Abstract
Background
Anti-Müllerian hormone (AMH) is one of the most reliable markers of ovarian reserve. Automated AMH assays are widely used in clinical laboratories, but reference intervals for the Elecsys AMH assay for Asian populations have not yet been determined. We aimed to determine reference intervals in healthy Korean women.
Methods
The study included 1,450 women aged 19 to 54 years who participated in the Korea National Health and Nutrition Examination Survey between 2013 and 2016. The study participants were divided into seven 5-year age groups. AMH and progesterone concentrations were measured using Roche Elecsys assays, and bone morphogenetic protein-15 (BMP15) was genotyped for the detection of major variants. Age group-specific reference intervals for AMH were established as recommended by the CLSI EP28-A3c guidelines.
Results
The mean age was 37.4 years. AMH concentrations decreased with increasing age, especially after 40 years, with the median AMH decreasing from 30.9 pmol/L in participants of 19–24 years to 0.071 pmol/L in participants of 50–54 years. The mid-95 percentile AMH reference intervals decreased from 7.93–81.21 pmol/L in participants of 19–24 years to 0.07–3.86 pmol/L in participants of 50–54 years. Disease-associated BMP15 variants were not detected.
Anti-Müllerian hormone (AMH) is a glycoprotein of the transforming growth factor beta superfamily. AMH is released by ovary granulosa cells in pre-antral and small antral follicles and regulates mature follicle quantities [1, 2]. Early in a woman’s fertile period, AMH concentrations are relatively high, but they decrease with age until menopause, when AMH is no longer detected. AMH testing is used to assess ovarian function, assist in the diagnosis of relevant diseases, predict responses to infertility treatment, and as a companion diagnostic [2-4]. AMH concentration is a good marker for the ovarian reserve, which also declines with age [2-4].
The Immunotec and Diagnostic Systems Laboratory ELISAs were widely used in the past for measuring AMH [3, 4]. However, these assays were insufficient in terms of precision and accuracy and required a long hands-on time. In 2011, a second-generation (Gen II) ELISA, which integrated the original two assays, was launched by Beckman Coulter [4]. Since 2015, automated platforms that use sandwich immunoassays have been developed [5-9]. The automated AMH assays include the Roche Diagnostics Elecsys AMH (Roche Diagnostics GmbH, Mannheim, Germany) and Beckman Coulter Access AMH (Beckman Coulter, Brea, CA, USA) immunoassays, both being currently used in clinical laboratories [6-12]. According to the 2021 College of American Pathologist AMH-B survey, 56.7% of facilities use Elecsys AMH and 37.3% use Access AMH. According to the 2021 Korean Association of External Quality Assessment Service Hormones III program, 72.2% use Elecsys AMH and 27.8% use Access AMH in Korea [13].
AMH assays are yet to be harmonized, and thus, test results vary greatly among assays. AMH is not listed in the International Consortium for Harmonization of Clinical Laboratory Results program, which covers the harmonization status of variable measurands [14]. AMH concentrations measured using automated assays reportedly tend to be lower than those measured using ELISAs, although the degree of difference is variable [6–10]. Additionally, AMH concentrations vary by ethnicity, and the degree of difference varies by age within the same ethnic group [2, 15-17]. There are insufficient data from Asian populations, including Korean populations.
Current AMH reference intervals in the Korean population were derived from ELISA data, which failed to reflect the latest trend of using automated platforms, and some studies were limited to patients visiting an infertility center and thus are vulnerable to selection bias [18–20]. Reference intervals are the basis for determining clinical decision limits in various diseases or conditions [21]. Therefore, we aimed to establish reference intervals for the Elecsys AMH assay in healthy Korean women by recruiting participants of the Korea National Health and Nutrition Examination Survey (KNHANES) [22, 23]. The bone morphogenetic protein-15 gene (BMP15) was genotyped as it is closely related to ovarian function [24, 25].
The study course is outlined in Fig. 1. Among the KNHANES participants between 2013 and 2016, 1,450 participants who met the following criteria were included in this study: (1) provide consent to donate samples (blood and urine), (2) women of 19–54 years of age, (3) non-smokers and no history of hypertension, diabetes mellitus, or hypercholesterolemia, and (4) availability of serum and DNA samples. The participants were divided into seven 5-year age groups: 19–24 years, 25–29 years, 30–34 years, 35–39 years, 40–44 years, 45–49 years, and 50–54 years [7, 10-12]. There was a minimum of 120 participants in each group. The participants were considered generally healthy.
This study was exempted from review by the Institutional Review Board of the Asan Medical Center, Seoul, Korea (IRB No: 2018-0886, 2019-0211).
Serum and DNA samples from the participants were obtained from the National Biobank of Korea in January and February 2019 anonymously. Serum volumes were up to 300 μL, and DNA concentrations were 62–550 ng/μL. The samples were stored at –70°C until testing. For each participant, anthropometric and laboratory test results were obtained, including height, body weight, systolic and diastolic blood pressures, hemoglobin A1c, fasting glucose, Hb, creatinine, total cholesterol, and triglyceride concentrations. The body mass index (BMI) was calculated by dividing the body weight in kilograms by the square of the height in meters. Obesity was defined as a BMI ≥25 kg/m2, according to the Asia-Pacific criteria of the WHO guidelines [26, 27]. Anemia was defined as an Hb concentration ≤120 g/L, according to the WHO recommendations to diagnose anemia in non-pregnant women [28].
