This article has been
cited by other articles in ScienceCentral.
Abstract
Purpose
The purpose of this study was to investigate antimicrobial activity of extracts from shiitake mushroom against periodontopathogens and its cytotoxicity for human gingival fibroblast.
Materials and Methods
Shiitake mushroom was soaked in water and acetone, and the supernatant was dried to collect its extract. The susceptibility of periodontopathogens for the extracts was investigated. Human gingival fibroblast was treated with the extracts, and the cell viability was measured CCK-8 solution.
Results
The water extract from shiitake mushroom significantly reduced the growth of periodontopathogens at 2.5 mg/ml (P < 0.05). The acetone extract significantly inhibited the growth of Porphyromonas gingivalis and Tannerella forsythia at 0.32 mg/ml and Treponema denticola growth at 0.64 mg/ml (P < 0.05). The cytotoxicity of the extract was shown at a concentration of 2.5 mg/ml. The extracts with a concentration of 1.25 mg/ml appeared to be reduce cell viability after 4 h.
Conclusion
The extracts of shiitake mushroom have antimicrobial activity against periodontitis-causing bacteria and relieving inflammation. Therefore, the extracts may be a candidate for preventing and treating periodontal disease.
Go to :

초록
목적
이 연구의 목적은 표고 버섯 추출물의 치주 병원체에 대한 항균 활성과 인간 치은 섬유아세포에 대한 세포 독성을 조사하는 것입니다.
연구 재료 및 방법
표고버섯을 물과 아세톤에 닮근 후, 상등액을 건조하여 추출물을 채취하였다. 추출물에 대한 치주 병원체의 감수성을 조사하였다. 인간 치은 섬유아세포에 추출물을 처리하고 CCK-8 용액을 이용하여 세포 생존율을 측정하였다.
결과
표고버섯 물추출물은 2.5 mg/ml에서 치주병원체의 증식을 유의하게 감소시켰다(P < 0.05). 아세톤 추출물은 0.32 mg/ml에서 Porphyromonas gingivalis와 Tannerella forsythia의 성장을 유의하게 억제하였고, 0.64 mg/ml에서 Treponema denticola의 성장을 유의하게 억제하였다(P < 0.05). 추출물의 세포독성은 2.5 mg/ml의 농도에서 나타났으며, 1.25 mg/ml 농도의 추출물이 4시간 후부터 세포 생존률을 감소시키는 것으로 나타났다.
결론
표고버섯 추출물은 치주염 유발 세균에 대한 항균 활성과 염증 완화 효과를 보였다. 따라서 추출물은 치주질환 예방 및 치료에 대한 후보물질이 될 수 있다.
Go to :

Keywords: antimicrobial activity, extract of shiitake mushroom, periodondopathogen
색인어: 항균 활성, 표고버섯 추출물, 치주염 세균
Introduction
Periodontal diseases such as periodontitis, periodontal tissue destruction, and loss of alveolar bone are related with Gram-negative anaerobic bacteria. Basis on epidemiological study,
Porphyromonas gingivalis,
Tannerella forsythia, and
Treponema denticola are closely associated with the periodontal diseases.
1 These bacteria, are asaccharolytic species, produced diverse and abundant enzymes to cleave protein for uptake,
2 by which halitosis is induced. Furthermore, the bacteria have lipopolysaccharide as endotoxin,
3-5 and lipopolysaccharide induces inflammatory cytokines from human gingival fibroblast and immune cells through Toll-like receptor 2 or 4.
4,6
Mushrooms have been used for tea and nutritional food due to their special fragrance and texture.
7 Furthermore, some mushrooms are applied as alternative medicine in Northeast Asia.
8 Especially,
Lentinula edodes as shiitake mushroom is the most famous and has medicinal compounds,
9 which are effective in treating and preventing tumors, inflammation, and microbial infection.
10,11 Also, the extract of
L. edodes showed anti-caries activity.
12 The components in shiitake mushroom consist of soluble- and insoluble-dietary fiber and aroma components. The soluble-dietary fibers are heteroglycans, heterogalactans, heteromannans, and xyloglucans. The insoluble-dietary fibers are heteroglycan and plyuronide, β-glucan, and chitin.
7 The aroma components, which are most fatty acid, are linoleic, palmitic, tetradecenoic, oleic, stearic, and myristic acid.
