INTRODUCTION
Blood bank autoanalyzers can save technician time and prevent various errors associated with manual testing. Since August 2015, the Korea Health Insurance Review & Assessment Service instituted a higher reimbursement fee for automated ABO blood grouping than for manual tests. Since this change, the number of hospitals that routinely use autoanalyzers for ABO blood grouping has significantly increased.
When results from blood bank autoanalyzers are rejected because of failure to determine ABO blood groups due to discrepant results or serologically suspicious ABO subgroups, the laboratory must have a manual process in place to investigate the problem. Thus, although blood bank autoanalyzers may have several advantages, the constant need for follow-up investigations may increase the laboratory workload and risk of errors. Therefore, it is necessary to identify the characteristics of rejected cases and develop a strategy to minimize their occurrence.
Erytra Eflexis (Eflexis; Grifols, Barcelona, Spain) is an immunohematology autoanalyzer suitable for pre-transfusion routine testing in small-to-medium sized laboratories [
1,
2]. Ajou University Hospital, Suwon, Korea, was the first to introduce Eflexis analyzers in Korea in 2019. There are several reports on the performance and reliability of Erytra or Erytra Eflexis in pre-transfusion testing [
1-
4]. However, there is a paucity of comprehensive studies evaluating failed cases of ABO grouping using Eflexis or autoanalyzers from other companies [
5-
9].
Cis-AB, a rare ABO subgroup, is caused by a gene mutation resulting in a single glycosyltransferase enzyme with dual A and B glycosyltransferase activities [
10]. The typical phenotype of
cis-AB is A
2B
3. The
cis-AB phenotype is the most frequent ABO subgroup in Korea (0.0354%) [
11]. It also occurs more frequently in Northeast Asia than in other regions [
12]. During our evaluation of Eflexis during 2019, we found several cases where the analyzers had misidentified the A
2B
W phenotype (presumed to be
cis-AB) as normal AB groups. Similar results of ABO mistyping of the
cis-AB group by other autoanalyzers have been reported [
13]. Subsequently in 2019, our laboratory requested that Grifols make modifications to the autoanalyzer, such that it is able to more clearly differentiate 4+ and 3+ hemagglutination reaction scores when using anti-B anti-sera, to allow for improved differentiation of the A
2B
W phenotype from that of the normal AB group. Consequently, the manufacturer modified the Eflexis software, and an upgraded version (v.1.2.2) was released in August 2020.
Since the introduction of the Eflexis analyzers at Ajou University Hospital, we have included extra criteria to prevent automatic interfacing of discrepant ABO grouping results to the Laboratory Information System (LIS) and to carry out further investigations using the conventional tube technique (TUBE). Further review was required to manage the accumulated data from the additional rejection criteria to detect serologically suspicious ABO subgroups, including the cis-AB phenotype.
The aim of this study was to inform laboratories on how to use immunohematology autoanalyzers more efficiently for ABO grouping by reviewing cases with a serologically weakened A or B antigen to detect ABO subgroups or analyzing rejected cases of ABO grouping that were not automatically interfaced by the Eflexis analyzer.
MATERIALS AND METHODS
Subjects
This retrospective study was approved by the Institutional Review Board of Ajou University Hospital (AJIRB-BMR-SMP-20-299). Samples from all patients who requested ABO blood grouping between January 1 and December 31, 2020, were tested using either of the two Eflexis analyzers. EDTA-anticoagulated whole blood samples were used for ABO grouping. ABO blood grouping results, patient’s ward, test request time, ABO grouping hemagglutination reaction strengths, or any flag results for forward or reverse ABO grouping were retrospectively extracted from both Eflexis analyzers as an Excel file (Microsoft 2019, Microsoft, Redmond, WA, USA). For the rejected cases, patient age and sex were additionally extracted. Babies ≤12 months of age were classified as neonates.
To evaluate the ability of the Eflexis analyzers to detect serologically suspicious ABO subgroups, samples with weakened A and/or B antigen reactions identified in our laboratory between January 2020 and April 2021 were also included in the study. These samples were confirmed by TUBE, not by molecular testing. Aw was defined as serologically weakened group A other than A1 and A2, and Bw was defined as serologically weakened group B.