The 1,450 vials of frozen sera were thawed and tested at the Asan Medical Center. AMH testing was performed using a Cobas e602 modular analyzer (Roche Diagnostics) and Elecsys AMH reagent (Roche Diagnostics). Because sample amounts were limited (<300 μL), the AMH concentration was measured only once. Staff members performing the assays were familiar with the test protocol, including calibration and quality control, equipment maintenance, and troubleshooting.
PreciControl AMH (Roche Diagnostics) was used for internal quality control of the AMH assay. The coefficients of variation in January and February 2019 were 7.3% and 5.2%, respectively, for level 1 (4.29 pmol/L), and 3.2% and 2.9%, respectively, for level 2 (34.71 pmol/L). One lot of AMH reagent was used for approximately 950 vials, and a second lot was used for the remaining vials. A parallel test using patient samples was conducted to assess comparability. Differences between the lots satisfied the cutoff with a variance of <10%. The limit of detection (LOD) and limit of quantitation of Elecsys AMH were 0.07 and 0.21 pmol/L, respectively. The manufacturer specified 0.07–164.00 pmol/L as a measuring interval [6, 10]. For statistical analysis, we set concentrations <0.07 pmol/L as 0.07 pmol/L.
As information on the menstrual cycle was not available for the KNHANES participants, we measured progesterone concentration to determine the menstrual phases and conditions of the study participants. Progesterone concentrations were measured using a Cobas e602 modular analyzer (Roche Diagnostics) and Elecsys progesterone reagent (Roche Diagnostics) with the residual amount after completion of AMH testing. Menstrual cycle phases were broadly classified according to the progesterone concentrations provided by the manufacturer as follows: follicular (0.16–0.38 nmol/L), follicular or ovulatory (0.39–2.84 nmol/L), ovulatory (2.85–5.81 nmol/L), ovulatory or luteal (5.82–38.47 nmol/L), and luteal (38.48–76.00 nmol/L). The manufacturer specifies the LOD as 0.16 nmol/L. Two additional conditions were set for participants with a progesterone concentration >76.00 nmol/L, classified as the high-progesterone group, and for participants aged ≥45 years with a progesterone concentration <0.18 nmol/L, classified as the postmenopausal group. Median AMH concentrations were compared among groups with different menstrual phases and conditions. All menstrual phases and conditions were included in the analysis.
BMP15 genetic testing using PCR amplification from DNA samples and direct sequencing was outsourced to Macrogen (Seoul, Korea). Forward sequencing was performed for BMP15 exon 1, and both forward and reverse sequencing were performed for exon 2. The samples were analyzed for c.-9C>G, c.202C>T (p.Arg68Trp), c.226C>T (p.Arg76Cys), c.631C>T (p.Gln211Ter), c.704A>G (p.Tyr235Cys), c.783_785TCT[3] (p.Leu263dup), and c.852C>T (p.Ser284=) [29-31].
MedCalc Statistical Software version 15.2.2 (MedCalc Software, Ostend, Belgium) was used to determine outliers and percentiles and to establish AMH reference intervals. As the AMH concentrations had a left-skewed distribution, values exceeding the third quartile plus three times the interquartile range (IQR) in each age group were defined as outliers [32]. After eliminating outliers per Tukey’s rules, age group mid-90 percentile and mid-95 percentile reference intervals with 90% confidence intervals (CIs) and age-specific percentiles (5th, 10th, 25th, 50th, 75th, 90th, and 95th) were calculated. As the 40–44 years, 45–49 years, and 50–54 years age groups exhibited extreme distributions, values within the 95% CI of the upper percentile in the package insert for the corresponding age groups were not excluded. We confirmed the appropriateness of the exclusion by applying the Box–Cox transformation [12]. Reference intervals were calculated using nonparametric methods according to recommendations in the CLSI EP28-A3c guidelines [33]. The percentile rank [R(p)] was calculated using the following equation:
R(p)=p÷100×(n+1),
where p is the percentile and n the number of values. Differences in AMH concentrations based on the menstrual cycle phase were analyzed using the Kruskal–Wallis test.
Anthropometric data and laboratory results by age group are shown in Table 1. The mean participant age was 37.4±10.4 years. The mean BMI was 21.7 kg/m2 in the 19–24 years group and slightly increased up to 23.2 kg/m2 in the 45–49 years and 50–54 years groups. The overall obesity prevalence was 18.3%. The mean blood pressure, fasting glucose, total cholesterol, and triglyceride concentrations tended to increase slightly with increasing age. However, the differences were not substantial, and the average values were within the normal ranges, even in the oldest age group. The overall anemia prevalence was 13.2%, with a relatively higher prevalence in the 35–39 years (17.5%), 40–44 years (19.1%), and 45–49 years (18.6%) groups, and a lower prevalence in the 19–24 years (4.4%), 25–29 years (7.6%), 30–34 years (11.5%), and 50–54 years (9.5%) groups.
AMH results were obtained from 1,429 of the 1,450 participants. For 21 participants, results were not obtained because of insufficient sample amounts. AMH concentrations were extremely skewed to the lower values, where 30% of the participants had a concentration ≤3.57 pmol/L. There was one outlier in the 19–24 years group, one in the 30–34 years group, two in the 35–39 years group, five in the 40–44 years group, two in the 45–49 years group, and two in the 50–54 years group, adding up to a total of 13 outliers. The percentage of participants with an AMH concentration ≤3.57 pmol/L started to exceed 20% in the 40–44 years group, with the percentage exceeding 70% in the 45–49 years group and 90% in the 50–54 years group.