13,14 Therefore, shiitake efficacy is different depending on the extraction method.
Despite advances in dental treatment, the number of patients with periodontitis is increasing, and the number of patients with peri-implantitis after implant surgery is also increasing.. Therefore, the purpose of present study was to investigate antimicrobial activity of soluble- and insoluble-extract from shiitake against periodontopathogens and to evaluate cytotoxicity of the soluble- and the insoluble-extract.
Go to :

Materials and Methods
Lentinula edodes (Shiitake mushroom) is purchased in oriental medicine market and used in this study. Shiitake mushroom was sliced, and 100 g soaked in 100 ml of distilled water, ethyl alcohol, and acetone with magnetic bar to isolate soluble and insoluble extract for shiitake mushroom, repectively. The preparations were incubated at room temperature for 24 h on magnetic stirrer. The extracts from the mushroom were collected by filtering with Whatman filter paper (GE healthcare, Chicago, USA), and the solvent was evaporated with vacuum evaporator (IKA, Staufen, Germany). The weight of the extract was measured and solved at 10 mg/ml with pyrogen-free distilled water and dimethyl sulfoxide (DMSO, Sigma-aldrich Co., San Jose, USA). The prepared solutions were filtrated with polyvinylidene fluoride (PVDF) filter (0.25 μm of pore size) (Millipore, Billerica, USA).
Porphyromonas gingivalis ATCC 33277,
Tannerella forsythia ATCC 43037, and
Treponema denticola ATCC 35405 were used in this study, and the bacteria were cultured under 5% H
2, 10% CO
2, and 85% N
2 atmosphere.
P. gingivalis was cultivated in brain heart infusion (BD biosciences, San jose, USA),
T. forsythia was cultured in modified New Oral Spirochete medium,
4 and
T. denticola was cultured in tryptone-yeast extractgelatin volatile fatty acid-serum (TYGVS) medium.
15 The rabbit serum concentration of
T. denticola medium was used at 2% less than suggested concentration.
The susceptibility assay of periodontopathogens for shiitake extracts was performed according to the method suggested by Clinical Laboratory Standard Institute.
16 Each specific medium (180 μl) was dispensed into 96-well plate (SPL lifescience, Pocheon, Korea), and each extract solution (180 μl) was dispensed into the 12
th row well of a 96-well plate. The extract was performed 2-fold serial dilution to the 11
th column with multi-micropipette. The cultured periodontopathogens were counted with bacterial counting chamber (Marienfeld, Lauda-Konigshöfen, Germany), and its concentration was adjusted at 3 × 10
6 cells/ml using the respective media. The bacterial suspension (20 μl) was inoculated into prepared well, and the bacteria-inoculated plate was incubated at 37°C under aerobic condition (H
2 5%, CO
2 10%, N
2 85%) for 48 h. The bacterial growth was measured using optical density at a 660 nm wavelength by a spectrophotometer (Biotek, Winooski, USA).
Human gingival fibroblasts (HGF-1; CRL-2014) was cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS; Hyclone), penicillin (100 U/ml), and streptomycin sulfate (100 μg/ml). The HGFs were plated in 100 mm diameter cell culture dish (SPL Lifescience) and subcultured two times. The cells were detached using trypsin-EDTA (Welgene Co., Gyeongsan, Korea), and the cell number was counted by a cell counting chamber (Marienfeld). 1 × 105 cells were seeded into 96-well plate and grown to 90% confluence. After starvating for 4 h, the cells were treated with each shiitake extract for 12 h. The spent medium was removed, and fresh medium (100 μl) was inoculated. After incubating for 1 h in a CO2 incubator, 10 μl of CCK-8 solution (Dojindo Laboratories, Mashiki, Kumamoto, Japan) was added into each well of the plate to measure cell viability, and the cells were incubated for 2 h in the incubator. After adding 1% sodium dodecyl sulfate (SDS) solution, the optical density of the well was measured at 450 nm wavelength by a microplate reader (Biotek).
Statistical analysis was processed by IBM SPSS statistics ver. 23 software (IBM, Armonk, USA). Data was analyzed by the non-parametric Kruskal-Wallis and Mann-Whitney test. Statistical significance was defined by P value of less than 0.05.
