Introduction
Materials and Methods
Results
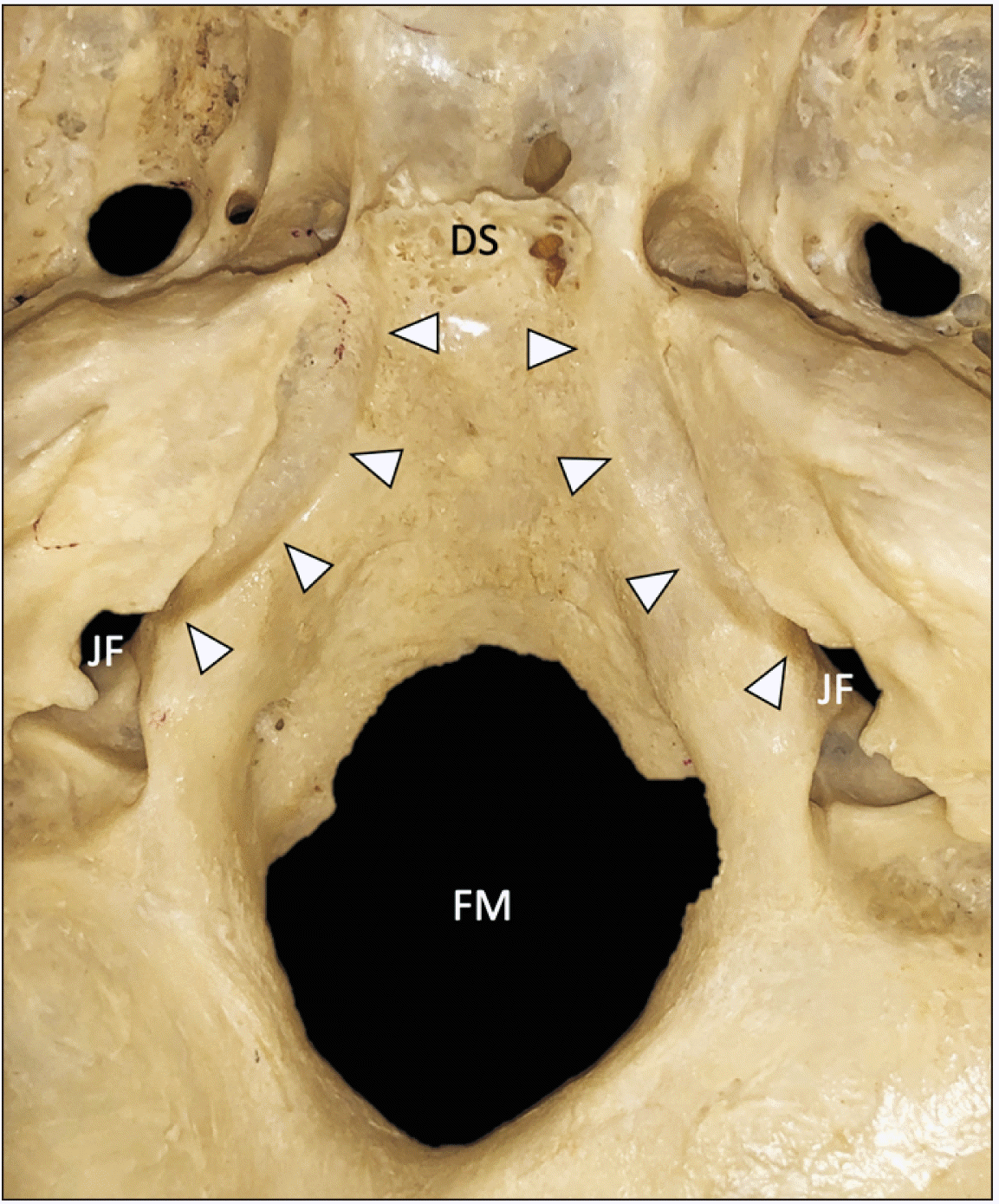 | Fig. 1Superior view of the skull base in a bony specimen. The inferior petrosal sinus grooves (arrowheads) are seen traveling from anteriorly just lateral to the dorsum sellae (DS) to posteriorly into the jugular foramen (JF). FM, foramen magnum. |
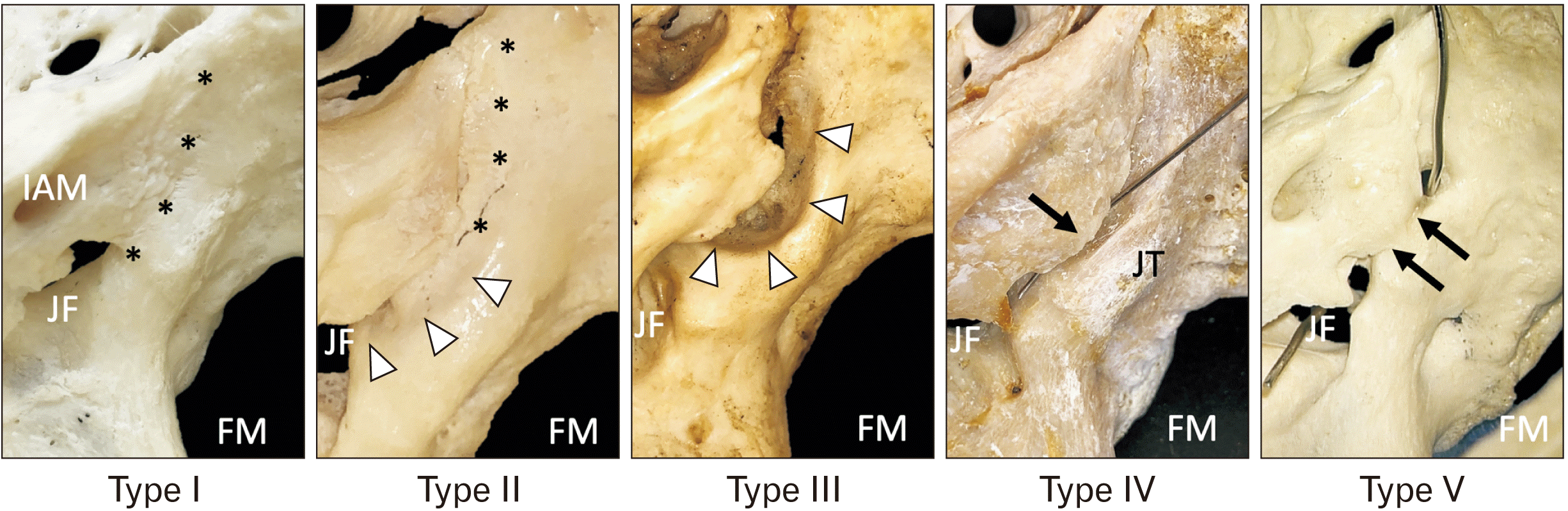 | Fig. 2Five types of inferior petrosal sinus groove (superior view on left sides). Type I: No inferior petrosal sinus