Introduction
Materials and Methods
Preparation of ECM‐derived mesenteric scaffold
Preparation of blastema tissue
Assembly and culture of ECM‐derived mesenteric scaffold with blastema tissue
Histological analysis
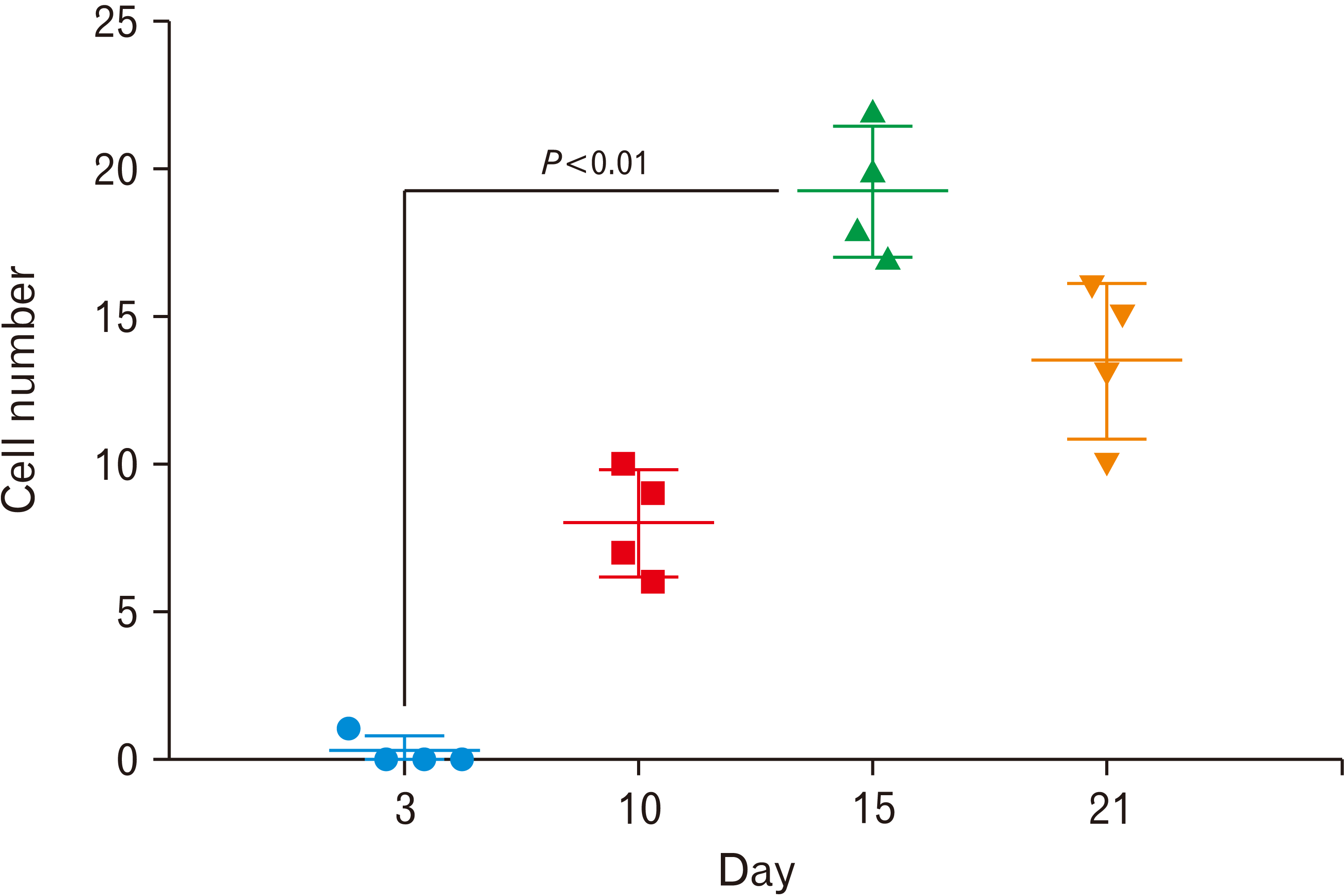
Cell number assay and statistical analysis
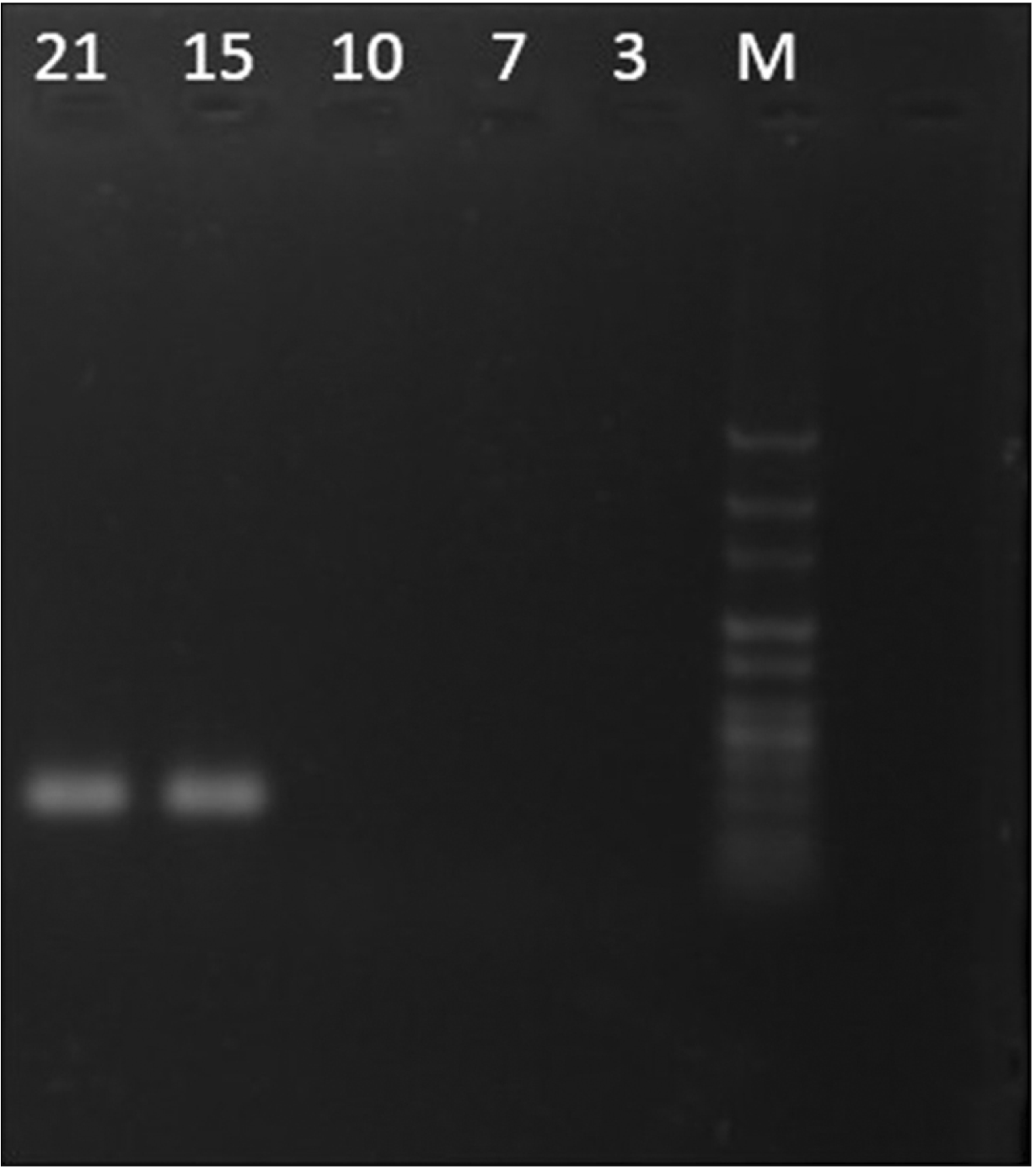
RNA isolation and RT-PCR
Results
Characterization of the decellularized mesenteric tissue by histological analysis
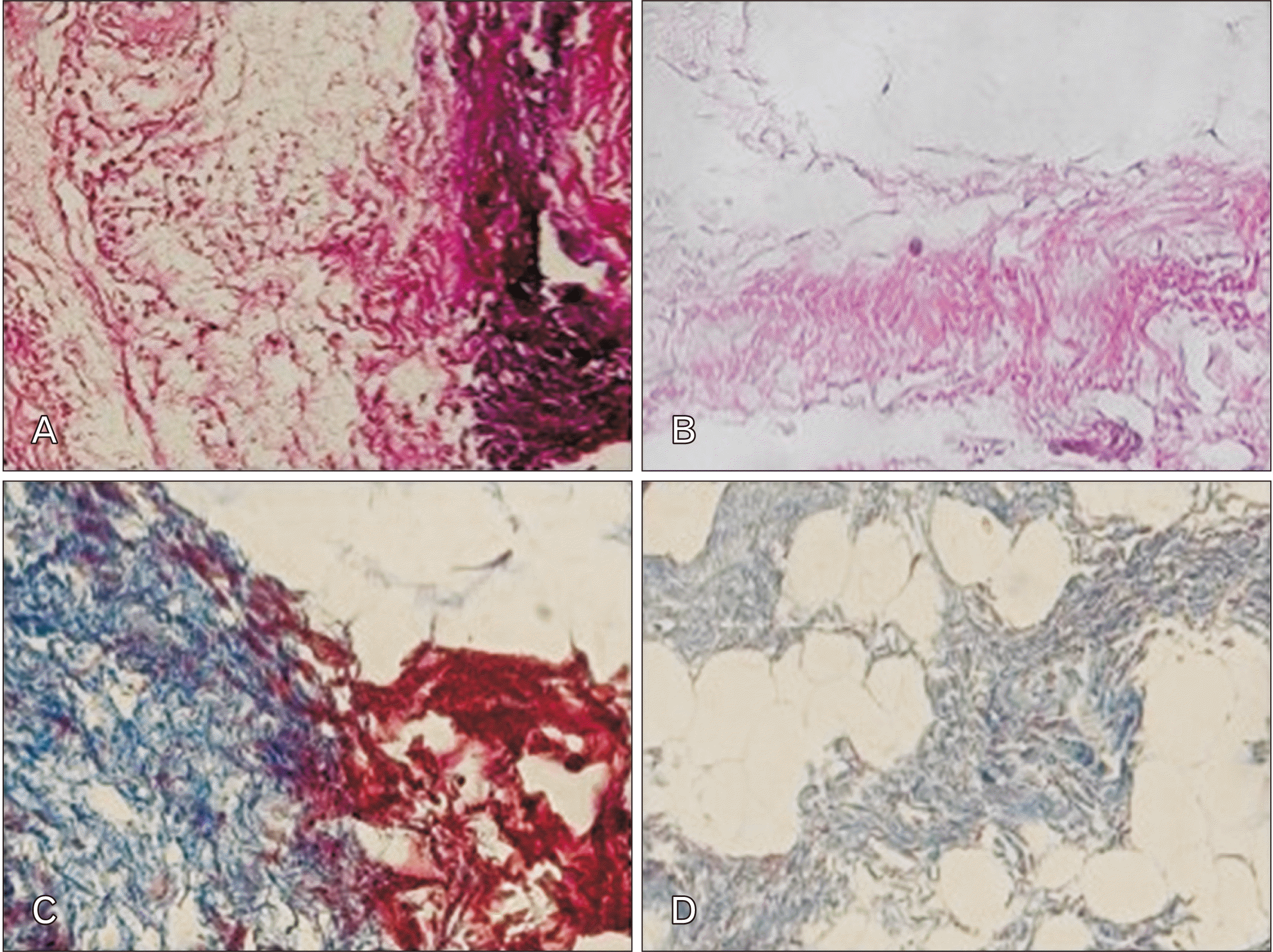 | Fig. 1Control and decellularized mesenteric samples after H&E and Masson's trichrome staining methods. (A, B) The construct of the decellularized mesenteric is displayed in comparison with the native mesenteric tissue using H&E staining. (C, D) Masson’s