Go to :

Results
Antimicrobial effect of the extracts from shiitake mushroom on periodontopathogens such as
P. gingivalis,
T. forsythia, and
T. denticola were examined. Water and acetone extracts from shiitake mushroom showed antimicrobial activity. In case of
P. gingivalis, the water extract inhibited above the concentration of 2.5 mg/ml, and the acetone extract significantly inhibited above the concentration of 0.32 mg/ml (
P < 0.05)(
Fig. 1). Next, in the investigation for
T. forsythia, the water and the acetone extract significantly inhibited the growth of
T. forsythia above the concentration of 2.5 mg/ml and 0.32 mg/ml, respectively (
P < 0.05)(
Fig. 2).
T. denticola showed less sensitive for the extracts than other bacteria. the water extract inhibited the growth of
T. denticola above 2.5 mg/ml, and the acetone extract significantly reduced the growth above 0.64 mg/ml of concentration (
P < 0.05)(
Fig. 3). Special thing is that the absorbance is increased in the acetone extract at a concentration of 5 mg/ml.
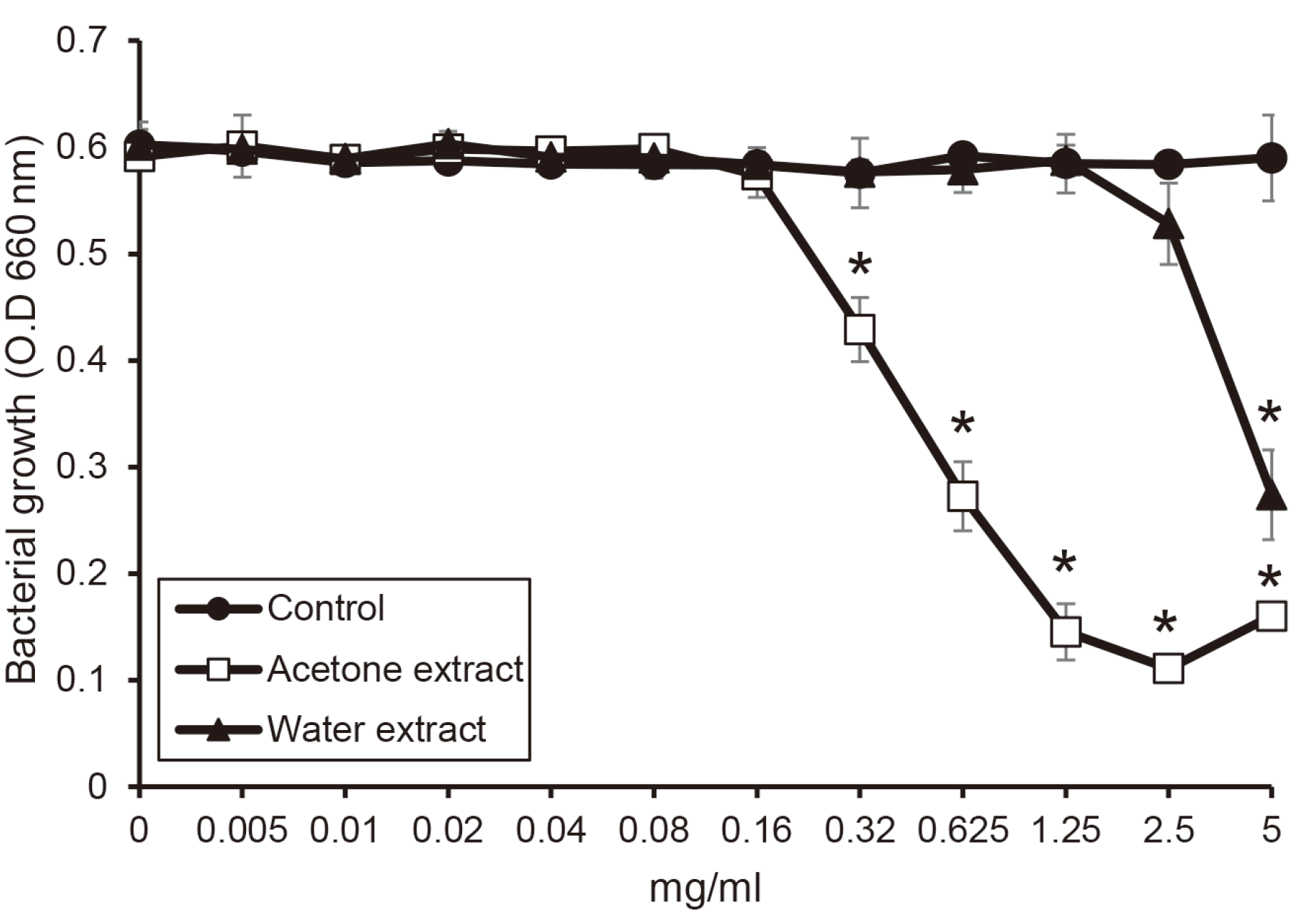 | Fig. 1
The susceptibility of P. gingivalis for the extracts from shiitake mushroom. P. gingivalis was incubated with or without the extracts in the various concentrations. The growth of P. gingivalis was measured by a spectrophotometer at 660 nm wavelength. Asterix (*) indicates significant difference compared with control (P < 0.05).
