Abstract
Variations of the ovarian veins can impact imaging diagnosis, surgical procedures of the region, and can be related to clinical findings such as compression of the ureter. Therefore, a good working knowledge of such variants is important to the clinician who interprets imaging of the posterior abdominopelvic region of women and surgeons who operate in this region. Herein, we present a comprehensive review of duplicated ovarian veins and provide a case illustration.
The ovarian veins originate from the pampiniform plexus surrounding the ovaries. The ovarian veins travel from this plexus in the mesovarium and the broad ligament between the ovary and uterine tube [1-4]. Alongside the ovarian arteries, the ovarian veins ascend through the suspensory ligament (infundibulopelvic) and are positioned anterior to the psoas major muscle [1, 3, 4]. The ovarian veins are anatomically asymmetric and drain at different sites. The right ovarian vein ascends parallel to the right ureter and crosses the ureter anteromedially halfway between the bifurcation of the inferior vena cava (IVC) to drain into the IVC anterolaterally and below its bifurcation at a level between T12 and L2. The left ovarian vein ascends similarly, parallel to the left ureter, but drains into the left renal vein [1-6]. Variations in drainage have occurred including the right ovarian vein draining into the right renal vein [1, 7] and the left ovarian vein draining into the left suprarenal vein [8].
Reports in the literature present limited knowledge concerning variations of the ovarian vein, particularly its duplication. Duplication and other variants of the ovarian vein are largely incidental findings during surgeries, anatomy course dissections, radiological procedures, and at autopsy [3]. To understand and discuss the anatomical and clinical implications of a duplicated ovarian vein, it is important to analyze the information currently available throughout the literature. We reviewed previously reported studies on duplication of the ovarian vein and presented an additional case illustration.
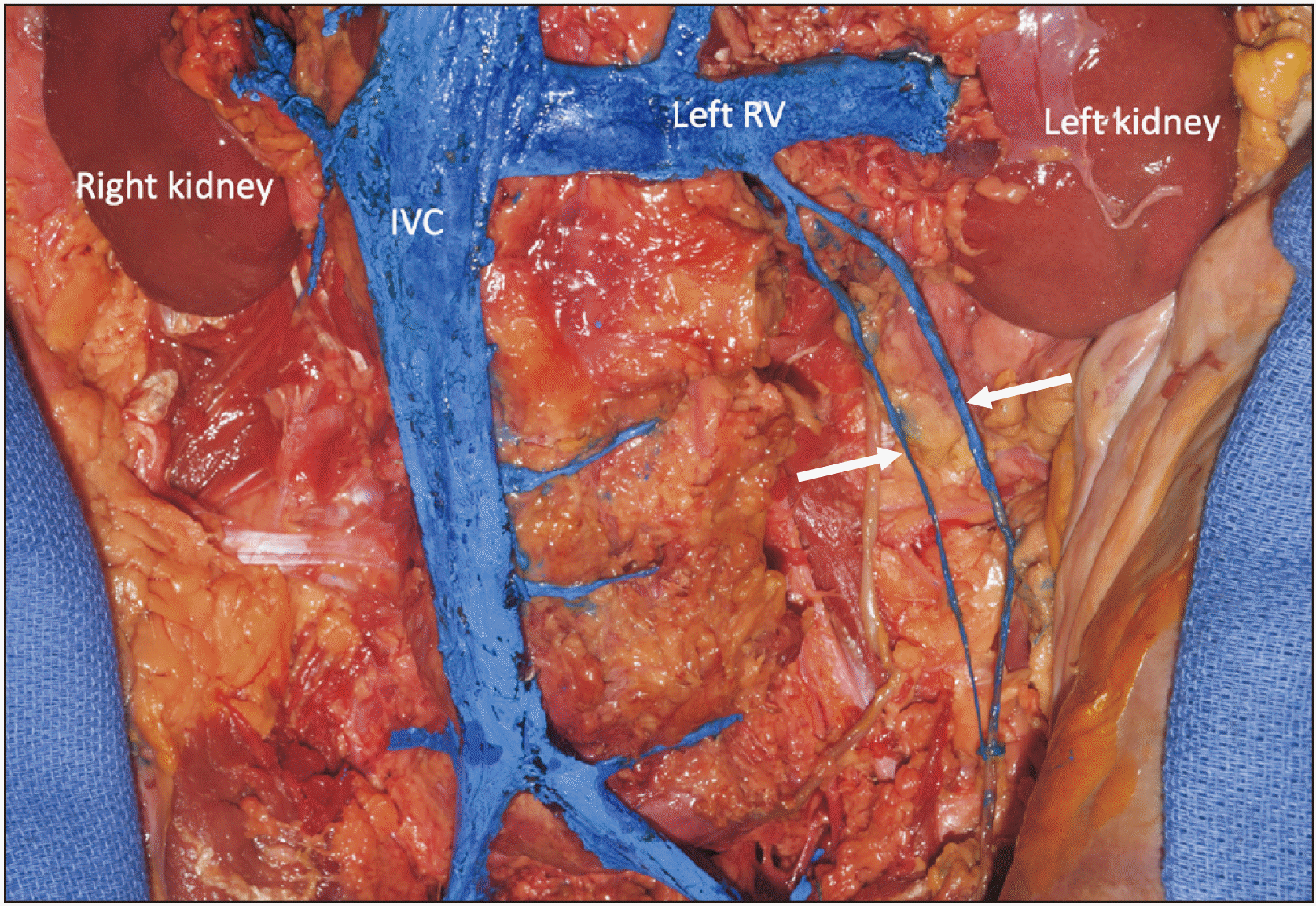
During the routine dissection of a fresh frozen adult female cadaver aged 56 years at death, an unusual finding of the left retroperitoneum was observed. In this specimen, a duplicated ovarian vein was identified (Fig. 1). The two veins traveled in a diagonal fashion from the left ovary in the pelvis to unite 2.2 cm inferior to the left renal vein (level of the superior part of the L2). This united vein then drained into the left renal vein just lateral to the drainage of the left suprarenal vein into the left renal vein. The two veins were similar in size with the more medial vein having a diameter of 2.34 mm and the more lateral vein having a diameter of 2.71 mm. Both veins crossed just anterior to the ipsilateral ureter with the medial vein crossing more inferiorly compared to the lateral vein that crossed the ureter more superiorly. Very small tributaries from the left ureter and posterior abdominal wall adipose tissue drained into both ovarian veins. The greatest distance between the veins measured 2.64 cm. The contralateral ovarian vein drained in normal fashion into the IVC. No other grossly visible anatomical variations were noted in the retroperitoneum of this specimen.