groove. Type II: A partial inferior petrosal sinus groove. Type III: A complete inferior petrosal sinus groove. Type IV: A partial inferior petrosal sinus tunnel (the arrow marks the ala of the petrous part of the temporal bone that is contributing to a partial tunnel here with a needle placed within it). Type V: A complete inferior petrosal sinus tunnel (the arrows marks the ala. A metal wire is placed into the complete tunnel created by the ala). IAM, internal acoustic meatus; FM, foramen magnum; JF, jugular foramen; JT, jugular tubercle. The asterisk marks the predicted course of the inferior petrosal sinus but without a bony groove. Arrowheads, inferior petrosal sinus groove. |
Type I: No IPSG (20; 10.0%)
Type II: A partial IPSG (13; 6.5%)
Type III: A complete IPSG (160; 80.0%)
Type IV: A partial IPS tunnel (5; 2.5%)
Type V: A complete tunnel (2; 1.0%)
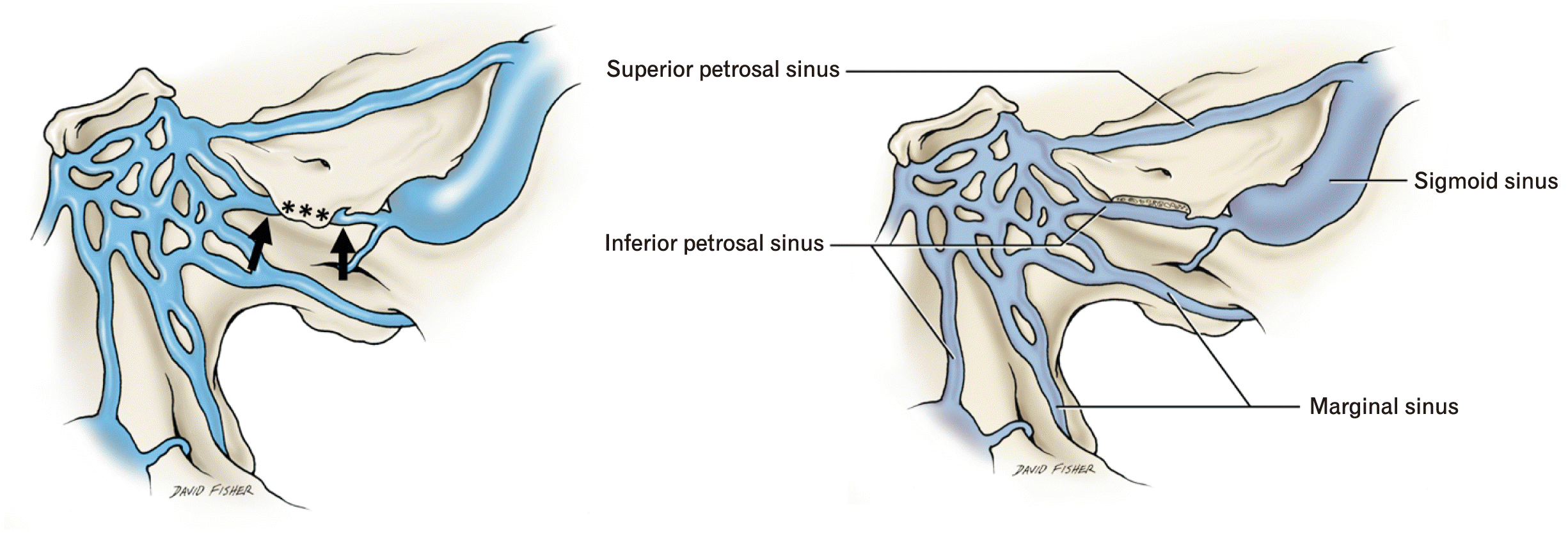 | Fig. 3Schematic drawings of the relationship of the inferior petrosal sinus. On the