1. Whitcomb DC, Frulloni L, Garg P, Greer JB, Schneider A, Yadav D, Shimosegawa T. 2016; Chronic pancreatitis: an international draft consensus proposal for a new mechanistic definition. Pancreatology. 16:218–24. DOI:
10.1016/j.pan.2016.02.001. PMID:
26924663. PMCID:
PMC6042966.

5. Melki G, Laham L, Karim G, Komal F, Kumar V, Barham S, Grossman M, Kuru S, Mohamed H, Garris R, Baddoura W. 2019; Chronic pancreatitis leading to pancreatogenic diabetes presenting in diabetic ketoacidosis: a rare entity. Gastroenterology Res. 12:208–10. DOI:
10.14740/gr1203. PMID:
31523331. PMCID:
PMC6731042.

7. Dong JY, Qin LQ, Zhang Z, Zhao Y, Wang J, Arigoni F, Zhang W. 2011; Effect of oral L-arginine supplementation on blood pressure: a meta-analysis of randomized, double-blind, placebo-controlled trials. Am Heart J. 162:959–65. DOI:
10.1016/j.ahj.2011.09.012. PMID:
22137067.

8. Meshram A, ivastava N Sr. 2015; Diverse potential and pharmacological studies of arginine. J Proteins Proteomics. 6:237–43.
9. Malins LR, Cergol KM, Payne RJ. 2013; Peptide ligation-desulfurization chemistry at arginine. Chembiochem. 14:559–63. DOI:
10.1002/cbic.201300049. PMID:
23426906.

10. Sharma S, Rana SV, Nada R, Malhotra S, Rana S, Bhasin DK. 2017; Wheatgrass (Triticum Aestivum): an effective anti-oxidant in L-arginine induced chronic pancreatitis model of rat: a dose dependent study. Imp J Interdiscip Res. 3:245–51.
12. Mayerle J, Hoffmeister A, Werner J, Witt H, Lerch MM, Mössner J. 2013; Chronic pancreatitis--definition, etiology, investigation and treatment. Dtsch Arztebl Int. 110:387–93. DOI:
10.3238/arztebl.2013.0387. PMID:
23826027. PMCID:
PMC3698906.
14. Tang D, Wang D, Yuan Z, Xue X, Zhang Y, An Y, Chen J, Tu M, Lu Z, Wei J, Jiang K, Miao Y. 2013; Persistent activation of pancreatic stellate cells creates a microenvironment favorable for the malignant behavior of pancreatic ductal adenocarcinoma. Int J Cancer. 132:993–1003. DOI:
10.1002/ijc.27715. PMID:
22777597.

15. Pothula SP, Xu Z, Goldstein D, Pirola RC, Wilson JS, Apte MV. 2016; Key role of pancreatic stellate cells in pancreatic cancer. Cancer Lett. 381:194–200. DOI:
10.1016/j.canlet.2015.10.035. PMID:
26571462.

16. Eidi A, Eidi M, Darzi R. 2009; Antidiabetic effect of Olea europaea L. in normal and diabetic rats. Phytother Res. 23:347–50. DOI:
10.1002/ptr.2629. PMID:
18844257.
19. Burja B, Kuret T, Janko T, Topalović D, Živković L, Mrak-Poljšak K, Spremo-Potparević B, Žigon P, Distler O, Čučnik S, Sodin-Semrl S, Lakota K, Frank-Bertoncelj M. 2019; Olive leaf extract attenuates inflammatory activation and DNA damage in human arterial endothelial cells. Front Cardiovasc Med. 6:56. DOI:
10.3389/fcvm.2019.00056. PMID:
31157238. PMCID:
PMC6531989.

20. Boss A, Bishop KS, Marlow G, Barnett MP, Ferguson LR. 2016; Evidence to support the anti-cancer effect of olive leaf extract and future directions. Nutrients. 8:513. DOI:
10.3390/nu8080513. PMID:
27548217. PMCID:
PMC4997426.

21. Al-Basher GI. 2018; Anti-fibrogentic and hepatoprotective potential of methanolic olive extract on cadmium induced toxicity in rats. Life Sci J. 15:1–11.
22. Lin Z, Zheng LC, Zhang HJ, Tsang SW, Bian ZX. 2015; Anti-fibrotic effects of phenolic compounds on pancreatic stellate cells. BMC Complement Altern Med. 15:259. DOI:
10.1186/s12906-015-0789-y. PMID:
26223780. PMCID:
PMC4520255.