trichrome staining demonstrated that in decellularized mesenteric ECM, the components remained intact. Magnifications for A, B, C, and D are ×200. ECM, extracellular matrix. |
Investigation of interactions between Blastema tissue and decellularized mesenteric tissue
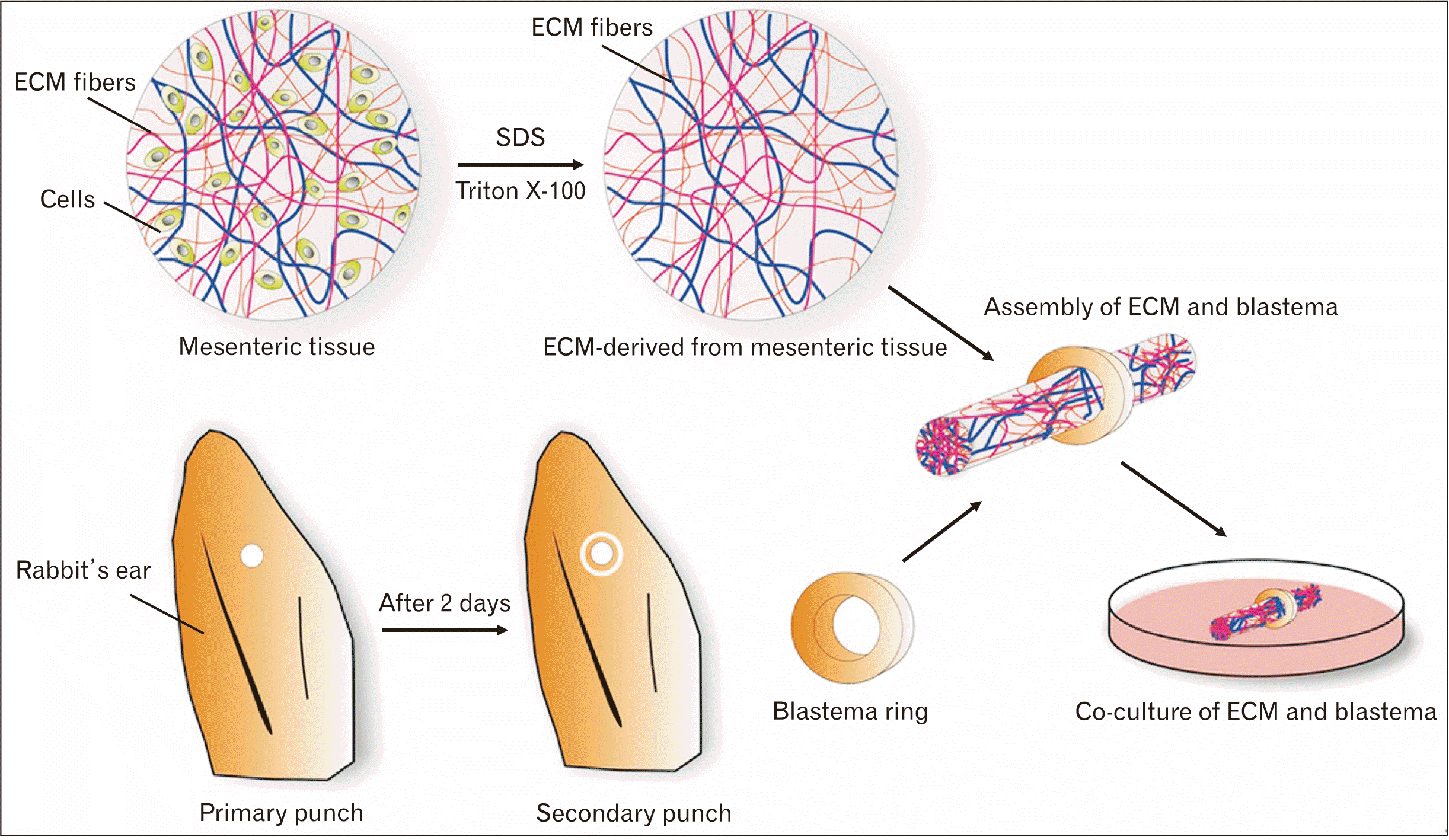 | Fig. 2Schematic overview of the preparation, assembly, and co-culture of the mesenteric ECM and blastema tissue ring. SDS and triton X-100 solutions decellularize the mesenteric tissue. As well as, to prepare blastema tissue, the New Zealand rabbit’s ears were punched, and two days after punching the ears, the blastema ring derived from the periphery of the primary hole. Next, the blastema rings and the ECM scaffolds were assembled