O.D, optical density.

|
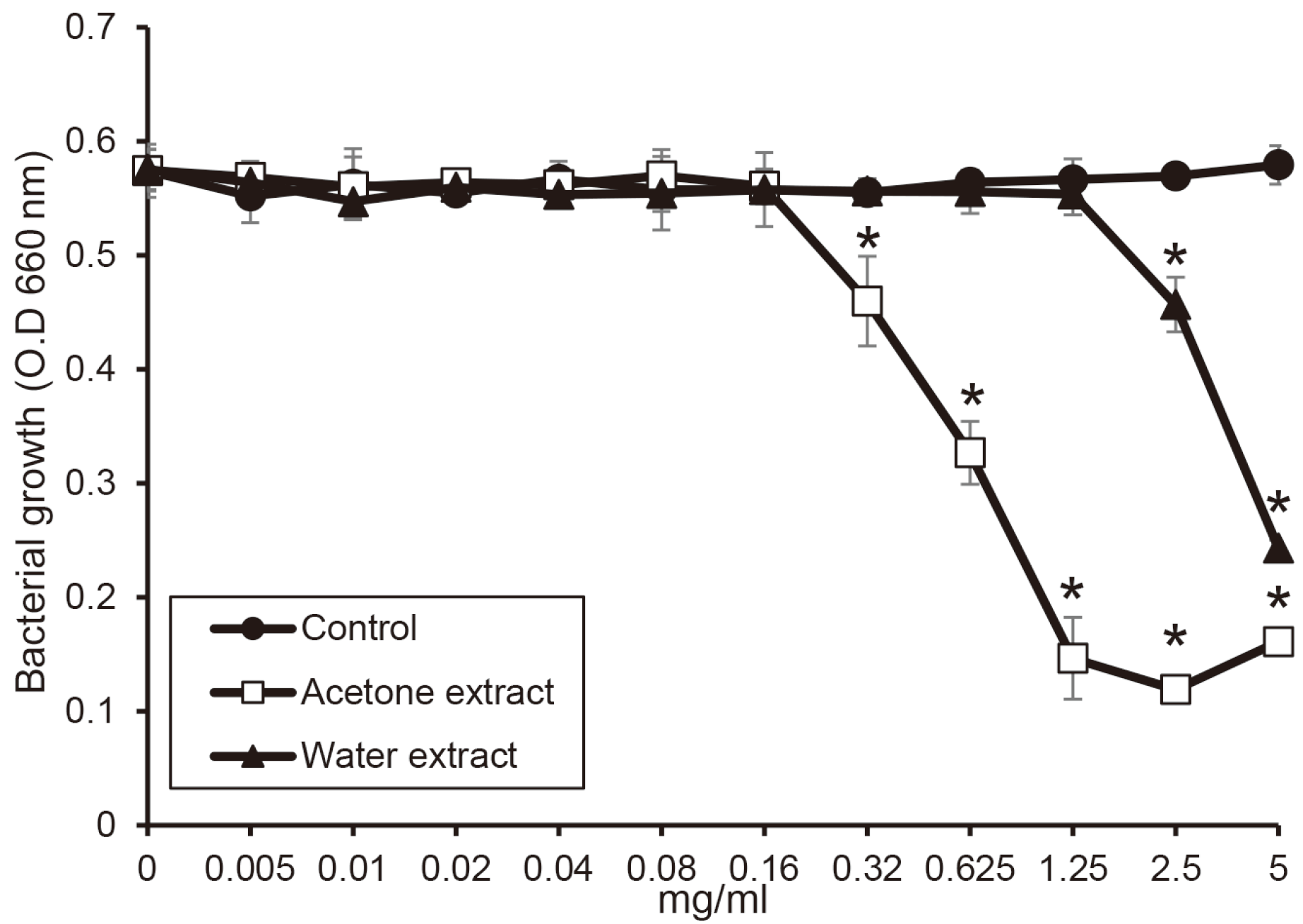 | Fig. 2
The antimicrobial activity of the extracts from shiitake mushroom against T. forsythia. T. forsythia was cultured with or without the extracts in the various concentrations. The growth of T. forsythia was measured by a spectrophotometer at 660 nm wavelength. Asterix (*) indicates significant difference compared with control (P < 0.05).
