.6%), followed by types 64, 37, 3, 53, 4, and 56. The largest number of positive samples was observed in July and August, and the infections in this period were mainly caused by HAdV-D8 (Table 1). The median age of the patients was 42.0 (range, 3–88) yrs, and there was no specific association between patient age and adenovirus genotype (Supplemental Data Table S1). The phylogenetic tree (Fig. 1, Supplemental Data Table S2) showed that samples with the same genotypes had similar sequences.
HAdV genotypes in keratoconjunctivitis have not been widely examined; however, one study in Busan, Korea, conducted jointly with researchers in Japan and Taiwan in 1983, showed that almost all cases were caused by the D8 type, with no cases caused by the D37 type (Table 2) [12]. However, types D37 and D64 (formerly D19) were detected at high frequency in our study. The new genotypes D53 and D56 were also identified in our study. D54 was the most prevalent type in Japan from 1995 to 2010; D53 was the most prevalent type in China from 2011 to 2013 (Table 2) [13, 14]. The D37 type showed a higher frequency than the D8 type in Japan and China (Table 2) [13, 14]. In Japan, the prevalence of keratoconjunctivitis caused by HAdV-D8 has remarkably reduced in recent years and that of HAdV-D53 and HAdV-D54 has increased [8, 13]. Thus, the genotype of HAdV causing keratoconjunctivitis varies over time and location.
Traditionally, HAdV-D8, -D19, and -D37 have been considered the major causes of epidemic keratoconjunctivitis. However, HAdV-D53, -D54, and -D56 have recently emerged as new agents causing this disease [2, 15–17]. HAdV-D53 is an intertype formed by recombination among HAdV-D8, -D37, and -D22 strains. HAdV-D54 is a common cause of epidemic keratoconjunctivitis in Japan and has not been reported in any other country [13]. HAdV-D56 is a recombinant type detected in only a few cases of epidemic keratoconjunctivitis and one case of fetal neonatal pneumonia. HAdV-D64 (previously known as D19) originates from recombination among HAdV-D19, -D37, and -D22 [18].
HAdVs attach to host cells through an interaction between the knob domain of the fiber and cell-surface receptors, and the types causing keratoconjunctivitis may have affinity for ocular epithelial cells [3]. The ocular tropism of these HAdV types may be related to the fact that HAdV-D37 and a few other HAdV-D types interact with sialic acid-containing glycans [19], and regions of the HAdV-D8, -D53, -D54, and -D56 fiber genes are highly similar to each other [20]. In this study, seven cases of HAdV-B3 and two cases of HAdV-E4 were found. HAdV-E4 has been associated with pharyngoconjunctival fever and epidemic keratoconjunctivitis, while HAdV-B3 with pharyngoconjunctival fever [2].
The majority of samples were collected in July and August 2019, when the positivity rate was ≥70%, confirming dominance of the D8 type (Table 1, Fig. 1). The HAdV genotype exhibits seasonal variation considering all samples; however, epidemic keratoconjunctivitis is acquired mainly from swimming pools in summer, with the D8 type as the main cause [1, 3]. There was minimal nucleotide sequence variation among the HAdV-D8 genotype strains detected in this study, which was further confirmed by the phylogenetic analysis. Therefore, the double-stranded DNA virus HAdV shows fewer mutations compared with single-stranded RNA viruses, such as rotavirus, norovirus, and coronavirus [5, 6].
In conclusion, the most common cause of adenoviral keratoconjunctivitis in Korea in 2019 was HAdV type 8 (group D), followed by type 64 and type 37 (group D). Adenoviral keratoconjunctivitis showed the highest frequency in July and August in 2019 and was mainly caused by type 8 (HAdV-D8). There was little sequence variation within the same type of HAdV. The genotype distribution of adenoviral keratoconjunctivitis may vary according to the time period and geographic area. Our results provide a foundation for further studies of adenoviral keratoconjunctivitis by geographic region and time period, which can help to better understand the relationships among genotypes and symptoms, ultimately providing guidance for drug resistance, treatment, and vaccine development.
Notes
AUTHOR CONTRIBUTIONS
Kim HS and Seo JW designed the study and wrote the manuscript. Lee SK performed the experiments and helped to analyze the data. Seo JW, Hong IH, Choi SH, Lee JY, and Kim HS collected samples and analyzed the data. All authors reviewed and approved the final version of the manuscript.
REFERENCES
1. Ruuskanen O, Metcalf JP, et al. 2009. Adenoviruses. Clinical virology. 3rd ed. American Society of Microbiology;Washington, DC: p. 559–79.