and co-cultured in a medium containing fibronectin. ECM, extracellular matrix; SDS, sodium dodecyl sulfate. |
 | Fig. 3H&E staining. (A, B) Three days after the cell culture, only mesenteric decellularized tissue was observed (magnification: A, ×100 and B, ×200). (C, D) Cells on day 10 at the site of interaction between blastema tissue and mesenteric extracellular matrix (ECM) are observed (magnification: C, ×200 and D, ×400). (E, F) The cell polarity is shown at the site of interaction between blastema tissue and the mesenteric matrix on day 15 (magnification: E, ×200 and F, ×400). (G, H) Cell migration indicates blastemal cells, along with the mesenteric matrix and proliferating cells at this site on day 21 (magnification: G, ×200 and H, ×400). Arrows show adherence and maintenance of blastema cells in the ECM scaffold during culture after the 10th day. |
 | Fig. 4Masson’s trichrome staining. (A, B) On day 3, only mesenteric decellularized tissue was evident (magnification: A, ×100 and B, ×200). (C, D) Cell proliferation in blastema tissue, especially in the peripheral areas on day 10 of the cell culture (magnification: C, ×100 and D, ×200). (E, F) On day 15, the polar structures of cells are detectable at the point of the interaction between blastema tissue and the mesenteric matrix (magnification: E, ×200 and F, ×400). (G, H) Blastema cells are observed along with the mesenteric matrix with vascular-like structures on day 21 (magnification: G, ×200 and