ABO grouping using the Erytra Eflexis analyzer
ABO grouping was performed using the Erytra Eflexis instrument and DG Gel 8 ABO/Rh cards (Diagnostic Grifols, Barcelona, Spain), according to the manufacturer’s recommendations. Neonatal cards were not used. The ABO cards consisted of eight columns, i.e., anti-A, anti-B, anti-AB, anti-D, anti-DVI clone, control, and two empty wells for reverse grouping using 0.8% A1 and B reagent red blood cells (RBCs) (Serigrup Diana A1/B, Diagnostic Grifols), respectively. We used 0.8% patient RBCs suspended in low ionic salt solution for ABO forward grouping and RhD determination. Automated hemagglutination reaction strengths were graded as –, 1+, 2+, 3+, or 4+. Alert flags, such as “trace,” were returned by the Eflexis analyzer when the reaction could not be graded or interpreted. For comparison of the reaction strength between the autoanalyzers and TUBE, “–” and “trace” reactions were considered 0 and 0.5, respectively.
Each sample was classified into a specific ABO group according to the results of forward and reverse grouping interpreted by the system’s software, and the final ABO grouping result was automatically interfaced to the LIS. When the autoanalyzers could not classify the ABO group, the results were withheld by the analyzer and not automatically interfaced to the LIS. Such results were identified as “Eflexis rejections.”
ABO blood group confirmation for rejected cases from the analyzers or serologically suspicious ABO subgroups was determined in the following four ways. 1) For patients who previously underwent ABO grouping in our hospital, the historical ABO group was considered the final ABO group, except for patients who had undergone an ABO-mismatched peripheral blood stem cell transplantation (PBSCT). 2) For new patients, forward and reverse ABO grouping using TUBE was performed, except for neonates, for whom only forward grouping was performed. 3) For cases with discrepant results from previous ABO blood grouping results or discrepant results between forward and reverse grouping, or a suspected ABO subgroup, the ABO group was determined by repeating the ABO blood grouping using the autoanalyzers and TUBE, as well as by reviewing the patient’s diagnosis. When necessary, additional tests, including antibody screening and identification, were performed using the autoanalyzers. Further tests using manual methods were performed, including the saline replacement method, washing RBCs with warm saline, and testing RBCs with anti-A1 (Lorne Laboratories Limited, Lower Earley, UK) and/or anti-H lectins (Lorne Laboratories Limited) using TUBE. 4) If it was difficult to determine the ABO group even after TUBE and additional tests, the result was reported as “Unclassified ABO group.”
ABO blood grouping using TUBE
ABO blood grouping using TUBE was performed following the laboratory’s standard operating procedure and manufacturer’s recommendations for all new patients, irrespective of the autoanalyzer results. IgM monoclonal anti-A and anti-B anti-sera (Shinyang Chemical, Seoul, Korea; raw materials from Bioscot, Millipore, Livingston, UK) were used for forward grouping. For reverse grouping, pooled A
1 and pooled B RBCs (7–10 random donors each for A
1 and B RBCs) suspended in 2%–5% saline were used. Incubation at room temperature for 15 minutes was standard for TUBE-based reverse grouping. The hemagglutination reactions for forward and reverse TUBE-based grouping were scored as follows: 0: negative, 0.5+: weak, 1+, 2+, 3+, or 4+ [
14].