23. Lins PG, Marina Piccoli Pugine S, Scatolini AM, de Melo MP. 2018; In vitro antioxidant activity of olive leaf extract (Olea europaea L.) and its protective effect on oxidative damage in human erythrocytes. Heliyon. 4:e00805. DOI:
10.1016/j.heliyon.2018.e00805. PMID:
30255162. PMCID:
PMC6148714.
24. Fredstrom SB, Jessurun J, Gallaher DD. 2009; Pancreatitis induced in rats by repetitive administration of L-arginine. Pancreas. 38:344–5. DOI:
10.1097/MPA.0b013e318184ff83. PMID:
19307932.

25. Al-Attar AM, Abu Zeid IM. 2013; Effect of tea (Camellia sinensis) and olive (Olea europaea L.) leaves extracts on male mice exposed to diazinon. Biomed Res Int. 2013:461415. DOI:
10.1155/2013/461415. PMID:
23691503. PMCID:
PMC3652132.
26. Al-Attar AM, Alsalmi FA. 2019; Effect of Olea europaea leaves extract on streptozotocin induced diabetes in male albino rats. Saudi J Biol Sci. 26:118–28. DOI:
10.1016/j.sjbs.2017.03.002. PMID:
30622415. PMCID:
PMC6318816.

27. Judzewitsch RG, Pfeifer MA, Best JD, Beard JC, Halter JB, Porte D Jr. 1982; Chronic chlorpropamide therapy of noninsulin-dependent diabetes augments basal and stimulated insulin secretion by increasing islet sensitivity to glucose. J Clin Endocrinol Metab. 55:321–8. DOI:
10.1210/jcem-55-2-321. PMID:
7045153.

28. Foo AY, Bais R. 1998; Amylase measurement with 2-chloro-4-nitrophenyl maltotrioside as substrate. Clin Chim Acta. 272:137–47. DOI:
10.1016/S0009-8981(98)00009-6. PMID:
9641355.

29. Haffter D, Meyer N, Scholer A, Gyr K. 1983; Diagnostic value of the determination of serum amylase and serum lipase in suspected acute onset of acute or chronic pancreatitis. Schweiz Med Wochenschr. 113:184–8. German. PMID:
6188210.
30. Kono Y. 1978; Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys. 186:189–95. DOI:
10.1016/0003-9861(78)90479-4. PMID:
24422.

31. Liu J, Xia Q, Zhang Q, Li H, Zhang J, Li A, Xiu R. 2009; Peroxisome proliferator-activated receptor-gamma ligands 15-deoxy-delta(12,14)-prostaglandin J2 and pioglitazone inhibit hydroxyl peroxide-induced TNF-alpha and lipopolysaccharide-induced CXC chemokine expression in neonatal rat cardiac myocytes. Shock. 32:317–24. DOI:
10.1097/SHK.0b013e31819c374c. PMID:
19174742.
32. Koivukoski S. 2015. Assessing goblet cell metaplasia and expression of peptidase inhibitor 15 in mouse prostate tissue [Thesis]. Tampere University;Tampere:
33. Christley RM. 2010; Power and error: increased risk of false positive results in underpowered studies. Open Epidemiol J. 3:16–9. DOI:
10.2174/1874297101003010016.

34. Valente R, Waldthaler A, Scandavini CM, Vujasinovic M, Del Chiaro M, Arnelo U, Löhr JM. 2020; Conservative treatment of chronic pancreatitis: a practical approach. Scand J Surg. 109:59–68. DOI:
10.1177/1457496920905559. PMID:
32192418.

35. Ramakrishnan P, Loh WM, Gopinath SCB, Bonam SR, Fareez IM, Mac Guad R, Sim MS, Wu YS. 2020; Selective phytochemicals targeting pancreatic stellate cells as new anti-fibrotic agents for chronic pancreatitis and pancreatic cancer. Acta Pharm Sin B. 10:399–413. DOI:
10.1016/j.apsb.2019.11.008. PMID:
32140388. PMCID:
PMC7049637.

36. Khan FY, Matar I. 2007; Chylous ascites secondary to hyperlipidemic pancreatitis with normal serum amylase and lipase. World J Gastroenterol. 13:480–2. DOI:
10.3748/wjg.v13.i3.480. PMID:
17230625. PMCID:
PMC4065911.