Variations in ovarian vein morphology including partial and complete duplication and various drainage sites have previously been reported in the literature (Table 1). Ghosh and Chaudhury (2019) [1] performed dissections on 47 female cadavers and reported on the course and termination of the right and left ovarian veins with two cadavers found to have duplicated right ovarian veins draining separately into the IVC, one cadaver with a partially duplicated left ovarian vein that bifurcated 40 mm distal to its termination at the left renal vein, and one cadaver with a single right ovarian vein draining to the right renal vein. Forte et al. (2002) [9] performed dissection of a 75-year-old at death female cadaver and reported an incomplete left ovarian vein duplication with a unique anastomosis with an inferior polar renal vein. Ito and Ikeda (2018) [10] documented a rare case of double IVC and double right ovarian vein in an 81-year-old at death Japanese female cadaver with both right ovarian veins running parallel to one another and terminating separately at the right IVC and right renal vein. Dissections of the paraaortic region in 18 cadavers from Klemm et al. (2005) [11] reported a case of bifurcation of the left renal vein with each trunk receiving one of a pair of duplicated left ovarian veins. Sonje et al. [7] performed dissection of 30 cadavers and observed one case with two variations including a duplicated left ovarian vein and drainage of the right side—right ovarian vein joining the right renal vein.
Duplicated ovarian veins have also been reported in the literature from inspection of the ovaries and related structures in ovarian disorders. Abrantes et al. (2019) [12] presented a case of a duplicated right ovarian vein in a 34-year-old patient, 72 hours postpartum, with postpartum ovarian vein thrombosis. In outlining conservative management in three cases of placenta percreta, Barber et al. (2011) [13] reported one case of a 34-year-old woman with abdominal pain and fever 11 weeks after delivery and upon computed tomography angiography (CTA), a duplicated right ovarian vein that drained into the IVC, a dilated left ovarian vein, and an enlarged uterus were observed. Rao et al. (2017) [3] reported duplication of the right ovarian vein at the level of S1 through L3 vertebrae during routine computed tomography (CT) in a patient with recurrent menorrhagia and pelvic pain.
Duplication of the ovarian vein may be explained by embryological disturbances that occur between the 5th and 7th weeks of venous development [9]. Normal venous development follows the sequence of development, regression, anastomosis, and replacement of the posterior cardinal, subcardinal, and supracardinal veins [2, 3]. The gonadal veins—testicular or ovarian veins, emerge from the caudal portion of the subcardinal veins and drain into the supracardinal and subcardinal anastomosis. Normal right gonadal vein drainage into the IVC occurs from merging of the supra-subcardinal anastomosis and a portion of the subcardinal vein which are incorporated into the IVC. Normal drainage of the left gonadal vein occurs when the supra-subcardinal anastomosis forms part of the left renal vein [1-3, 7, 9, 10, 14]. A duplicated ovarian vein and other vascular variations including a duplicated IVC can result from changes in the venous developmental sequence such as persistent connections with the lower subcardinal veins and the ipsilateral sacrocardinal veins [9].