2. Chandra N, Frängsmyr L, Imhof S, Caraballo R, Elofsson M, Arnberg N. 2019; Sialic acid-containing glycans as cellular receptors for ocular human adenoviruses: implications for tropism and treatment. Viruses. 11:395. DOI: 10.3390/v11050395. PMID: 31035532. PMCID: PMC6563162.

3. Akello JO, Kamgang R, Barbani MT, Suter-Riniker F, Leib SL, Ramette A. 2020; Epidemiology of human adenoviruses: A 20-year retrospective observational study in hospitalized patients in Bern, Switzerland. Clin Epidemiol. 12:353–66. DOI: 10.2147/CLEP.S246352. PMID: 32308491. PMCID: PMC7147615.
4. Kim JS, Lee SK, Ko DH, Hyun J, Kim HS, Song W, et al. 2017; Associations of adenovirus genotypes in Korean acute gastroenteritis patients with respiratory symptoms and intussusception. Biomed Res Int. 2017:1602054. DOI: 10.1155/2017/1602054. PMID: 28255553. PMCID: PMC5309414.

5. Kim JS, Kim HS, Hyun J, Kim HS, Song W. 2015; Molecular epidemiology of human Norovirus in Korea in 2013. Biomed Res Int. 2015:468304. DOI: 10.1155/2015/468304. PMID: 26421289. PMCID: PMC4572438.

6. Kim JS, Kim HS, Hyun J, Kim HS, Song W, Lee KM, et al. Analysis of rotavirus genotypes in Korea during 2013: an increase in the G2P[4] genotype after the introduction of rotavirus vaccines. Vaccine. 2014; 32:6396–402. DOI: 10.1016/j.vaccine.2014.09.067. PMID: 25312273.

7. Kim JS, Lee WJ, Lee SK, Lee EJ, Hyun J, Kim HS, et al. 2019; Molecular epidemiology of human astrovirus in stool samples from patients with acute gastroenteritis in Korea, 2013-2017. Ann Lab Med. 39:367–72. DOI: 10.3343/alm.2019.39.4.367. PMID: 30809982. PMCID: PMC6400717.

8. Adhikary AK, Banik U. 2014; Human adenovirus type 8: the major agent of epidemic keratoconjunctivitis (EKC). J Clin Virol. 61:477–86. DOI: 10.1016/j.jcv.2014.10.015. PMID: 25464969.

9. Casas I, Avellon A, Mosquera M, Jabado O, Echevarria JE, Campos RH, et al. 2005; Molecular identification of adenoviruses in clinical samples by analyzing a partial hexon genomic region. J Clin Microbiol. 43:6176–82. DOI: 10.1128/JCM.43.12.6176-6182.2005. PMID: 16333124. PMCID: PMC1317187.

10. Xu W, McDonough MC, Erdman DD. 2000; Species-specific identification of human adenoviruses by a multiplex PCR assay. J Clin Microbiol. 38:4114–20. DOI: 10.1128/JCM.38.11.4114-4120.2000. PMID: 11060077. PMCID: PMC87550.

11. Tamura K, Stecher G, Kumar S. 2022; MEGA11: Molecular evolutionary genetics analysis version 11. Mol Biol Evol. 38:3022–7. DOI: 10.1093/molbev/msab120. PMID: 33892491. PMCID: PMC8233496.

12. Ishii K, Nakazono N, Fujinaga K, Fujii S, Kato M, Ohtsuka H, et al. 1987; Comparative studies on aetiology and epidemiology of viral conjunctivitis in three countries of East Asia-Japan, Taiwan and South Korea. Int J Epidemiol. 16:98–103. DOI: 10.1093/ije/16.1.98. PMID: 3032816.

13. Nakamura M, Hirano E, Kowada K, Ishiguro F, Yamagishi Z, Adhikary AK, et al. 2012; Surveillance of adenovirus D in patients with epidemic keratoconjunctivitis from Fukui Prefecture, Japan, 1995-2010. J Med Virol. 84:81–6. DOI: 10.1002/jmv.22252. PMID: 22052618.

14. Le J, Lu X, Jiang B, Du Y, Yang Y, Qian H, et al. 2018; Adenovirus-associated acute conjunctivitis in Beijing, China, 2011-2013. BMC Infect Dis. 18:135. DOI: 10.1186/s12879-018-3014-z. PMID: 29558885. PMCID: PMC5859447.

15. Jhanji V, Chan TC, Li EY, Agarwal K, Vajpayee RB. 2015; Adenoviral keratoconjunctivitis. Surv Ophthalmol. 60:435–43. DOI: 10.1016/j.survophthal.2015.04.001. PMID: 26077630.