38. Zari TA, Al-Attar AM. 2011; Therapeutic effects of olive leaves extract on rats treated with a sublethal concentration of carbendazim. Eur Rev Med Pharmacol Sci. 15:413–26. PMID:
21608437.
39. Bombardo M, Chen R, Malagola E, Saponara E, Hills AP, Graf R, Sonda S. 2018; Inhibition of class I histone deacetylases abrogates tumor growth factor β expression and development of fibrosis during chronic pancreatitis. Mol Pharmacol. 94:793–801. DOI:
10.1124/mol.117.110924. PMID:
29880639.

40. Geyikoglu F, Emir M, Colak S, Koc K, Turkez H, Bakir M, Hosseinigouzdagani M, Cerig S, Keles ON, Ozek NS. 2017; Effect of oleuropein against chemotherapy drug-induced histological changes, oxidative stress, and DNA damages in rat kidney injury. J Food Drug Anal. 25:447–59. DOI:
10.1016/j.jfda.2016.07.002. PMID:
28911689.

41. Bello M, Basilio-Antonio L, Fragoso-Vázquez J, Avalos-Soriano A, Correa-Basurto J. 2017; Molecular recognition between pancreatic lipase and natural and synthetic inhibitors. Int J Biol Macromol. 98:855–68. DOI:
10.1016/j.ijbiomac.2017.01.150. PMID:
28212930.

42. Schneider E, Schmid-Kotsas A, Zhao J, Weidenbach H, Schmid RM, Menke A, Adler G, Waltenberger J, Grünert A, Bachem MG. 2001; Identification of mediators stimulating proliferation and matrix synthesis of rat pancreatic stellate cells. Am J Physiol Cell Physiol. 281:C532–43. DOI:
10.1152/ajpcell.2001.281.2.C532. PMID:
11443052.

43. McCarroll JA, Naim S, Sharbeen G, Russia N, Lee J, Kavallaris M, Goldstein D, Phillips PA. 2014; Role of pancreatic stellate cells in chemoresistance in pancreatic cancer. Front Physiol. 5:141. DOI:
10.3389/fphys.2014.00141. PMID:
24782785. PMCID:
PMC3988387.

44. Gong Z, Yuan Y, Lou K, Tu S, Zhai Z, Xu J. 2002; Mechanisms of Chinese herb emodin and somatostatin analogs on pancreatic regeneration in acute pancreatitis in rats. Pancreas. 25:154–60. DOI:
10.1097/00006676-200208000-00007. PMID:
12142738.

45. Mews P, Phillips P, Fahmy R, Korsten M, Pirola R, Wilson J, Apte M. 2002; Pancreatic stellate cells respond to inflammatory cytokines: potential role in chronic pancreatitis. Gut. 50:535–41. DOI:
10.1136/gut.50.4.535. PMID:
11889076. PMCID:
PMC1773172.

46. Omary MB, Lugea A, Lowe AW, Pandol SJ. 2007; The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest. 117:50–9. DOI:
10.1172/JCI30082. PMID:
17200706. PMCID:
PMC1716214.

47. Buchwalow I, Schnekenburger J, Tiemann K, Samoilova V, Bankfalvi A, Poremba C, Schleicher C, Neumann J, Boecker W. 2013; L-arginine-NO-cGMP signalling pathway in pancreatitis. Sci Rep. 3:1899. DOI:
10.1038/srep01899. PMID:
23712581. PMCID:
PMC3664897.

48. Abdin AA, El-Hamid MA, El-Seoud SH, Balaha MF. 2010; Effect of pentoxifylline and/or alpha lipoic acid on experimentally induced acute pancreatitis. Eur J Pharmacol. 643:289–96. DOI:
10.1016/j.ejphar.2010.06.020. PMID:
20599924.

49. Al-Azzawie HF, Alhamdani MS. 2006; Hypoglycemic and antioxidant effect of oleuropein in alloxan-diabetic rabbits. Life Sci. 78:1371–7. DOI:
10.1016/j.lfs.2005.07.029. PMID:
16236331.

50. Abd El-Azim AO. 2014; Antioxidant effect of olive leaf extract on methotrexate-induced hepatic injury in rats. Canadian J Clin Nutr. 2:4–14. DOI:
10.14206/canad.j.clin.nutr.2014.01.02.