left, the arrows mark the sinus before and after entering the tunnel of a type V and created by the ala (asterisk). On the right side, the ala is removed to show the inferior petrosal sinus along its entire course. |
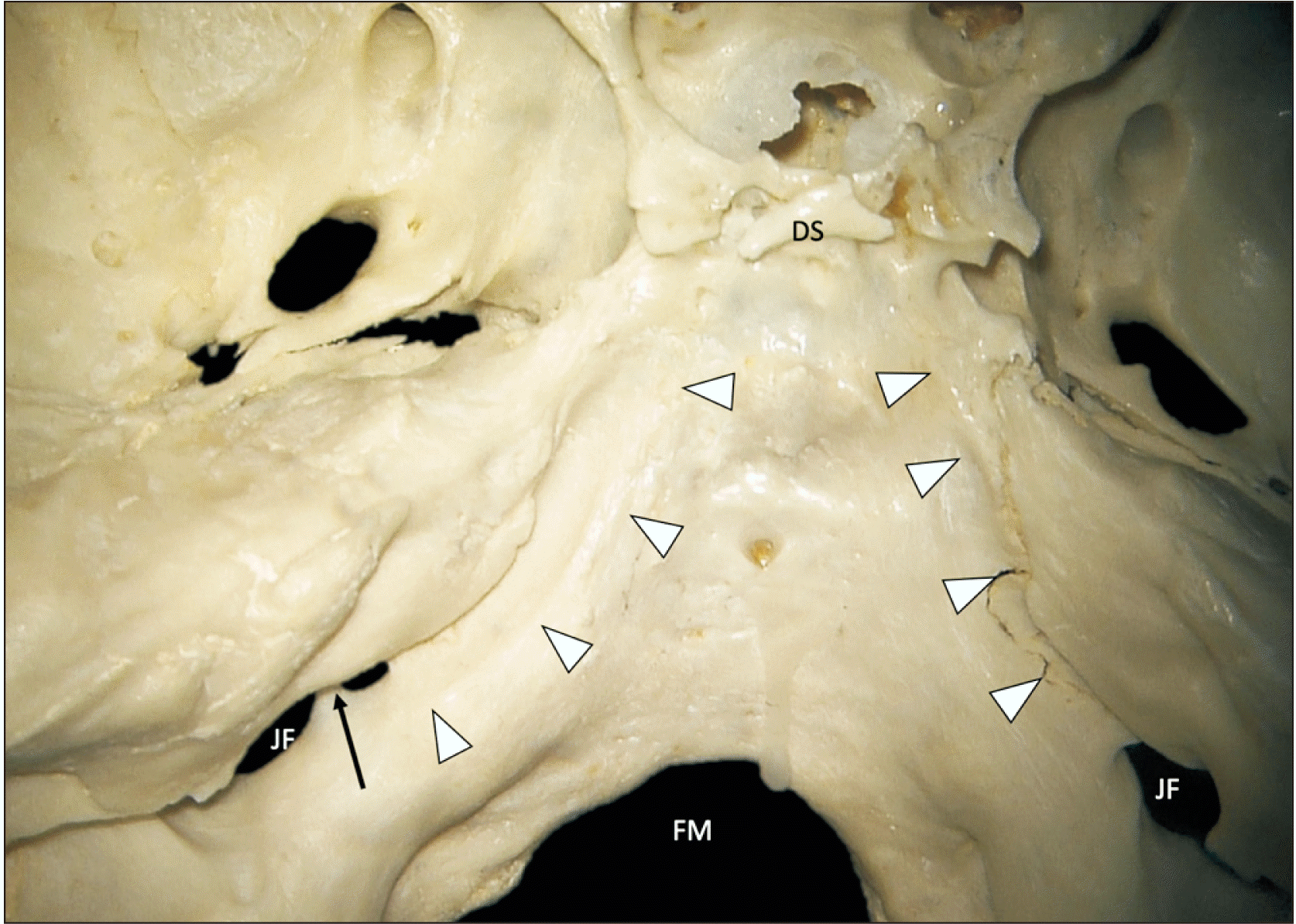 | Fig. 4Skull base sample with an inferior petrosal sinus groove (arrowheads) connecting posteriorly into a bony septated jugular foramen (arrow). DS, dorsum sellae; FM, foramen magnum; JF, jugular foramen. |