Study design
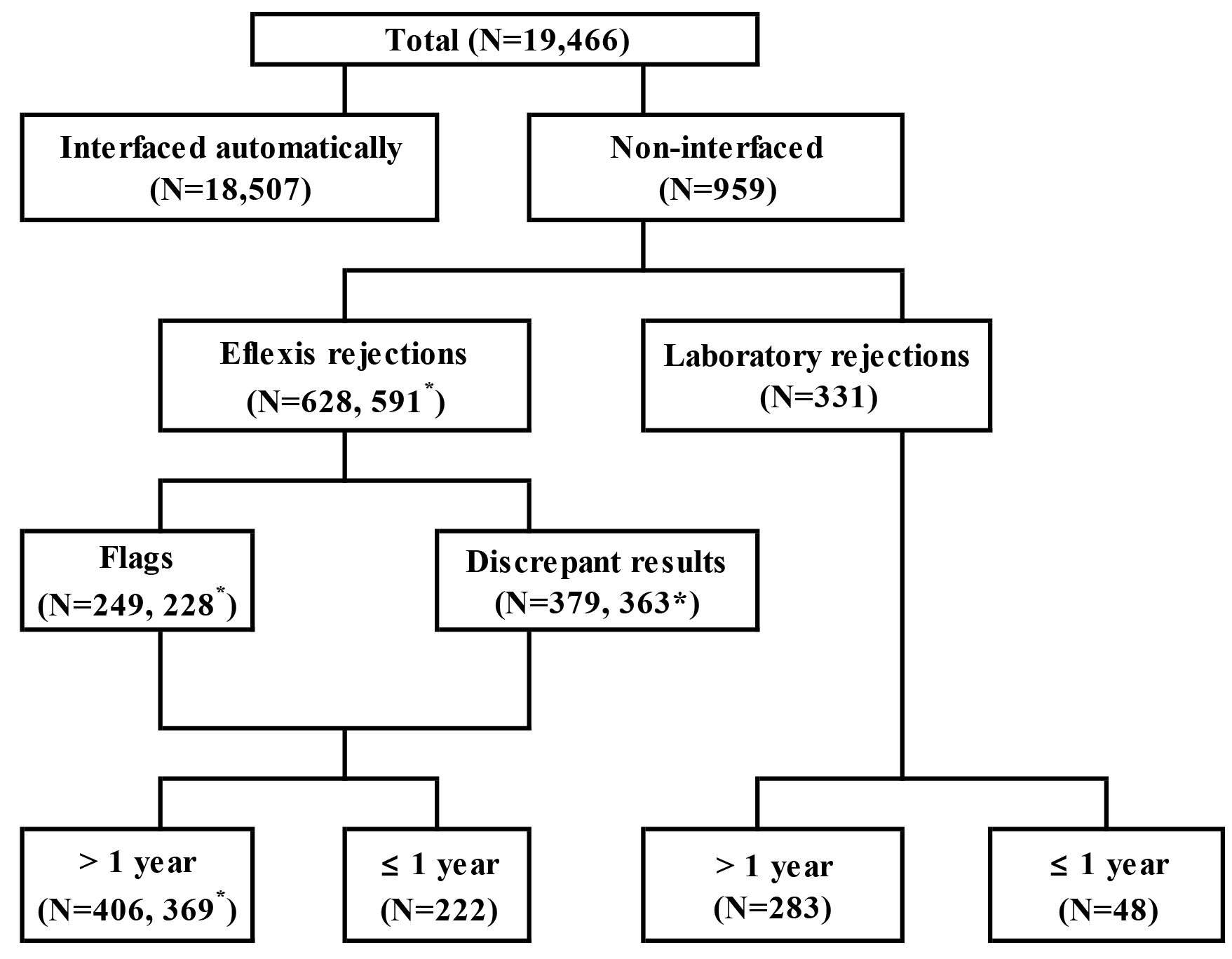
Rejected, non-interfaced cases from the autoanalyzers were classified into the following two groups. 1) Eflexis rejection group: failed analyzer ABO blood grouping and failed automatic interfacing because of the presence of any flag, such as “trace,” “double population (DP),” or “?” (defined as the Flag group) or a discrepancy between forward and reverse grouping without any flag (defined as the Discrepant result group). 2) Laboratory rejection group: extra criteria of our laboratory, including cases with ABO hemagglutination reaction scores from 1+ to 3+ for forward grouping or 1+ for reverse grouping, preventing automatic interfacing, although an ABO group was determined by the analyzers (
Fig. 1). The extra criteria were used for the detection of A
2B
W with weak reaction of anti-B antibodies, which was typical for the
cis-AB group.