51. Al-Attar AM, Shawush NA. 2015; Influence of olive and rosemary leaves extracts on chemically induced liver cirrhosis in male rats. Saudi J Biol Sci. 22:157–63. DOI:
10.1016/j.sjbs.2014.08.005. PMID:
25737646. PMCID:
PMC4336450.

52. Lobo V, Patil A, Phatak A, Chandra N. 2010; Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 4:118–26. DOI:
10.4103/0973-7847.70902. PMID:
22228951. PMCID:
PMC3249911.

53. Cohen Z, Wilson J, Ritter L, McDonagh P. 2004; Caspase inhibition decreases both platelet phosphatidylserine exposure and aggregation: caspase inhibition of platelets. Thromb Res. 113:387–93. DOI:
10.1016/j.thromres.2004.03.020. PMID:
15226093.
54. Mohammed HA, Okail HA, Ibrahim MA, Emam NM. 2018; Influences of olive leaf extract in the kidney of diabetic pregnant mice and their offspring. J Basic Appl Zool. 79:1–13. DOI:
10.1186/s41936-018-0024-8.

55. Pretnar-Oblak J, Sabovic M, Vidmar G, Zaletel M. 2007; Evaluation of L-arginine reactivity in comparison with flow-mediated dilatation and intima-media thickness. Ultrasound Med Biol. 33:1546–51. DOI:
10.1016/j.ultrasmedbio.2007.04.011. PMID:
17618037.

56. Badr A, Fouad D. 2016; Anti-apoptotic and anti-inflammatory effects of olive leaf extract against cisplatin-induced nephrotoxicity in male rats. Int J Pharmacol. 12:675–88. DOI:
10.3923/ijp.2016.675.688.

57. Rahman SH, Ammori BJ, Larvin M, McMahon MJ. 2003; Increased nitric oxide excretion in patients with severe acute pancreatitis: evidence of an endotoxin mediated inflammatory response? Gut. 52:270–4. DOI:
10.1136/gut.52.2.270. PMID:
12524412. PMCID:
PMC1774972.

58. Eriksson JW. 2007; Metabolic stress in insulin's target cells leads to ROS accumulation - a hypothetical common pathway causing insulin resistance. FEBS Lett. 581:3734–42. DOI:
10.1016/j.febslet.2007.06.044. PMID:
17628546.