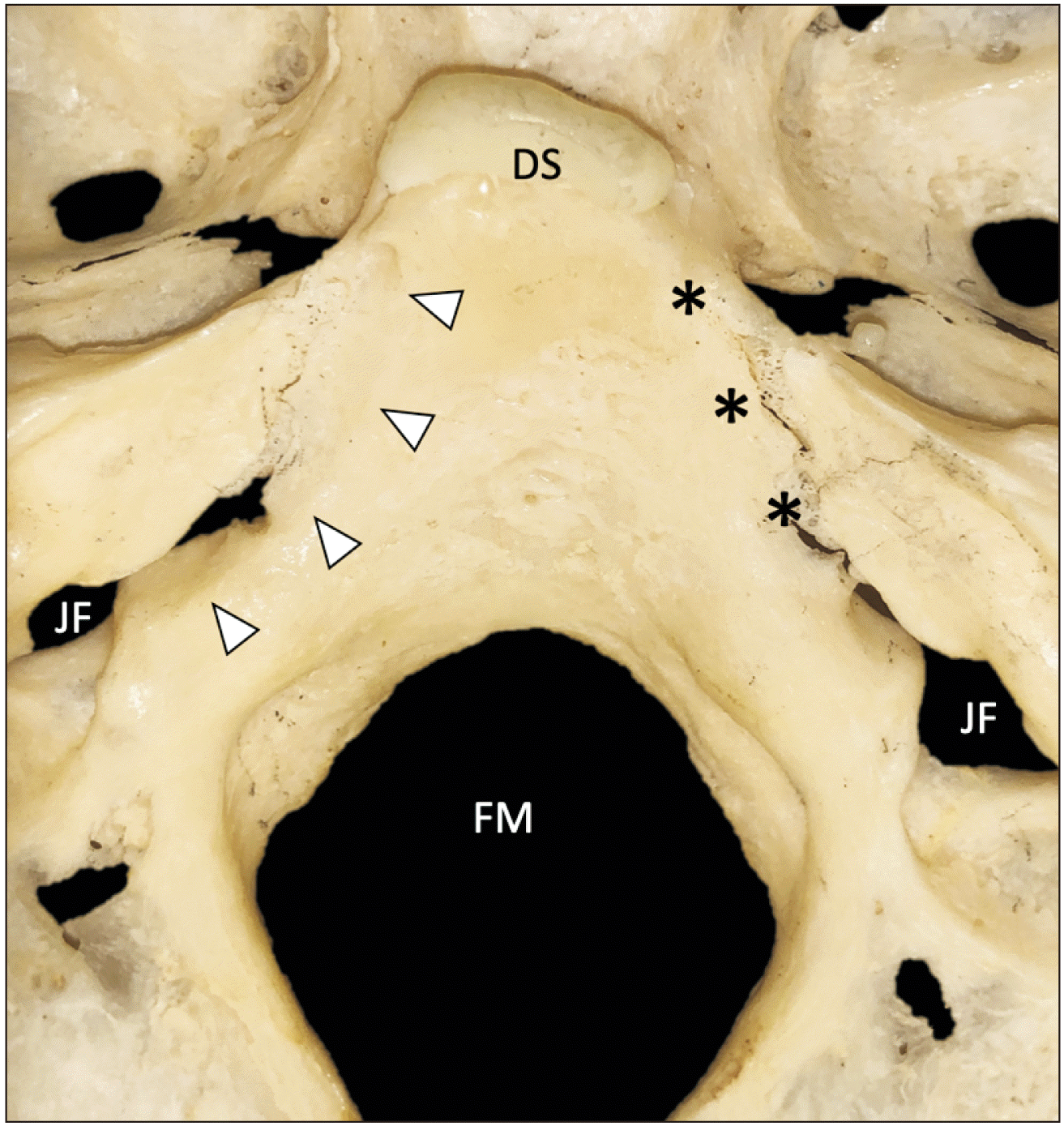 | Fig. 5Skull base sample without an inferior petrosal sinus groove on the right (asterisk, type I) and a groove on the left (arrowheads, type II). DS, dorsum sellae; FM, foramen magnum; JF, jugular foramen. |
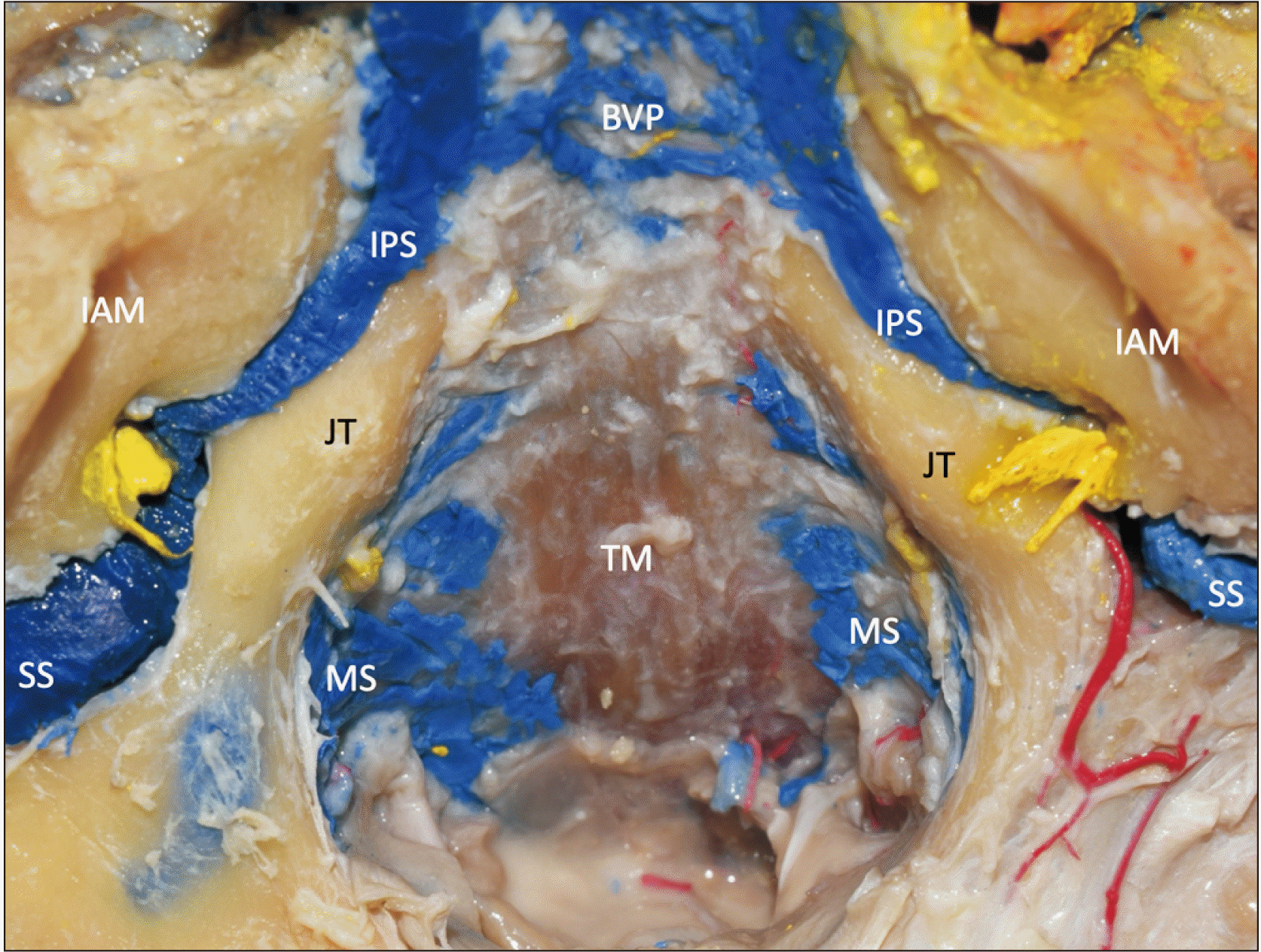 | Fig. 6An example of skull base with blue latex injected into the venous sinuses of the posterior cranial fossa. Note the asymmetry between the left and right inferior petrosal sinuses (IPS). The yellow structures are the stumps of the