Several diagnostic imaging modalities have been successfully used to assess ovarian vein duplication. Abdominopelvic CT, multidetector CT (MDCT), and CTA have been the most frequently used for confirmation of duplicated ovarian veins [3, 13, 15, 16]. CT/CTA/MDCT can be used to visualize normal ovarian vein anatomy, its variations and disorders [15]. In a case with postpartum ovarian vein thrombosis, an abdominopelvic CT with iodine contrast identified a duplicated right ovarian vein [12]. A CTA scan of a 34-year-old woman eight weeks after delivery with complete placenta percreta and a uterine septum revealed an arteriovenous malformation and a duplicated right ovarian vein draining into the IVC [13]. Pelvic venography (phlebography) has also been used to visualize ovarian duplication and to perform therapeutic embolization of the duplicated vein to treat pelvic venous congestion syndrome [6]. Other imaging modalities including transabdominal or transvaginal ultrasound, color Doppler ultrasound, and magnetic resonance imaging have also been used to diagnose ovarian disorders [16-18].
Knowledge of variations of the ovarian veins is useful in performing laparoscopic procedures and understanding the relationship of certain vascular disorders to variations of venous development. Ovarian venous reflux contributes to pelvic pain in women and is a common therapy is pelvic vein embolization for treating pelvic congestion syndrome. Pelvic venous congestion syndrome can result from variations in the ovarian veins and their drainage [16]. Therefore, understanding of such variations can guide clinicians in successful embolization and prevent complications [1, 6]. During other laparoscopic procedures, knowledge of the frequency and variants in the vasculature can aid in preventing vascular injury from hemorrhage and avoid transfusion or conversion to laparotomy [11]. Prior identification of gonadal vein variants in patients can also allow for further planning of abdominopelvic surgeries by radiologists and gynecologists in order to reduce the chance of failure. The gonadal veins are also particularly useful for kidney transplantation and vascular reconstruction. These veins can assist in elongation of renal veins during transplantation [2, 8]. Duplication of the ovarian vein has multiple implications and its visualization and assessment help to establish a clearer understanding of the abdominopelvic venous anatomy. Lastly, the site of crossing of the gonadal veins anterior to the ureter is a known site of potential renal calculus location. Therefore, in cases of duplicated veins, two potential sites of obstruction might be identified.
Variations of the ovarian veins can impact imaging diagnosis, surgical procedures of the region, and can be related to clinical findings such as compression of the ureter. Therefore, a good working knowledge of such variants is important to the clinician who interprets imaging of the posterior abdominopelvic region of women and surgeons who operate in this region.
Acknowledgements
The authors sincerely thank those who donated their bodies to science so that anatomical research could be performed. Results from such research can potentially increase mankind’s overall knowledge that can then improve patient care. Therefore, these donors and their families deserve our highest gratitude [19].
Notes
References
1. Ghosh A, Chaudhury S. 2019; A cadaveric study of ovarian veins: variations, measurements and clinical significance. Anat Cell Biol. 52:385–9. DOI: 10.5115/acb.19.062. PMID: 31949976. PMCID: PMC6952686.