The rejection rate (RR) for non-interfaced cases was determined as the percentage of the number of rejected cases divided by the total number of automatically interfaced cases plus rejected cases from the autoanalyzers during 2020. Cases with identical results obtained from the same sample after retesting within 10 minutes were excluded from the rejected cases and were defined as “same repeated cases.” The frequency of rejected results was defined as the percentage of the number of rejected results divided by the total number of cases with rejected results from the autoanalyzers during 2020.
Statistical analysis
The chi-square test was used to compare sample RRs among ABO blood groups. The paired t-test was used to compare the average strength of reverse grouping reactions between TUBE and the autoanalyzers. Statistical analysis was performed using Excel (Microsoft), and P<0.05 was considered statistically significant.
RESULTS
The sample RR was 3.2% (628/19,466) in the Eflexis rejection group and 1.7% (331/19,466) in the Laboratory rejection group (
Fig. 1). The Eflexis rejection group comprised 249 Flag group cases (1.3%) and 379 Discrepant result group cases (1.9%). Thirty-seven cases were excluded from ABO grouping due to previous mismatched PBSCT, causing discrepant results between forward and reverse grouping. The remaining 591 cases in the Eflexis rejection group could be classified into an ABO group. Neonates accounted for 35.4% (222/628) of the Eflexis rejected cases and 270/959 (28.1%) of the total rejected cases.
The sample RR significantly differed according to the ABO group in the Eflexis rejections (
P<0.001), laboratory rejections (
P<0.001), and total rejections (
P<0.001). Blood group A showed the highest RR, and group O the lowest. Group AB had the lowest RR in neonates (0.3%) (
Table 1).
The results (N=682) of the Eflexis rejection group from 628 rejected samples are shown in
Table 2. According to the ABO blood grouping reaction, 682 rejected results resulted from anti-A (9.2%) and anti-B anti-sera (7.8%), and reactions against A
1 (28.4%) and B RBCs (54.5%). Rejections for ABO blood grouping by the autoanalyzers were caused by a lack of the expected reverse reaction or an unexpected reaction associated with cold auto-antibodies, allo-antibodies, or rouleaux formation (
Table 2).
There were 263 flags (106 “DP,” 135 “trace,” and 22 “?” flags) among the 249 flagged cases (
Fig. 1,
Table 2). “DP” flags were reported only in forward grouping, whereas “trace” and “?” flags were also reported in reverse grouping, except for two cases of “trace” in forward grouping. While “DP” occurred in 26.4% (28/106) of results following ABO-mismatched PBSCT, this flag was also observed in cases with serologically weakened A and/or B groups, neonates, and patients who received emergency transfusions of O RBCs. The two cases with a “trace” flag in forward grouping were group A and were considered nonspecific reactions in the forward group using anti-B anti-sera. The “trace” and “?” flags represented weak reactions in reverse grouping, and none of these were false reactions. Forty-nine percent (129/263) of the flags were observed in group A. The frequencies of flagged cases were 16.7% (37/222) for patients aged ≤1 year old and 55.7% (226/406) for those >1 year old.
In the Laboratory rejection group, there were 336 rejected results from 331 patients. The rejected results comprised 55 results with a 3+ reaction strength, one result with a 1+ reaction strength in forward grouping, and 280 results with a 1+ reaction strength in reverse grouping, including 190 results from reverse grouping using B RBCs. One hundred eighty-six (55.4%) results were from group A, 88 (26.2%) from group B, 39 (11.6%) from group AB, and only 19 (6.8%) from group O. Except for one case, group AB rejections were because of reaction strengths ≤3+ for forward grouping using anti-B anti-sera.
Reverse grouping using TUBE was performed in 112 patients. The average hemagglutination reaction strengths for reverse grouping by TUBE with immediate spin and following a 15-minute incubation at room temperature were significantly higher than those for the autoanalyzers (
Table 3).