The 25th percentile, median, and 75th percentile by age group are shown in Fig. 2. AMH concentrations gradually decreased with increasing age. While the median AMH concentration was >28.57 pmol/L in the 20–29 years group, it neared 21.43 pmol/L in the 30–34 years group and rapidly declined toward 7.14 pmol/L starting at age 35. Most participants aged ≥45 years had AMH concentrations <7.14 pmol/L.
The AMH reference intervals are shown in Table 2 along with median. The 30–34 years group showed a wide range of values. The 2.5th percentile value (1.14 pmol/L) was below the 4.11 pmol/L noted in the package insert, whereas the 97.5th percentile value of 78.43 pmol/L was higher than the package insert value of 58.0 pmol/L. The percentiles and number of participants at each age between 19 and 54 years are shown in Supplemental Data Table S1. The mean number of participants by age was 39.
Progesterone results were obtained from 1,427 of the 1,450 participants. For 23 participants, results were not obtained because of insufficient sample amounts. Two hundred participants were in the follicular phase (median 0.29 nmol/L), 656 in the follicular or ovulatory phase (median 0.83 nmol/L), 92 in the ovulatory phase (median 3.82 nmol/L), 238 in the luteal or ovulatory phase (median 20.03 nmol/L), and 168 in the luteal phase (median 49.93 nmol/L). In addition, 28 participants were classified into the high-progesterone group (median 85.23 nmol/L) and 45 into the postmenopausal group (median 0.16 nmol/L). There were no significant differences in median AMH concentrations according to the progesterone concentration (P=0.158, Kruskal–Wallis test), except for the postmenopausal group. The median AMH concentrations based on the menstrual cycle phases and conditions are shown in Supplemental Data Table S2.
BMP15 genetic testing was performed for all 1,450 participants. The four BMP15 variants considered to be clinically important were not detected in the study population. Regarding the other three variants assessed, there were no significant differences in AMH concentrations between two groups divided according to the presence or absence of each variant.
AMH concentration is a marker of the ovarian reserve, which is closely associated with ovarian function. Although reference intervals in the healthy population are essential for the appropriate use of AMH, relevant studies on the Elecsys AMH assay in the Korean population are lacking. Only one study has been conducted on the Elecsys AMH assay, and this study included only Caucasian populations [10]. We established reference intervals for the Elecsys AMH assay in healthy Korean women of childbearing age according to age groups. Compared to Caucasian populations, the AMH concentrations varied widely in the 30–34 year group of the Korean population, with lower values in the lower percentiles (1.14 pmol/L for 2.5th) and higher values in the upper percentiles (78.43 pmol/L for 97.5th). In the same age group of a European population, the 2.5th and 97.5th percentiles of Elecsys AMH were 4.11 pmol/L and 58.0 pmol/L, respectively [10]. In our study, the AMH concentrations began to decline from age 35 and significantly decreased toward the LOD (0.07 pmol/L) from age 40. The age-specific percentile data showed that AMH concentrations markedly declined particularly from age 43. Age-specific percentile data can provide approximate information regarding the ovarian function of an individual [19, 20].
A few previous studies have attempted to establish AMH reference intervals in the Korean population. These studies used the Immunotec AMH ELISA or modified Gen II ELISA, not automated assays, to measure AMH concentrations and included women visiting infertility centers [18–20]. In these previous Korean studies, the AMH concentrations in similar age groups were higher than those in our study. This is consistent with previous results that the AMH concentrations measured using the Elecsys AMH assay were 12%–28% lower and those measured using the Access AMH assay were 9%–22% lower than those measured using ELISA [6–10]. Differences in calibration and/or the high inter-laboratory variability of manual ELISAs may contribute to this discordance [8, 9]. In a recent Korean study comparing automated AMH assays using serum samples collected from an infertility clinic, the median AMH concentrations measured using the Elecsys AMH assay were 29.9 pmol/L for the 18–29 years, 24.6 pmol/L for the 30–34 years, and 14.1 pmol/L for the 35–39 years age groups [34]. These AMH concentrations are similar to those in our study, although they did not intend to provide the age-specific AMH reference intervals.
Our study is unique in that an automated AMH assay was used and the sera were collected from KNHANES participants. KNHANES is a nationwide survey conducted by the Korea Disease Control and Prevention Agency and provides reliable statistical data regarding the overall health and nutritional status in the general Korean population [22, 23]. In addition to a questionnaire survey on health and lifestyle, physical examination, blood and urine sampling, and follow-up are conducted. Further, our study included a wider age range of participants (19–54 years) than previous Korean studies (25–45 or 50 years). In addition to AMH, progesterone concentrations were obtained for menstrual cycle phase estimation. Median AMH concentrations did not significantly differ between menstrual cycle phases in non-postmenopausal participants. Thus, the menstrual cycle phase did not affect the AMH concentrations in this study, which is in line with the previous result that serum AMH concentrations were relatively stable across the menstrual cycle when compared with follicle-stimulating hormone concentrations [1–3]. In the postmenopausal group, the median AMH concentration was as low as 0.07 pmol/L, which is the LOD of the Elecsys assay. In BMP15 testing, the four clinically important variants (p.Arg68Trp, p.Arg76Cys, p.Gln211Ter, and p.Tyr235Cys) were not detected in the study population. BMP15 plays an important role in early human folliculogenesis and is considered a candidate gene for premature ovarian insufficiency [24, 25]. By analyzing this gene, we aimed to ascertain if there were any clinically important variants that may have affected ovarian function in the study participants.