2. Gupta R, Gupta A, Aggarwal N. 2015; Variations of gonadal veins: embryological prospective and clinical significance. J Clin Diagn Res. 9:AC08–10. DOI: 10.7860/JCDR/2015/9493.5578. PMID: 25859438. PMCID: PMC4378720. PMID: 4abcacbe00a24cfbab3062f193cdd576.

3. Rao S, Konduru S, Rao T. 2017; Duplication of right ovarian vein. Arch Curr Res Int. 7:1–4. DOI: 10.9734/ACRI/2017/33294.

4. Patel M, Iwanaga J, Oskouian RJ, Tubbs RS. 2018; Fenestration of the proximal left ovarian vein. Cureus. 10:e2343. DOI: 10.7759/cureus.2343. PMID: 29796355. PMCID: PMC5959309.

5. Han J, Sadiq NM. 2022. Anatomy, abdomen and pelvis, fallopian tube. StatPearls Publishing;Treasure Island:
6. Lopez AJ. 2015; Female pelvic vein embolization: indications, techniques, and outcomes. Cardiovasc Intervent Radiol. 38:806–20. DOI: 10.1007/s00270-015-1074-7. PMID: 25804635. PMCID: PMC4500858.

7. Sonje P, Kanasker N, Vatsalaswamy P. 2020; Variations in the origin and draining pattern of gonadal vessels with their surgical significance and developmental correlations. Indian J Anat. 9:39–43. DOI: 10.21088/ija.2320.0022.9120.6.

8. Lalwani R, Athavale SA, Chauhan K, Nigam GL, Babu CSR, Kotgirwar S. 2017; Cadaveric study of mode of termination of gonadal veins: implications for procedures utilizing terminal ends of gonadal veins as entry portals. J Nat Sci Biol Med. 8:210–2. DOI: 10.4103/0976-9668.210005. PMID: 28781489. PMCID: PMC5523530.

9. Forte F, Farina C, Bronzetti E, Carbotta S, Germani S, Virgili G, Vespasiani G. 2002; Clinical implications of left ovarian vein incomplete duplicity with embryonic intersubcardinal anastomosis-derived branches. Surg Radiol Anat. 24:64–7. DOI: 10.1007/s00276-002-0017-6. PMID: 12197014.

10. Ito T, Ikeda Y. 2018; A case of double inferior vena cava with renal, ovarian and iliac vein variation. Anat Sci Int. 93:139–43. DOI: 10.1007/s12565-017-0397-7. PMID: 28283881.

11. Klemm P, Fröber R, Köhler C, Schneider A. 2005; Vascular anomalies in the paraaortic region diagnosed by laparoscopy in patients with gynaecologic malignancies. Gynecol Oncol. 96:278–82. DOI: 10.1016/j.ygyno.2004.09.056. PMID: 15661208.

12. Abrantes J, Teixeira E, Gomes F, Fernandes C. 2019; Postpartum ovarian vein thrombosis and venous anatomical variation. BMJ Case Rep. 12:e228399. DOI: 10.1136/bcr-2018-228399. PMID: 31229971. PMCID: PMC6605948.