16. Chigbu DI, Labib BA. 2018; Pathogenesis and management of adenoviral keratoconjunctivitis. Infect Drug Resist. 11:981–93. DOI: 10.2147/IDR.S162669. PMID: 30046247. PMCID: PMC6054290.

17. Huang G, Yao W, Yu W, Mao L, Sun H, Yao W, et al. 2014; Outbreak of epidemic keratoconjunctivitis caused by human adenovirus type 56, China, 2012. PLoS One. 9:e110781. DOI: 10.1371/journal.pone.0110781. PMID: 25343525. PMCID: PMC4208770.

18. Zhou X, Robinson CM, Rajaiya J, Dehghan S, Seto D, Jones MS, et al. 2012; Analysis of human adenovirus type 19 associated with epidemic keratoconjunctivitis and its reclassification as adenovirus type 64. Invest Ophthalmol Vis Sci. 53:2804–11. DOI: 10.1167/iovs.12-9656. PMID: 22467570. PMCID: PMC3367469.

19. Nilsson EC, Storm RJ, Bauer J, Johansson SM, Lookene A, Ångström J, et al. 2011; The GD1a glycan is a cellular receptor for adenoviruses causing epidemic keratoconjunctivitis. Nat Med. 17:105–9. DOI: 10.1038/nm.2267. PMID: 21151139.

20. Robinson CM, Singh G, Henquell C, Walsh MP, Peigue-Lafeuille H, Seto D, et al. 2011; Computational analysis and identification of an emergent human adenovirus pathogen implicated in a respiratory fatality. Virology. 409:141–7. DOI: 10.1016/j.virol.2010.10.020. PMID: 21056888. PMCID: PMC3006489.