AMH concentrations may vary according to ethnicity, and there are some reports about ethnic differences within specific age groups. Nelson, et al. [16], using the Elecsys assay, found that Chinese women tended to have higher AMH concentrations before the age of 25 years and lower concentrations from age 25 onward than European women. Bleil, et al. [17], using the Beckman Coulter ELISA, found that Hispanic women had lower AMH concentrations across all ages, whereas African–American and Chinese women had lower AMH concentrations only at younger (i.e., 25 and 30 years) and middle ages (i.e., 30, 35, and 40 years), respectively, than Caucasian women. In a recent study using the Elecsys assay in Egyptian women, the AMH concentrations across all ages were lower than those in our study or the manufacturer-provided values based on Caucasian populations [12]. In our study, the median AMH concentrations in Korean women of 19–34 years were slightly higher than those shown in the package insert. Using the Access assay in Chinese women, Cheng, et al. [11] reported a similar trend, which was even more evident in the age group 25–34 years. Korean women within a certain age group may show slightly higher AMH concentrations in automated AMH assays than Western women; however, current results are inconsistent or conflicting, warranting further studies on the matter [10].
Results of the two widely used automated AMH assays, Roche Elecsys and Beckman Coulter Access, reportedly show high agreement, with a difference of only 3% and no significant bias [8, 9, 35]. The Access AMH assay tended to produce slightly higher concentrations (average 2.1 pmol/L) than the Elecsys AMH assay in a European population [9]. However, AMH results obtained by the Roche and Beckman Coulter assays are not interchangeable, especially in certain circumstances, such as individualized dosing based on a companion diagnostic assay [35].
The current study had several limitations. Although we attempted to eliminate confounders that may impact AMH concentrations when selecting a healthy population, it is still possible that AMH concentrations were affected by unknown factors. First, the selection criteria did not include parameters related to women’s health, such as menstrual cycle regularity, current use of oral contraceptives or other medications, history of infertility or polycystic ovary syndrome (PCOS), or menstrual phase at the time of blood sampling. We were unable to obtain information about these parameters as they were not included in the interview or examination portions of the KNHANES. Instead, we included apparently healthy non-smoking women with no previous or current chronic diseases, such as hypertension, diabetes mellitus, or hypercholesterolemia, and serum progesterone measurement and BMP15 testing were deliberately performed. It is possible that some participants had PCOS. While we expect these patients would have been excluded as outliers, we cannot definitively confirm this to be the case. The use of oral contraceptives or gonadotropin-releasing hormone agonist may have reduced the AMH concentrations. Second, obesity may have affected the AMH concentrations [36]. While some reports suggest that obese women have lower AMH concentrations than non-obese women, the results are not consistent across studies [36, 37]. The prevalence of obesity in our study population was similar to or lower than that in the 2018 National Health Checkup database provided by the Korean National Health Insurance Service or 2013–2014 KNHANES reports [22, 23]. Hence, we reasoned that obesity would not have a deleterious impact on the AMH reference intervals, and accordingly, we did not exclude obese women from the study. Third, we used frozen sera for AMH measurements. Thus, sample storage conditions or freeze/thaw cycles may have affected the AMH stability. However, the stability issue is considered to be minimal as our samples had been stored in a deep-frozen state at the National Biobank of Korea and were thawed only once [6, 38, 39].
In summary, we established reference intervals for the Elecsys AMH assay according to 5-year incremental age groups in Korean women aged 19–54 years and presented age-specific percentiles. The AMH concentrations tended to decline with advancing age and markedly decreased starting from age 40, where the lower reference limit declined to near the LOD. The median AMH value in the 25–34 years group of our Korean cohort tended to be slightly higher than that in Caucasian populations. These results are insightful in interpreting AMH results and assessing ovarian reserve in Korean women and can be helpful for expanding the clinical utility of AMH in Asian populations.
ACKNOWLEDGMENTS
This study was conducted with bioresources from the National Biobank of Korea, Korea Disease Control and Prevention Agency (KBN-2018-049).
Notes
AUTHOR CONTRIBUTIONS
Ji M analyzed the data and wrote the draft. Kim KR designed the study, analyzed the data, and reviewed the manuscript. Chun S conceived and designed the study, analyzed the data, and finalized the draft. Kim HK analyzed data and participated in the drafting. Lee W, Yun YM, and Min WK discussed the data and reviewed the manuscript. All authors read and approved the entire content of the final manuscript.
REFERENCES
1. Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, et al. 2014; The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update. 20:370–85. DOI: 10.1093/humupd/dmt062. PMID: 24430863.
2. Tal R, Seifer DB. 2017; Ovarian reserve testing: a user's guide. Am J Obstet Gynecol. 217:129–40. DOI: 10.1016/j.ajog.2017.02.027. PMID: 28235465.