During the 16-month study period, 14 cases of serologically suspected ABO subgroups were observed: one Aw, four Bw, three ABw, and six A
2B
W (
Table 4). Thirteen cases were new patients who required retesting for ABO blood grouping using TUBE. Case No. 1 was a patient with AML whose ABO grouping returned to normal blood group A after three months. Because of a DP flag in forward grouping, the results were not automatically interfaced for six cases of Aw, Bw, and ABw, which were easily detected. However, six cases of A
2B
W and two cases of ABw were determined as group AB and would have been automatically interfaced by the autoanalyzers had our laboratory rejection criteria not been in place. The false but strong reactions in these cases were detected by laboratory staff by performing repeat TUBE not only for new patients but also for samples with reaction strengths of 1+ to 3+. All six A
2B
W cases showed 2+ reaction strengths and mixed-field reactions in TUBE-based forward grouping using anti-B anti-sera, which are typical results for
cis-AB. Anti-B antibody was detected after a 15-minute incubation at room temperature in four cases of A
2B
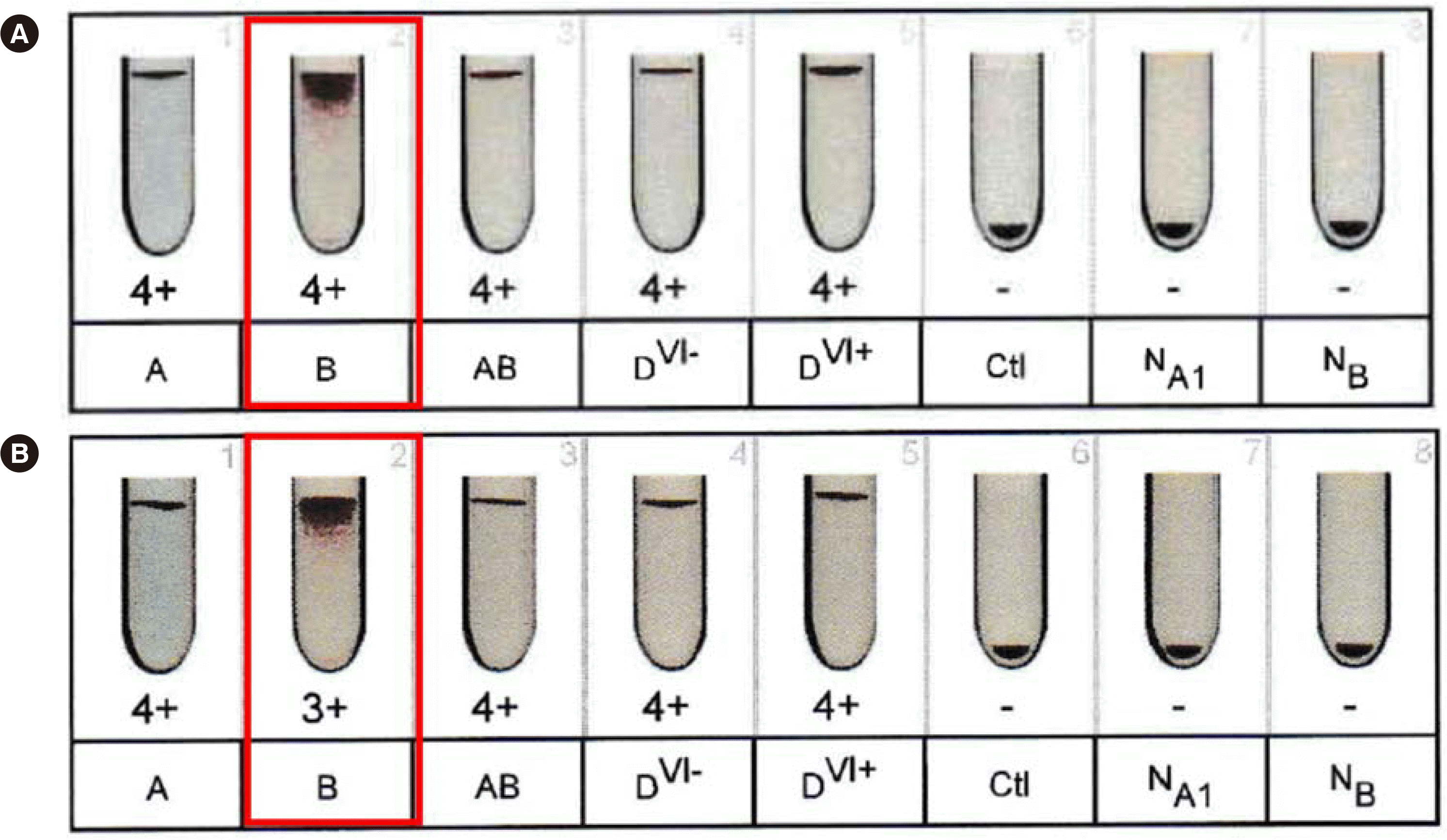
W, whereas the autoanalyzers did not detect any anti-B antibody. Using the Eflexis software v1.2.1, two out of four cases (case Nos. 6 and 7,
Table 4) showed 4+ hemagglutination in forward grouping using anti-B anti-sera, although the reaction strength was weaker than the typical 4+ strength for a normal AB group. Following the software upgrade (v1.2.2 or later versions), the reaction strength was revised to 3+ (
Fig. 2).
DISCUSSION
In our study, the Eflexis analyzers could not determine the ABO group in 628 out of 19,466 cases. The Eflexis RR was 3.2% (including 1.1% for neonates up to 1 year of age), which was higher than that of 2.3% in 13,113 samples (including 1.2% for error messages) reported for the AutoVue system (Ortho-Clinical Diagnostics, Raritan, NJ, USA) [
9]. However, in that study, samples from neonates up to 1 year of age were excluded, which may have affected the RR.
We did not use neonatal cards as these are more expensive than standard ABO RhD and reverse grouping cards. In total, 35.4% (222/628) of Eflexis rejected cases in our study were neonates. Anti-A and/or anti-B antibodies generally appear after the first 3–6 months of life, which explains why the AB group showed the lowest RR compared with the O group in neonates [