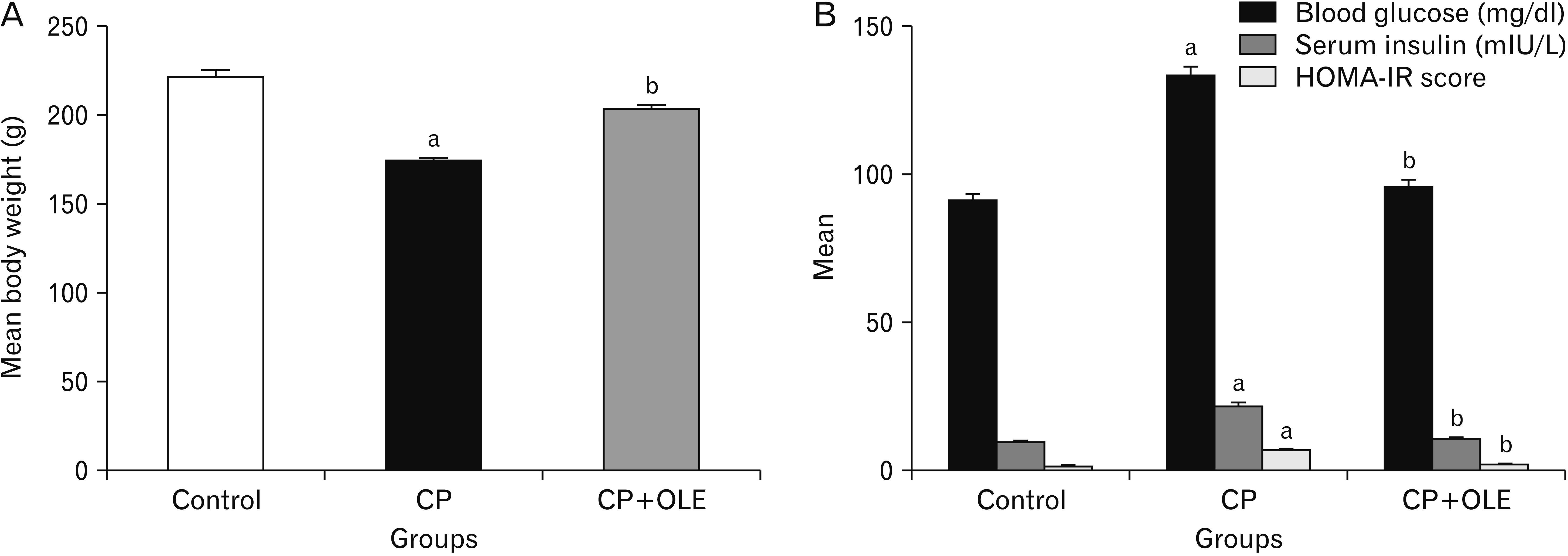
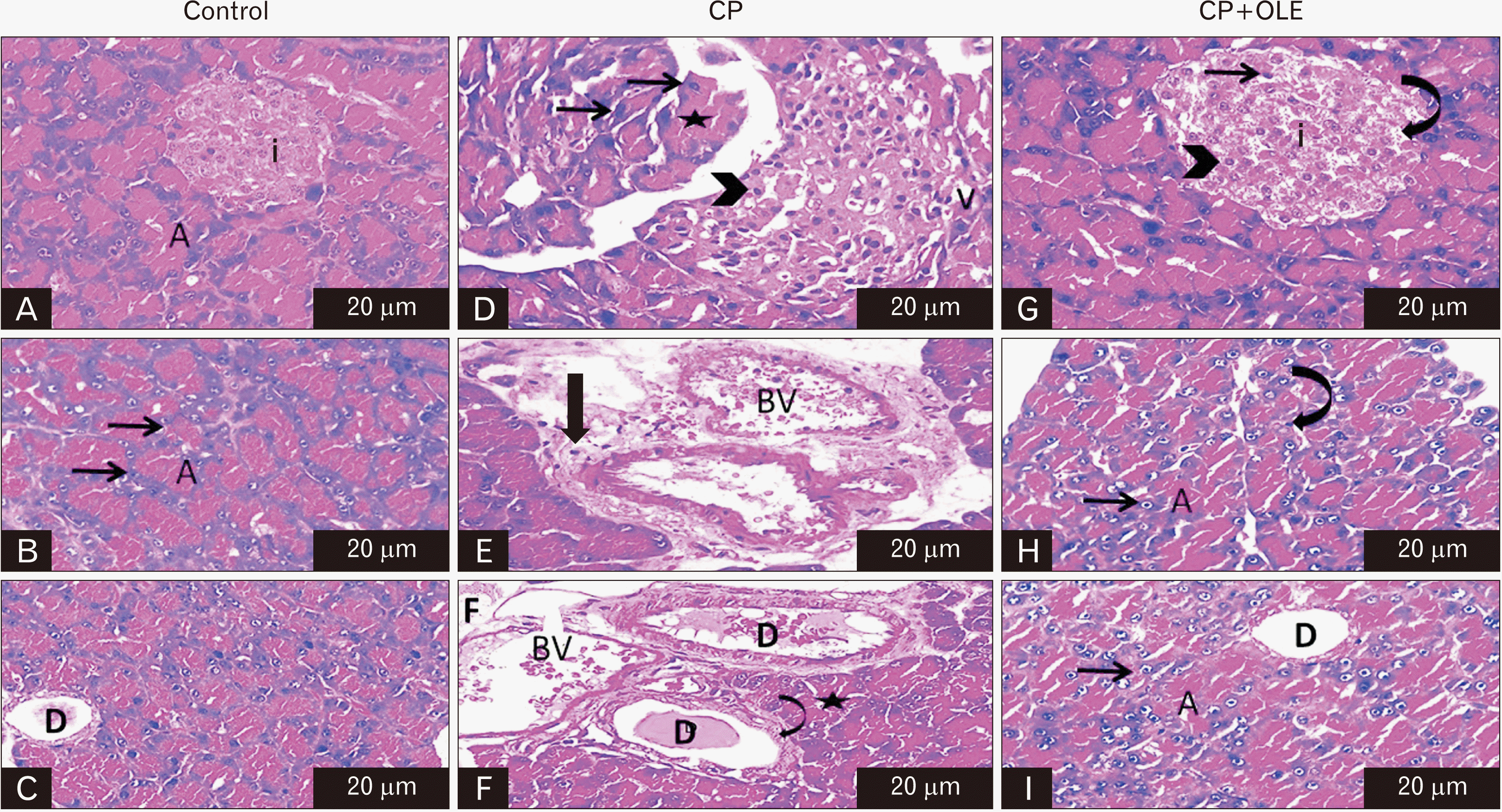