O.D, optical density.

|
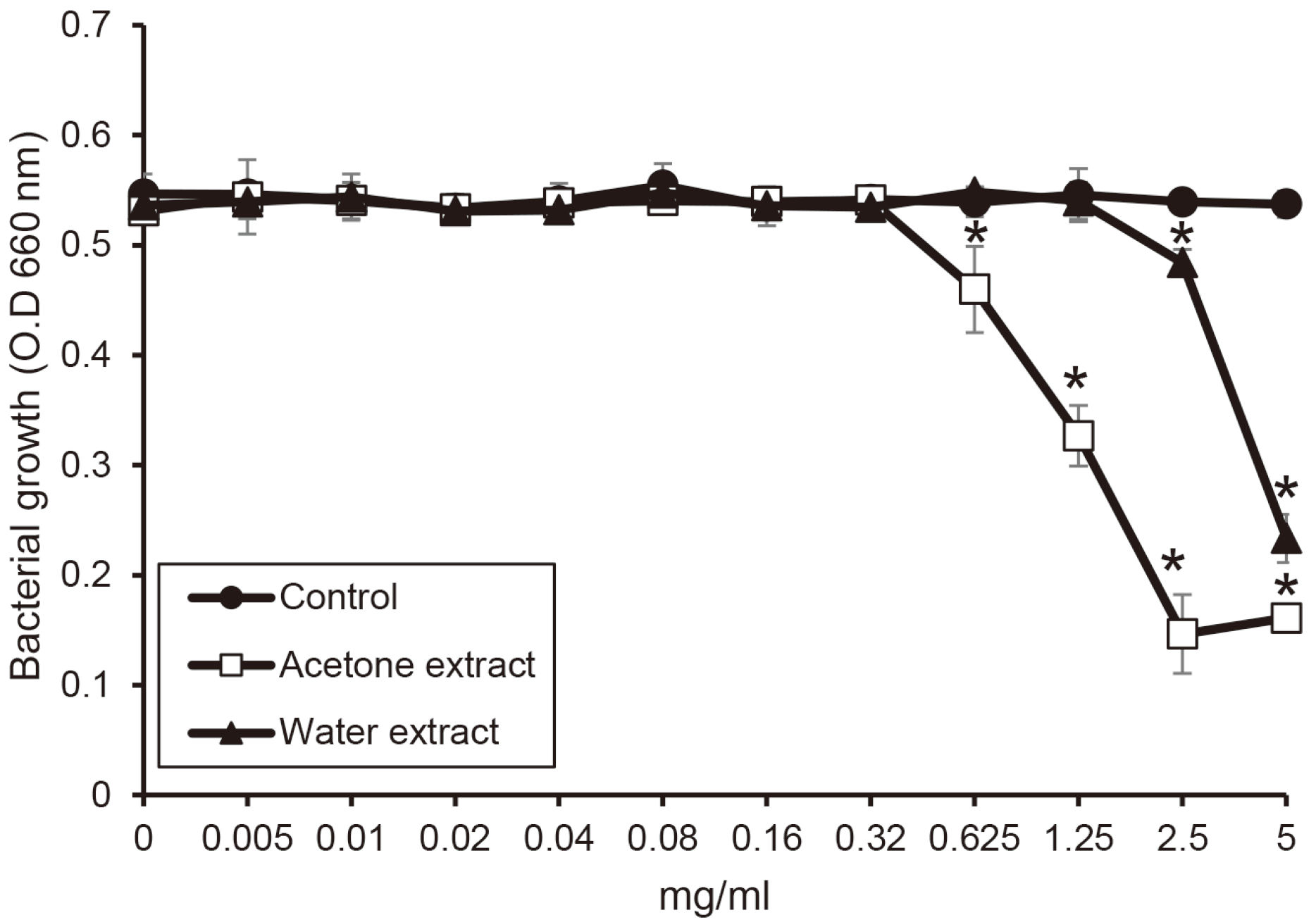 | Fig. 3
The susceptibility of T. denticola for the extract from shiitake mushroom. T. denticola was incubated with or without the extracts in the various concentrations. The growth of T. denticola was measured by a spectrophotometer at 660 nm wavelength. Asterix (*) indicates significant difference compared with control (P < 0.05).