3. Podfigurna A, Lukaszuk K, Czyzyk A, Kunicki M, Maciejewska-Jeske M, Jakiel G, et al. 2018; Testing ovarian reserve in pre-menopausal women: why, whom and how? Maturitas. 109:112–7. DOI: 10.1016/j.maturitas.2017.11.014. PMID: 29292013.

4. Broer SL, Broekmans FJ, Laven JS, Fauser BC. 2014; Anti-Müllerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update. 20:688–701. DOI: 10.1093/humupd/dmu020. PMID: 24821925.

5. Ghazal K, Brabant S, Prie D, Piketty ML. 2022; Hormone immunoassay interference: A 2021 update. Ann Lab Med. 42:3–23. DOI: 10.3343/alm.2022.42.1.3. PMID: 34374345. PMCID: PMC8368230.

6. Gassner D, Jung R. 2014; First fully automated immunoassay for anti-Müllerian hormone. Clin Chem Lab Med. 52:1143–52. DOI: 10.1515/cclm-2014-0022. PMID: 24622790.

7. Demirdjian G, Bord S, Lejeune C, Masica R, Rivière D, Nicouleau L, et al. 2016; Performance characteristics of the Access AMH assay for the quantitative determination of anti-Müllerian hormone (AMH) levels on the Access* family of automated immunoassay systems. Clin Biochem. 49:1267–73. DOI: 10.1016/j.clinbiochem.2016.08.005. PMID: 27542330.

8. van Helden J, Weiskirchen R. 2015; Performance of the two new fully automated anti-Müllerian hormone immunoassays compared with the clinical standard assay. Hum Reprod. 30:1918–26. DOI: 10.1093/humrep/dev127. PMID: 26093541.

9. Nelson SM, Pastuszek E, Kloss G, Malinowska I, Liss J, Lukaszuk A, et al. 2015; Two new automated, compared with two enzyme-linked immunosorbent, antimüllerian hormone assays. Fertil Steril. 104:1016–21.e6. DOI: 10.1016/j.fertnstert.2015.06.024. PMID: 26183313.

10. Anckaert E, Öktem M, Thies A, Cohen-Bacrie M, Daan NM, Schiettecatte J, et al. 2016; Multicenter analytical performance evaluation of a fully automated anti-Müllerian hormone assay and reference interval determination. Clin Biochem. 49:260–7. DOI: 10.1016/j.clinbiochem.2015.10.008. PMID: 26500002.

11. Cheng X, Zhang Q, Liu M, Li S, Tao Z, Ichihara K, et al. 2017; Establishing age-specific reference intervals for anti-Müllerian hormone in adult Chinese women based on a multicenter population. Clin Chim Acta. 474:70–5. DOI: 10.1016/j.cca.2017.09.008. PMID: 28911998.

12. El-Attar EA, Hosny TA, Ichihara K, Bedair RN, Tork ASE. 2021; Nomogram of age-specific anti-Müllerian hormone levels in healthy Egyptian females. PLoS One. 16:e0254858. DOI: 10.1371/journal.pone.0254858. PMID: 34310641. PMCID: PMC8312962.

13. Kim S, Lee K, Park HD, Lee YW, Chun S, Min WK. 2021; Schemes and performance evaluation criteria of Korean Association of External Quality Assessment (KEQAS) for improving laboratory testing. Ann Lab Med. 41:230–9. DOI: 10.3343/alm.2021.41.2.230. PMID: 33063686. PMCID: PMC7591290.

14. The International Consortium for Harmonization of Clinical Laboratory Results. Measurands. https://www.harmonization.net/measurands. Updated on Jan, 2022.
15. Kotlyar AM, Seifer DB. 2021; Ethnicity/race and age-specific variations of serum AMH in women-A review. Front Endocrinol (Lausanne). 11:593216. DOI: 10.3389/fendo.2020.593216. PMID: 33633682. PMCID: PMC7900163.

16. Nelson SM, Aijun S, Ling Q, Tengda X, Wei X, Yan D, et al. 2020; Ethnic discordance in serum anti-Müllerian hormone in healthy women: a population study from China and Europe. Reprod Biomed Online. 40:461–7. DOI: 10.1016/j.rbmo.2019.11.013. PMID: 32094052.