15]. The RR depends on the proportion of neonates in each hospital and on whether a specific neonatal/cord blood ABO grouping card is available or whether only the standard ABO/Rh grouping card is used. Thus, laboratories should consider implementing a special interpretation process for neonates if only the standard ABO/Rh grouping cards are used. After further analysis, we discovered that 93.8% of the rejected reverse typing results in our laboratory were from neonates. Therefore, we applied a process to interpret ABO blood group results using only forward grouping and to disregard the reverse typing results for neonates of four months old or younger, as the reverse grouping results were not helpful for this group.
We found that the ABO group also affects the autoanalyzer RRs. Group A showed the highest RR, which can be explained by the highly frequent rejected results of reverse grouping because of the absence of expected reactions against B RBCs (
Table 2). Likewise, weak plasma reactions on the AutoVue system causing ABO blood grouping discrepancies have been reported [
9]. The number of A antigen sites (0.81–1.17×10
6) per RBC is higher than that of B antigen sites (0.61–0.83×10
6) [
16]. This quantitative difference appears to be one of the reasons for the weaker reactions in reverse grouping using B RBCs than when using A RBCs.
Except for neonates, group O subjects showed the lowest RR (0.4%). Anti-A or anti-B in B or A subjects are mostly IgM in adults, but IgG anti-A and anti-B is far more common in O subjects [
17]. We previously reported that ABO antibody titers were higher in TUBE than in the gel card method using ID-Card (Dia-Med AG), except for the anti-A antibody in group O at the antiglobulin phase [
18]. This result supports the concept that gel card reactions are weaker in IgM anti-A and anti-B of groups B and A than in IgG anti-A and anti-B of group O. However, the proportion of ABO groups varies among populations [
19]. While group A is the most common group in most areas, most South American Indians have group O, and B is the most frequent group in Bengalese [
19]. Therefore, the RRs of the Eflexis analyzer may vary among populations.
Hemagglutination reactions for reverse grouping by TUBE were significantly stronger than those for the autoanalyzers (
Table 3); this phenomenon has also been reported for the AutoVue system [
9].