H, ×400). Arrows blastema cells’ attachment and penetration in the marginal zone of the extracellular matrix scaffold since the 10th day after culture. |
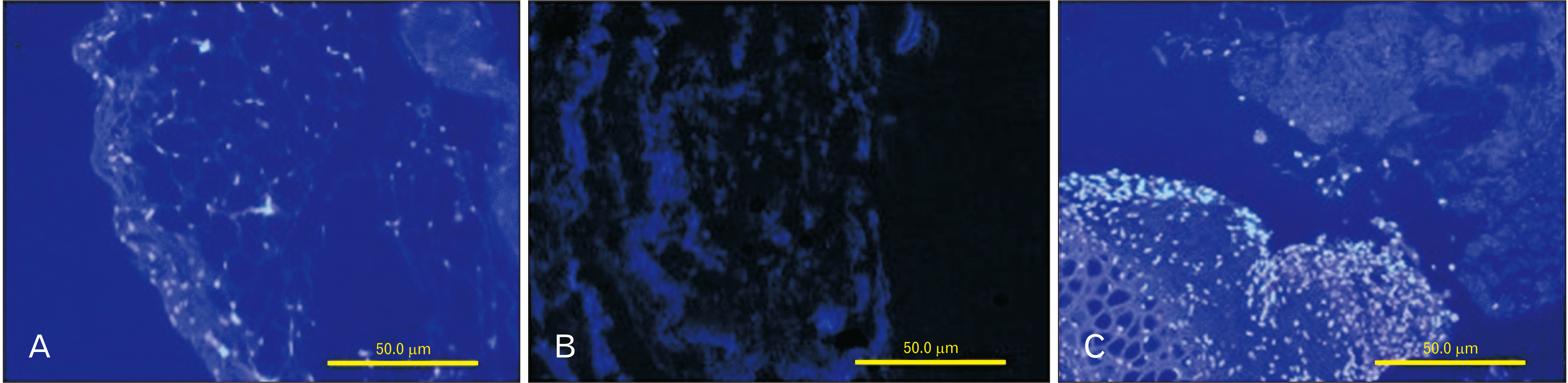 | Fig. 6Staining with DAPI before and after decellularization. Native bovine mesenteric tissue shown in bright nuclei represents the presence of intact cells in native tissue (A). The elimination of cell nuclei in obtained decellularized mesenteric compared with native mesenteric tissue (B). On day 15, blastema cells were depicted as bright spots in the interaction site with the decellularized mesenteric tissue (C). Magnifications for A, B, and C are ×200. DAPI, 4,6 diamidino-2-phenylindole. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download