17. Bleil ME, Gregorich SE, Adler NE, Sternfeld B, Rosen MP, Cedars MI. 2014; Race/ethnic disparities in reproductive age: an examination of ovarian reserve estimates across four race/ethnic groups of healthy, regularly cycling women. Fertil Steril. 101:199–207. DOI: 10.1016/j.fertnstert.2013.09.015. PMID: 24182412. PMCID: PMC3976042.

18. Yoo JH, Kim HO, Cha SW, Park CW, Yang KM, Song IO, et al. 2011; Age specific serum anti-Müllerian hormone levels in 1,298 Korean women with regular menstruation. Clin Exp Reprod Med. 38:93–7. DOI: 10.5653/cerm.2011.38.2.93. PMID: 22384425. PMCID: PMC3283054.

19. Lee JE, Park DS, Kim ML, Yoon BS, Song T, Kim MK, et al. 2012; Age-related distribution of anti-Müllerian hormone levels in 2,879 Korean women with regular menstruation. Korean J Obstet Gynecol. 55:920–8. DOI: 10.5468/KJOG.2012.55.12.920.

20. Lee JY, Ahn S, Lee JR, Jee BC, Kim CH, Seo S, et al. 2017; Reference values for the revised anti-Müllerian hormone generation II assay: infertile population-based study. J Korean Med Sci. 32:825–9. DOI: 10.3346/jkms.2017.32.5.825. PMID: 28378557. PMCID: PMC5383616.

21. Zou Y, Wang D, Cheng X, Ma C, Lin S, Hu Y, et al. 2021; Reference intervals for thyroid-associated hormones and the prevalence of thyroid diseases in the Chinese population. Ann Lab Med. 41:77–85. DOI: 10.3343/alm.2021.41.1.77. PMID: 32829582. PMCID: PMC7443523.

22. Kim Y. 2014; The Korea National Health and Nutrition Examination Survey (KNHANES): current status and challenges. Epidemiol Health. 36:e2014002. DOI: 10.4178/epih/e2014002. PMID: 24839580. PMCID: PMC4017741.

23. Kweon S, Kim Y, Jang MJ, Kim Y, Kim K, Choi S, et al. 2014; Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES). Int J Epidemiol. 43:69–77. DOI: 10.1093/ije/dyt228. PMID: 24585853. PMCID: PMC3937975.

24. Rossetti R, Di Pasquale E, Marozzi A, Bione S, Toniolo D, Grammatico P, et al. 2009; BMP15 mutations associated with primary ovarian insufficiency cause a defective production of bioactive protein. Hum Mutat. 30:804–10. DOI: 10.1002/humu.20961. PMID: 19263482. PMCID: PMC2677132.
25. Pu D, Xing Y, Gao Y, Gu L, Wu J. 2014; Gene variation and premature ovarian failure: a meta-analysis. Eur J Obstet Gynecol Reprod Biol. 182:226–37. DOI: 10.1016/j.ejogrb.2014.09.036. PMID: 25445105.

26. Nam GE, Kim YH, Han K, Jung JH, Rhee EJ, Lee SS, et al. Obesity fact sheet in Korea, 2019: prevalence of obesity and abdominal obesity from 2009 to 2018 and social factors. J Obes Metab Syndr. 2020; 29:124–32. DOI: 10.7570/jomes20058. PMID: 32581145. PMCID: PMC7338491.

27. Shin HY, Kang HT. 2017; Recent trends in the prevalence of underweight, overweight, and obesity in Korean adults: the Korean National Health and Nutrition Examination Survey from 1998 to 2014. J Epidemiol. 27:413–9. DOI: 10.1016/j.je.2016.08.014. PMID: 28420559. PMCID: PMC5565760.

28. WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. https://apps.who.int/iris/handle/10665/85839. Updated on 2011.
29. Di Pasquale E, Beck-Peccoz P, Persani L. 2004; Hypergonadotropic ovarian failure associated with an inherited mutation of human bone morphogenetic protein-15 (BMP15) gene. Am J Hum Genet. 75:106–11. DOI: 10.1086/422103. PMID: 15136966. PMCID: PMC1181993.
30. Di Pasquale E, Rossetti R, Marozzi A, Bodega B, Borgato S, Cavallo L, et al. 2006; Identification of new variants of human BMP15 gene in a large cohort of women with premature ovarian failure. J Clin Endocrinol Metab. 91:1976–9. DOI: 10.1210/jc.2005-2650. PMID: 16464940.
31. Dixit H, Rao LK, Padmalatha VV, Kanakavalli M, Deenadayal M, Gupta N, et al. 2006; Missense mutations in the BMP15 gene are associated with ovarian failure. Hum Genet. 119:408–15. DOI: 10.1007/s00439-006-0150-0. PMID: 16508750.