“DP” was observed only in forward grouping for cases with weakened expression of A or B antigen on RBCs associated with ABO subgroups, neonates, or a mixture of A or B group RBCs that can be observed following ABO-mismatched transfusion or PBSCT, as reported in other studies [
5,
9]. Therefore, when the Eflexis analyzer reports a “DP” flag, a weakened ABO group or mixture of different ABO groups should be considered. The “trace” and “?” flags are considered weak reactions in reverse grouping.
Depending on the co-inherited ABO allele, the
cis-AB phenotype typically is A
2B
3, A
2B, or A
1B
3 [
20]. Most
cis-AB phenotypes have a weaker B phenotype than the normal B phenotype. Mistyping of
cis-AB as normal AB was reported in 87.5% of cases when using the QWALYS-3 analyzer (Diagast, Loos, France) and 70.0% when using the Galileo NEO analyzer (Immucor Gamma, Norcross, GA, USA) [
13]. The authors recommended the manual tile method as a simple supplemental test for the detection of the
cis-AB phenotype, especially in countries with a relatively high prevalence of
cis-AB phenotypes [
13]. To prevent hemolytic transfusion reactions, O RBCs (or A RBCs when anti-A is not detectable in the plasma) and AB plasma or platelets are recommended for transfusion of patients with the
cis-AB phenotype in Korea [
21]. In our study, Aw or Bw other than
cis-AB could be easily detected by the presence of “DP” flags and a weakened reaction in forward grouping. This can explain why there has been no problem with using the Eflexis prior to the current software upgrade as shown in case A in
Fig. 2 in other countries. Since the software upgrade, the A
2B
W phenotype with 4+ strength in forward grouping using B RBCs has not yet been detected in our laboratory.
We also used arbitrary extra exclusion criteria that prevented automatic interfacing of the results. These criteria were designed to detect A
2B
W with weak reactions of anti-B antibodies. The autoanalyzers showed stronger reactions in forward grouping using anti-B anti-sera than TUBE for six cases of A
2B
W (
Table 4). Our laboratory has a policy to repeat ABO blood grouping by TUBE following analysis on the autoanalyzer for all patients with an initial ABO blood grouping test. This policy and our extra exclusion criteria for forward grouping were helpful in detecting serologically suspicious ABO subgroups and preventing automatic interfacing of results without any flags from the autoanalyzers (case Nos. 8–13,
Table 4). Although our laboratory policy for new patients is effective in identifying serologically suspicious ABO subgroups, additional criteria for forward grouping may help detect suspicious ABO subgroups that had previously been missed and grouped as a normal ABO group. Reviewing cases using the extra criteria regarding the hemagglutination reaction strength ≤3+ for forward grouping, at least when using anti-B anti-sera, appears to be helpful in detecting ABO subgroups in regions where the
cis-AB group occurs frequently [
5]. However, the extra criterion of ≤1+ hemagglutination reaction strength for reverse grouping did not help detect any extra plasma reactions of anti-B antibodies in four A
2B
W cases. Therefore, the extra criteria for reverse grouping other than those for forward grouping using Eflexis may be unproductive in increasing the probability of detecting A
2B
W, especially as it increases not only the laboratory workload but also the risk of clerical errors.
In summary, failed Eflexis analyzer ABO grouping was mainly due to various flags and discrepant ABO blood grouping with weak reactions in reverse grouping. This caused higher RRs in neonates and group A because our laboratory does not use neonatal cards; group O showed the lowest RR. As the cis-AB phenotype was overlooked by Eflexis, ABO grouping using TUBE in new patients and/or the implementation of extra rejection criteria in cases with ≤3+ reaction grades for forward grouping were helpful in detecting the cis-AB phenotype. Following changes to the Eflexis software for scoring and the interpretation of weak hemagglutination reactions, there was a significant improvement in the detection of serologically suspicious ABO subgroups. The results of this study will help other laboratories to efficiently use Eflexis, especially in cis-AB phenotype-prevalent regions.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download