glossopharyngeal, vagus, and accessory nerves. JT, jugular tubercle; IAM, internal acoustic meatus; TM, tectorial membrane; MS, marginal sinus; BVP, basilar venous plexus; SS, sigmoid sinus. |
Discussion
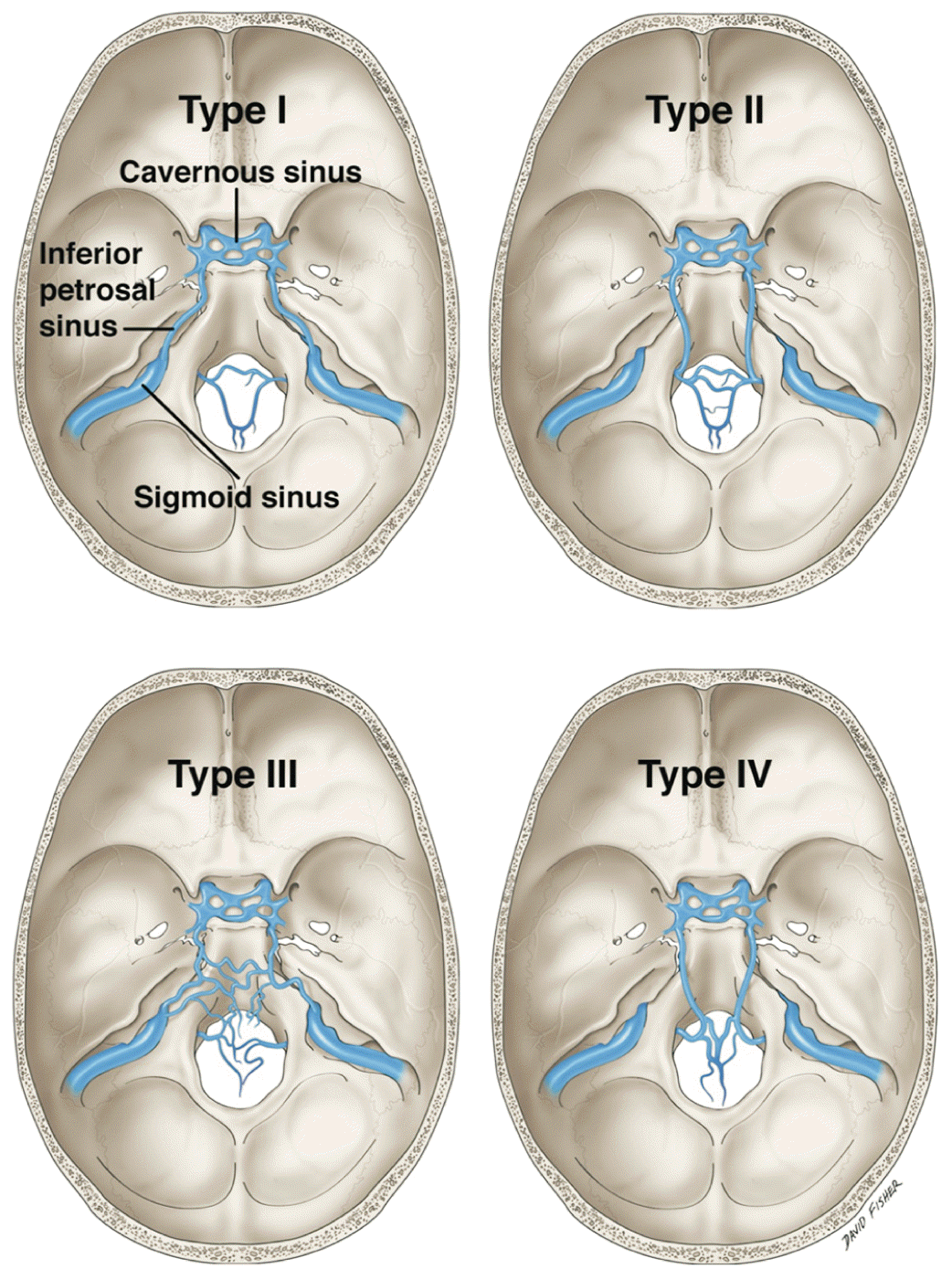 | Fig. 7Shiu et al. [20] classification of the four main variations of the inferior petrosal sinus. |
Type I (45% of patients): The most common version where the IPS drains directly in to the IJV
Type II (24% of patients): In these cases, the IPS drained into a communicating vein that united the internal jugular bulb with the deep cervical plexus
Type III (24% of patients): In type III variants, the IPS exists essentially as a plexus of veins. The cavernous sinus drains into various locations via this plexus
Type IV (7% of patients): The IPS, in these cases, is well formed. However, instead of draining into the superior jugular bulb, it drains into the deep cervical plexus of veins




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download