O.D, optical density.

|
A cytotoxicity test was evaluated using the cell line of HGF-1 to examine what kind of effect it has on cells at a concentration showing antibacterial activity. When HGF-1 was treated with the extract at a concentration in the range of 0.625 - 5 mg/ml for 12 h, both extracts showed significant cytotoxicity for the cell above the concentration of 2.5 mg/ml (
P < 0.05)(
Fig. 4). Since the remaining time of the extracts in oral cavity is shorter than 12 h, the cytotoxicity of the extracts was investigated for various times. When the extract of a concentration of 2.5 mg/ml as a concentration showing cytotoxicity was treated HGF-1, the cell viability was significantly decreased from 4 h (
P < 0.05)(
Fig. 5).
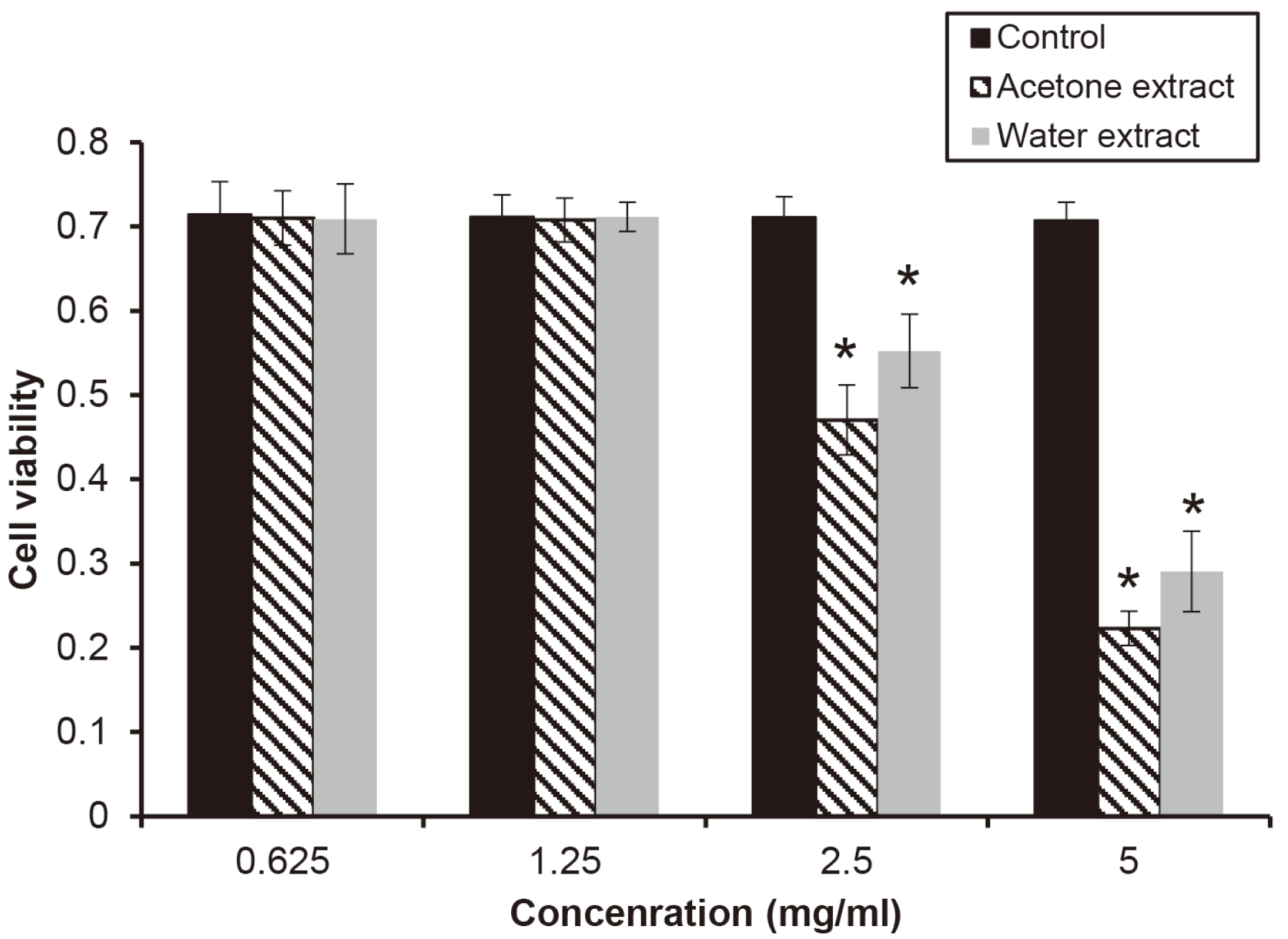 | Fig. 4The effect of the extracts on cell viability. HGF-1 treated with the extracts in the various concentrations for 12, and the cell viability was measured by CCK-8. Asterix (*) indicates significant difference compared with control (P < 0.05). 
|
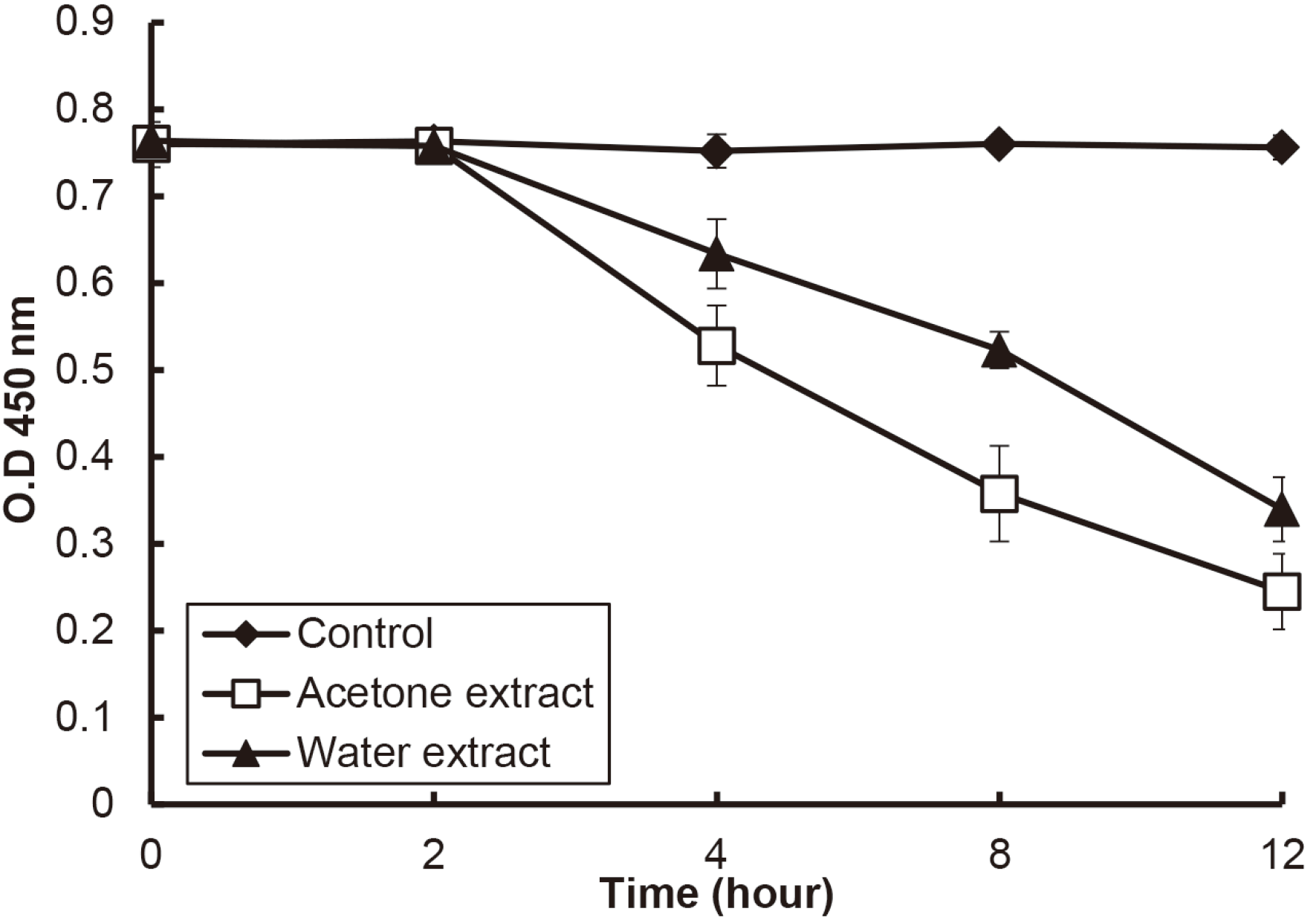 | Fig. 5
The cytotoxicity of the extracts over time. HGF-1 treated with the extracts at a concentration of 1.25 mg/ml for the various time, and the cell viability was measured by CCK-8. Asterix (*) indicates significant difference compared with control (P < 0.05).