13. Barber JT Jr, Tressler TB, Willis GS, Martinez FJ, Peisner DB, Goodman JD, Taboada CD. 2011; Arteriovenous malformation identification after conservative management of placenta percreta with uterine artery embolization and adjunctive therapy. Am J Obstet Gynecol. 204:e4–8. DOI: 10.1016/j.ajog.2011.01.001. PMID: 21349491.

14. Abraham J, Sharma A, Sharma M, Priyanka J P J. 2015; Duplication of right testicular vein: embryological and clinical consideration- a case report. J Clin Diagn Res. 9:AD03–4. DOI: 10.7860/JCDR/2015/14934.6723. PMID: 26673850. PMCID: PMC4668396. PMID: 917c131ca7bd494394e32006e49f7767.

15. Karaosmanoglu D, Karcaaltincaba M, Karcaaltincaba D, Akata D, Ozmen M. 2009; MDCT of the ovarian vein: normal anatomy and pathology. AJR Am J Roentgenol. 192:295–9. DOI: 10.2214/AJR.08.1015. PMID: 19098213.

16. Koc Z, Ulusan S, Oguzkurt L. 2006; Right ovarian vein drainage variant: is there a relationship with pelvic varices? Eur J Radiol. 59:465–71. DOI: 10.1016/j.ejrad.2006.03.021. PMID: 16644167.

17. Nascimento AB, Mitchell DG, Holland G. 2002; Ovarian veins: magnetic resonance imaging findings in an asymptomatic population. J Magn Reson Imaging. 15:551–6. DOI: 10.1002/jmri.10098. PMID: 11997896.

18. Park SJ, Lim JW, Ko YT, Lee DH, Yoon Y, Oh JH, Lee HK, Huh CY. 2004; Diagnosis of pelvic congestion syndrome using transabdominal and transvaginal sonography. AJR Am J Roentgenol. 182:683–8. DOI: 10.2214/ajr.182.3.1820683. PMID: 14975970.

19. Iwanaga J, Singh V, Ohtsuka A, Hwang Y, Kim HJ, Moryś J, Ravi KS, Ribatti D, Trainor PA, Sañudo JR, Apaydin N, Şengül G, Albertine KH, Walocha JA, Loukas M, Duparc F, Paulsen F, Del Sol M, Adds P, Hegazy A, Tubbs RS. 2021; Acknowledging the use of human cadaveric tissues in research papers: recommendations from anatomical journal editors. Clin Anat. 34:2–4. DOI: 10.1002/ca.23671. PMID: 32808702.

Table 1
Partial and complete duplication of ovarian vein
| Case no. | Author | Year | Age (yr) | Side | Method identification | Division pattern | Note |
|---|---|---|---|---|---|---|---|
| 1 | Forte et al. [9] | 2002 | 75 | L | Cadaveric dissection | Partial duplication | Partial duplication with unique anastomosis with inferior polar renal vein |
| 2 | Klemm et al. [11] | 2005 | Unknown | L | Cadaveric dissection | Duplication | Partially bifid LRV receives duplicated LOV |
| 3 | Barber et al. [13] | 2011 | 34 | R | CTA scan | Duplication | None |
| 4 | Lalwani et al. [8] | 2017 | Unknown | L | Cadaveric dissection | Duplication | None |
| 5 | Rao et al. [3] | 2017 | 33 | R | CT scan | Duplication | ROV duplication at level of S1 and reunited to form a single vein at L3 |
| 6 | Ito and Ikeda [10] | 2018 | 81 | R | Cadaveric dissection | Duplication | Duplicated IVC with duplicated ROV terminating at right IVC and RRV |
| 7 | Abrantes et al. [12] | 2019 | 34 | R | CT scan | Duplication | None |
| 8 | Ghosh and Chaudhury [1] | 2019 | 64 | R | Cadaveric dissection | Duplication | None |
| 9 | Ghosh and Chaudhury [1] | 2019 | 85 | L | Cadaveric dissection | Partial duplication | None |
| 10 | Sonje et al. [7] | 2020 | Unknown | L | Cadaveric dissection | Duplication | Normal drainage of duplicated LOV while drainage od ROV to RRV |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download