Fig. 1
Phylogenetic analysis of adenoviruses detected in this study. The sequences of adenoviruses and most genetically similar sequences from GenBank were included for analysis. The tree was inferred using the maximum-likelihood method and Tamura-Nei model with MEGA11. The tree is drawn to scale, with branch lengths measured according to the number of substitutions per site.
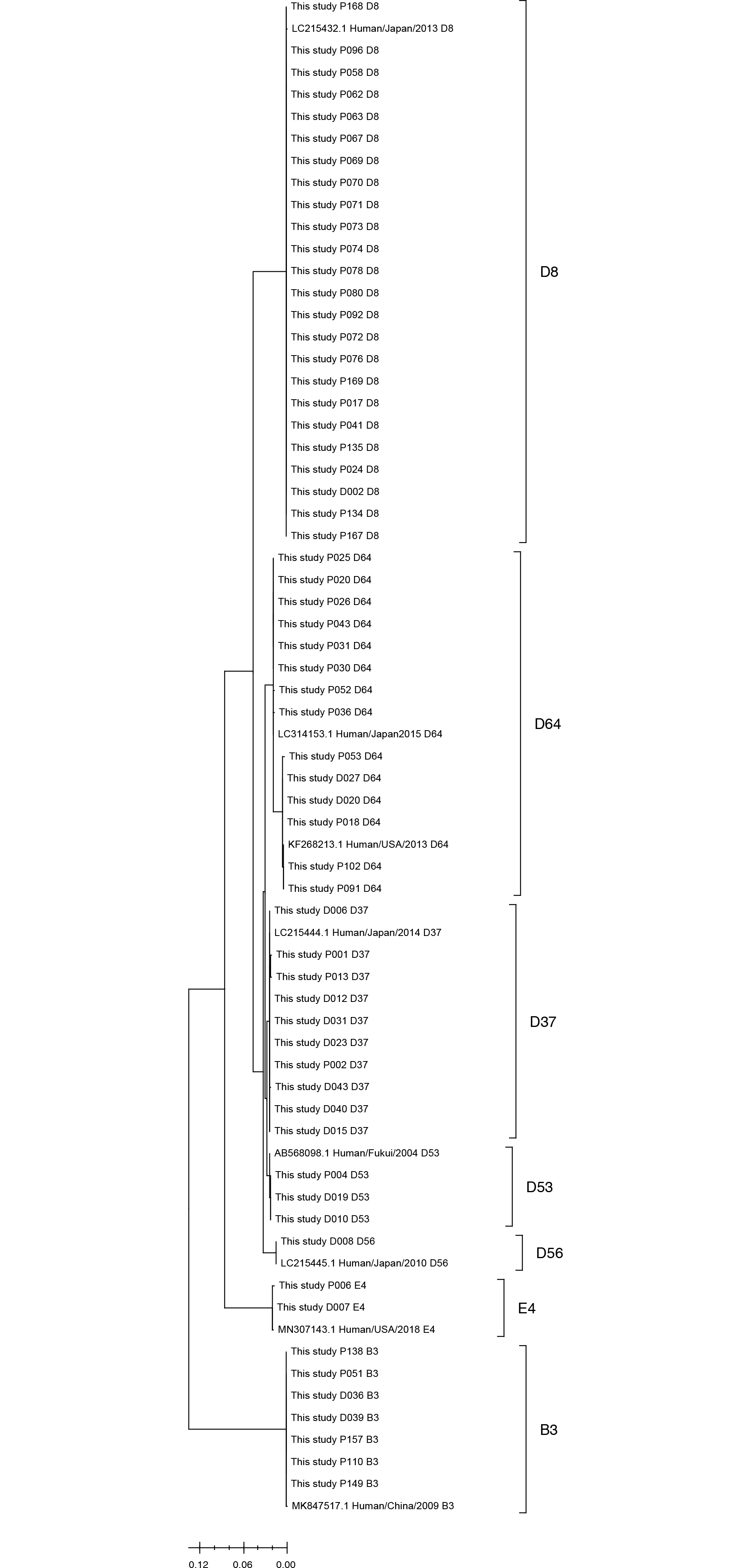
Table 1
Monthly positivity and genotypic distribution of acute adenoviral keratoconjunctivitis in 2019
| Type | N (%)* | Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HAdV B3 | 7 (5.5) | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 2 | 3 | 0 |
| HAdV E4 | 2 (1.6) | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| HAdV D8 | 84 (65.6) | 0 | 3 | 1 | 2 | 2 | 5 | 20 | 35 | 6 | 4 | 6 |
| HAdV D37 | 12 (9.4) | 2 | 2 | 1 | 2 | 0 | 2 | 0 | 1 | 0 | 1 | 1 |
| HAdV D53 | 4 (3.1) | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| HAdV D56 | 1 (0.8) | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| HAdV D64 | 16 (12.5) | 0 | 0 | 1 | 5 | 5 | 1 | 1 | 1 | 0 | 0 | 2 |
| Failed | 2 (1.6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| N of positive samples | 128 (100) | 3 | 8 | 4 | 9 | 9 | 8 | 21 | 40 | 8 | 9 | 9 |
| N of tested samples | 218 | 5 | 15 | 9 | 18 | 30 | 17 | 26 | 58 | 10 | 14 | 16 |
| Positive rate (%) | 58.7 | 60.0 | 53.3 | 44.4 | 50.0 | 30.0 | 47.1 | 80.8 | 69.0 | 80.0 | 64.3 | 56.3 |
Table 2
Comparison of results of this study with previously reported ocular adenovirus genotypes
| Country | Present study | Busan, Korea | Japan | Taiwan | Japan | China |
|---|---|---|---|---|---|---|
| Year of sample collection | 2019 | 1983 | 1981–1981, 1983 | 1981–1981, 1983 | 1995–2010 | 2011–2013 |
| N of typed samples | 218 | 123 | 354 | 628 | 124 (D type only) | 349 |
| HAdV C1 | 2 (0.6%) | |||||
| HAdV C2 | 2 (0.6%) | |||||
| HAdV B3 | 7 (3.2%) | 5 (4%) | 17 (5%) | 31 (5%) | 26 (7.4%) | |
| HAdV E4 | 2 (0.9%) | 1 (1%) | 12 (3%) | 1 (0.2%) | 65 (18.6%) | |
| HAdV C5 | 1 (0.3%) | |||||
| HAdV B7 | 1 (0.2%) | 19 (5.4%) | ||||
| HAdV D8 | 85 (39.0%) | 57 (46%) | 102 (29%) | 186 (30%) | 8 (6.5%) | 47 (13.5%) |
| HAdV B11 | 3 (2%) | 2 (1%) | 23 (4%) | 4 (1.1%) | ||
| HAdV B14 | 1 (0.3%) | |||||
| HAdV D37 | 12 (5.5%) | 16 (5%) | 18 (3%) | 40 (32.2%) | 61 (17.5%) | |
| HAdV D42 | 1 (0.3%) | |||||
| HAdV D48 | 2 (0.6%) | |||||
| HAdV D53 | 5 (2.3%) | 5 (4.0%) | 59 (16.9%) | |||
| HAdV D54 | 66 (53.2%) | |||||
| HAdV D56 | 1 (0.5%) | 1 (0.8%) | 1 (0.3%) | |||
| HAdV D64 (previously D19) | 14 (6.4%) | 3 (2%) | 14 (4%) | 27 (4%) | 4 (3.1%) | 51 (14.6%) |
| Untyped or others | 92 (42.2%) | 54 (44%) | 191 (56%) | 341 (55%) | 7 (2.0%) | |
| Reference | [12] | [12] | [12] | [13] | [14] |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download