32. Tukey JW. 1977. Exploratory data analysis. Addison-Wesley Publishing Company;Boston: DOI: 10.1007/s00439-006-0150-0.
33. CLSI. 2010. Defining, establishing, and verifying reference intervals in the Clinical Laboratory; approved guideline. 3rd ed. CLSI EP28-A3c. Clinical and Laboratory Standards Institute;Wayne, PA: DOI: 10.1007/s00439-006-0150-0.
34. Han A, Suh B, Yi G, Lee YJ, Kim SE. 2022; Comparison of the automated fluorescent immunoassay system with Roche Elecsys and Beckman Coulter access 2 assays for anti- Müllerian hormone measurement. Ann Lab Med. 42:47–53. DOI: 10.3343/alm.2022.42.1.47. PMID: 34374348. PMCID: PMC8368231.

35. Iliodromiti S, Salje B, Dewailly D, Fairburn C, Fanchin R, Fleming R, et al. 2017; Non-equivalence of anti-Müllerian hormone automated assays-clinical implications for use as a companion diagnostic for individualised gonadotrophin dosing. Hum Reprod. 32:1710–5. DOI: 10.1093/humrep/dex219. PMID: 28854583. PMCID: PMC5850658.

36. Shahrokhi SZ, Kazerouni F, Ghaffari F. 2018; Anti-Mullerian hormone: genetic and environmental effects. Clin Chim Acta. 476:123–9. DOI: 10.1016/j.cca.2017.11.027. PMID: 29175649.
37. Moslehi N, Shab-Bidar S, Ramezani Tehrani F, Mirmiran P, Azizi F. 2018; Is ovarian reserve associated with body mass index and obesity in reproductive aged women? A meta-analysis. Menopause. 25:1046–55. DOI: 10.1097/GME.0000000000001116. PMID: 29738413.

38. Rustamov O, Smith A, Roberts SA, Yates AP, Fitzgerald C, Krishnan M, et al. 2014; The measurement of anti-Müllerian hormone: a critical appraisal. J Clin Endocrinol Metab. 99:723–32. DOI: 10.1210/jc.2013-3476. PMID: 24423305.

39. Cho SY, Hong EJ, Nam JM, Han B, Chu C, Park O. 2012; Opening of the national biobank of Korea as the infrastructure of future biomedical science in Korea. Osong Public Health Res Perspect. 3:177–84. DOI: 10.1016/j.phrp.2012.07.004. PMID: 24159511. PMCID: PMC3738708.

Fig. 1
Outline of the study course.
Abbreviations: KNHANES, Korea National Health and Nutrition Examination Survey; AMH, anti-Müllerian hormone; BMP15, bone morphogenetic protein-15.