O.D, optical density.

|
Go to :

Discussion
Periodontitis is a chronic inflammation of the gingiva with polymicrobial ecology. In the ecology,
P. gingivalis,
T. forsythia, and
T. denticola are closely associated with the inflammation. They are called periodontopathogens and classified as red complex bacteria in oral cavity.
17 In addition, the periodontopathogens induce peri-implantitis.
18 Shiitake mushroom (
L. edodes) are consumed a lot in Northeast Asia including Korea and used more because of their nutritional and medical properties.
19 L. edodes is one of the well-known fungi used in various therapeutic applications.
20 Extracts from shiitake mushroom are active against Gram-positive and Gram-negative bacteria, yeasts and mycelial fungi.
21 Also, these extracts are used medicinally for diseases including cancer, diabetes, allergies, bronchial inflammation.
20 Therefore, this study was examined antimicrobial activity and cytotoxicity of shiitake extracts to prevent and treat periodontal disease.
The antimicrobial activity of the extracts from shiitake mushroom showed against
P. gingivalis,
T. forsythia, and
T. denticola. Comparing the antimicrobial activity of water and acetone extract from shiitake mushroom, the acetone extract inhibited the growth of periodontopathogens at a lower concentration than its water extract. Among extracts from shiitake mushroom, oil extracts have aromatic compounds that carvacrol, thymol, eugenol, and their components is known to show antimicrobial activity.
22 These aromatic molecules showed to disintegrate the outer membrane of Gram-negative bacteria by releasing lipopolysaccharide.
23 Basis on these characteristics of aromatic molecules, the acetone extract may show more antimicrobial activity against periodontopathogens compared to the water extract. Also, aromatic molecules can be predicted to release lipopolysaccharide from the outer membrane by binding to lipid A of lipopolysaccharide. Comparing lipid A structure of periodontopathogens,
T. denticola has two acyl chain and shorter acyl chain than
P. gingivalis and
T. forsythia. Therefore, the antimicrobial activity of the acetone extract may show less against
T. denticola than other periodontopathogens.
Finally, the cytotoxicity test was performed using HGF-1 for the extracts. The acetone extract in the concentration showing antimicrobial activity did not show cytotoxicity. When most of the components of the acetone extract may be predicted aroma molecules, these molecules are known to be good for health such as antioxidation and anti-cancer effect. 24,25 Since the acetone extract is also a lipid, it may have a good effect at moderate concentrations, but may show cytotoxicity at high concentrations. Furthermore, the acetone extract showed cytotoxicity from 4 h in high concentration as 2.5 mg/ml. Therefore, considering the length of the extract stays in the mouth, the extract may not adversely affect the oral cavity even at high concentrations in the end.
Go to :

Conclusion
The extracts from shiitake mushroom have the antimicrobial activity against periodontopathogens. Detailly, the acetone extract from shiitake mushroom has stronger antimicrobial activity than the water extract. Also, the acetone extract did not show cytotoxicity in the concentration showing antimicrobial activity. Basis on these results, the shiitake mushroom may help to prevent and treat periodontitis.
Go to :








 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download