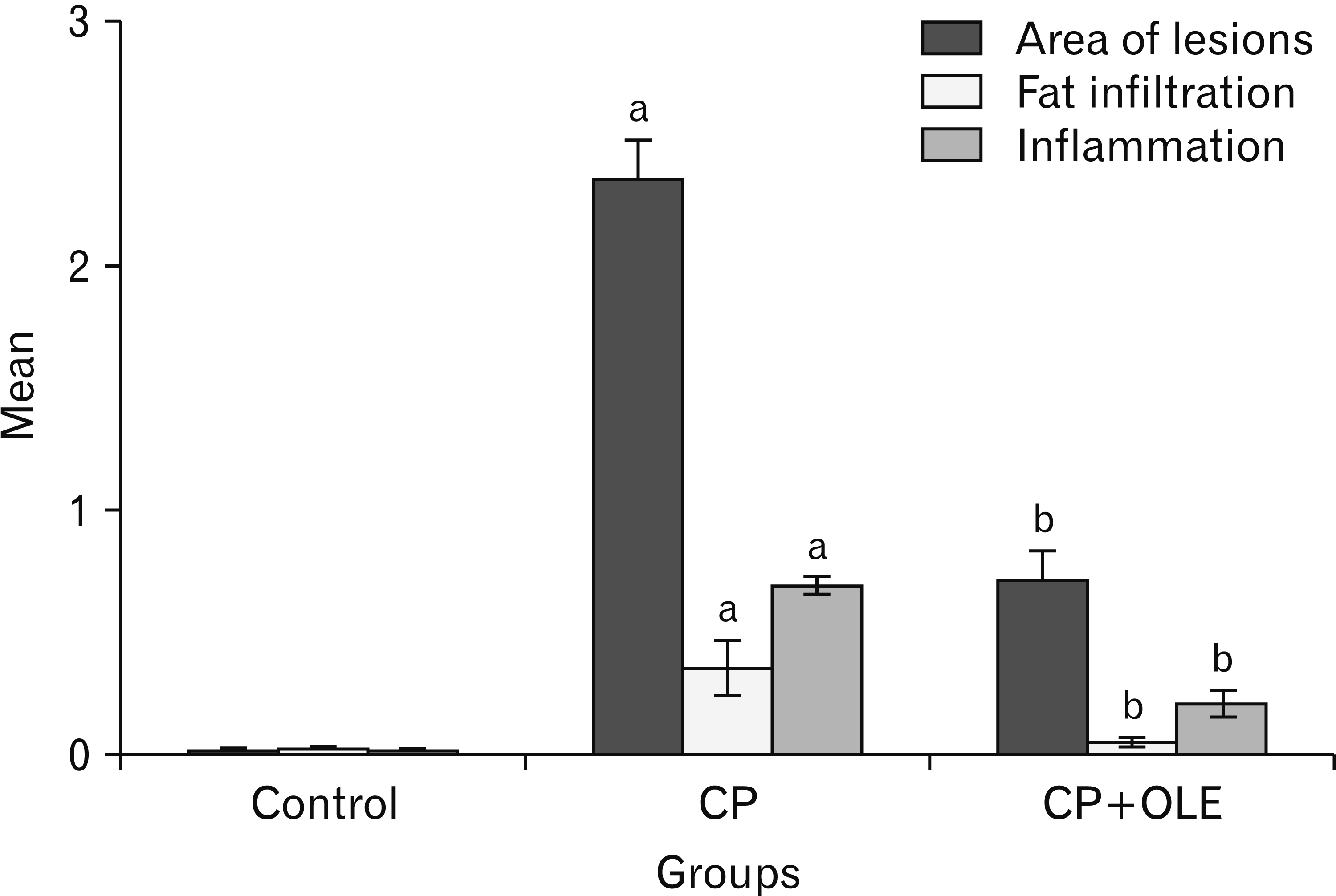






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download