Fig. 2
AMH concentrations according to different age groups of healthy Korean women. The 25th, 50th, and 75th percentiles are presented with the median values. AMH concentrations decreased with increasing age, especially after 40 years. In certain age groups, e.g., 25–29 years and 30–34 years, median values were slightly higher than those mentioned in the package insert, which were obtained from 887 healthy Caucasian women.
Abbreviation: AMH, anti-Müllerian hormone.
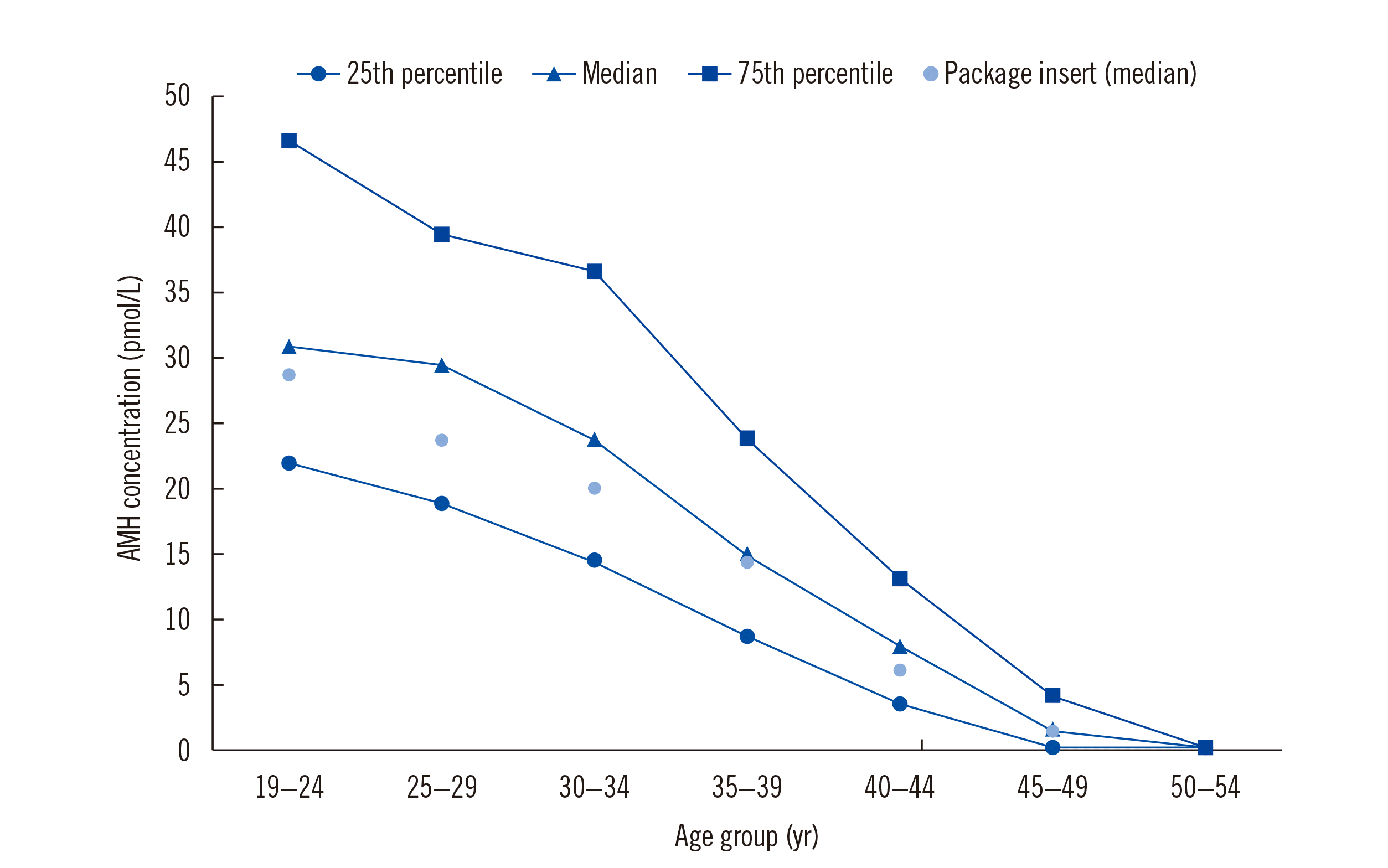
Table 1
Anthropometric and laboratory test results of the study participants
| Variable | Age group, yr (N participants) | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| 19–24 (160) | 25–29 (132) | 30–34 (238) | 35–39 (236) | 40–44 (231) | 45–49 (216) | 50–54 (216) | Total N (1,429) | |
| Age (yr)* | 21.2 (1.6) | 27.1 (1.4) | 32.2 (1.4) | 37.0 (1.5) | 41.9 (1.5) | 47.1 (1.5) | 52.0 (1.5) | 37.4 (10.4) |
| Height (cm)* | 161.6 (6.3) | 160.9 (6.0) | 161.3 (5.7) | 160.4 (5.2) | 160.4 (4.9) | 158.0 (5.7) | 157.0 (5.3) | 159.9 (5.8) |
| Weight (kg)* | 56.9 (9.3) | 58.3 (9.5) | 57.6 (9.2) | 58.2 (8.7) | 58.0 (7.4) | 58.0 (7.6) | 57.2 (7.4) | 57.8 (8.5) |
| BMI (kg/m2)* | 21.7 (3.4) | 22.4 (3.2) | 22.1 (3.5) | 22.6 (3.3) | 22.5 (2.8) | 23.2 (2.7) | 23.2 (2.7) | 22.6 (3.2) |
| Obesity (%)† | 13.1 | 17.6 | 20.1 | 15.8 | 15.3 | 23.2 | 21.8 | 18.3 |
| SBP (mmHg)* | 105 (9) | 104 (8) | 104 (9) | 105 (9) | 107 (11) | 108 (11) | 111 (12) | 107 (10) |
| DBP (mmHg)* | 69 (7) | 69 (7) | 69 (8) | 70 (7) | 72 (8) | 72 (8) | 73 (8) | 71 (8) |
| Fasting glucose (mmol/L)* | 4.9 (0.3) | 4.9 (0.4) | 5.0 (0.4) | 5.0 (0.4) | 5.1 (0.4) | 5.1 (0.4) | 5.2 (0.5) | 5.1 (0.4) |
| HbA1c (mmol/mol)* | 34 (3.3) | 34 (3.3) | 36 (3.3) | 37 (3.3) | 37 (3.3) | 37 (3.3) | 38 (4.4) | 36 (3.3) |
| Total cholesterol (mmol/L)* | 4.4 (0.6) | 4.7 (0.7) | 4.6 (0.7) | 4.6 (0.6) | 4.7 (0.6) | 4.9 (0.6) | 5.0 (0.6) | 4.7 (0.7) |
| Triglyceride (mmol/L)* | 0.88 (0.4) | 0.97 (0.7) | 0.97 (0.5) | 0.96 (0.5) | 1.10 (0.7) | 1.14 (0.7) | 1.29 (0.8) | 1.05 (0.6) |
| Creatinine (μmol/L)* | 62.8 (8.0) | 61.9 (8.0) | 61.0 (8.0) | 62.8 (7.1) | 63.6 (8.0) | 62.8 (8.0) | 61.9 (8.8) | 61.9 (8.0) |
| Hb (g/L)* | 132 (9) | 133 (9) | 130 (11) | 129 (12) | 128 (12) | 127 (13) | 132 (11) | 130 (11) |
Table 2
Elecsys AMH reference interval using the CLSI EP28-A3c percentile method (pmol/L)




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download