This article has been
cited by other articles in ScienceCentral.
Abstract
The vasculature of the pituitary gland is discussed briefly and the details of an anatomical discovery of the vessels supplying the pituitary gland provided. Twenty latex injected cadaveric heads were dissected. Any vessels that were found to penetrate the sella turcica and travel to the pituitary gland were documented and measured. Additionally, 25 adult skulls were evaluated for the presence, size, and sites of bony foramina in the floor of sella turcica. Trans-sellar vessels were identified in 65% of specimens. There was a mean of 1.5 vessels per specimen consisting usually of a mixture of veins and arteries. The mean diameter of these vessels was 0.3 mm and the mean length from the sella turcica to the pituitary gland was 2.3 mm. These vessels were concentrated in the most concave part of the sella turcica. In bony specimens, the mean number of trans-sellar foramina was four. The diameter of these foramina ranged from 0.3 to 0.6 mm in size. The trans-sellar foramina were concentrated near the center part of the sella turcica and had no regular pattern. The pituitary gland receives at least some blood supply and drainage via vessels traveling along the septum of the sphenoidal sinuses and through the sella turcica. Knowledge of such vessels might lead to a better understanding of the vascular supply and drainage of the pituitary gland and would be useful during skull base approaches such as trans-nasal approaches to the pituitary gland.
Go to :

Keywords: Anatomy, Cadaver, Sphenoid bone, Blood supply, Pituitary gland
Introduction
The arterial supply to the pituitary gland has been described in detail in the literature, most recently in a comprehensive review by Cironi et al. [
1] (
Fig. 1). Knowledge of this intricate blood supply is important for both clinicians and surgeons, and recent cadaveric dissection has demonstrated a potentially new trans-sellar artery traveling to the gland. The goal of this article is to review the blood supply of the pituitary gland briefly, and to highlight the anatomical discovery of vessels traveling through the sella turcica to the gland, an anatomical structure that, to our knowledge, has not previously been described in the literature.
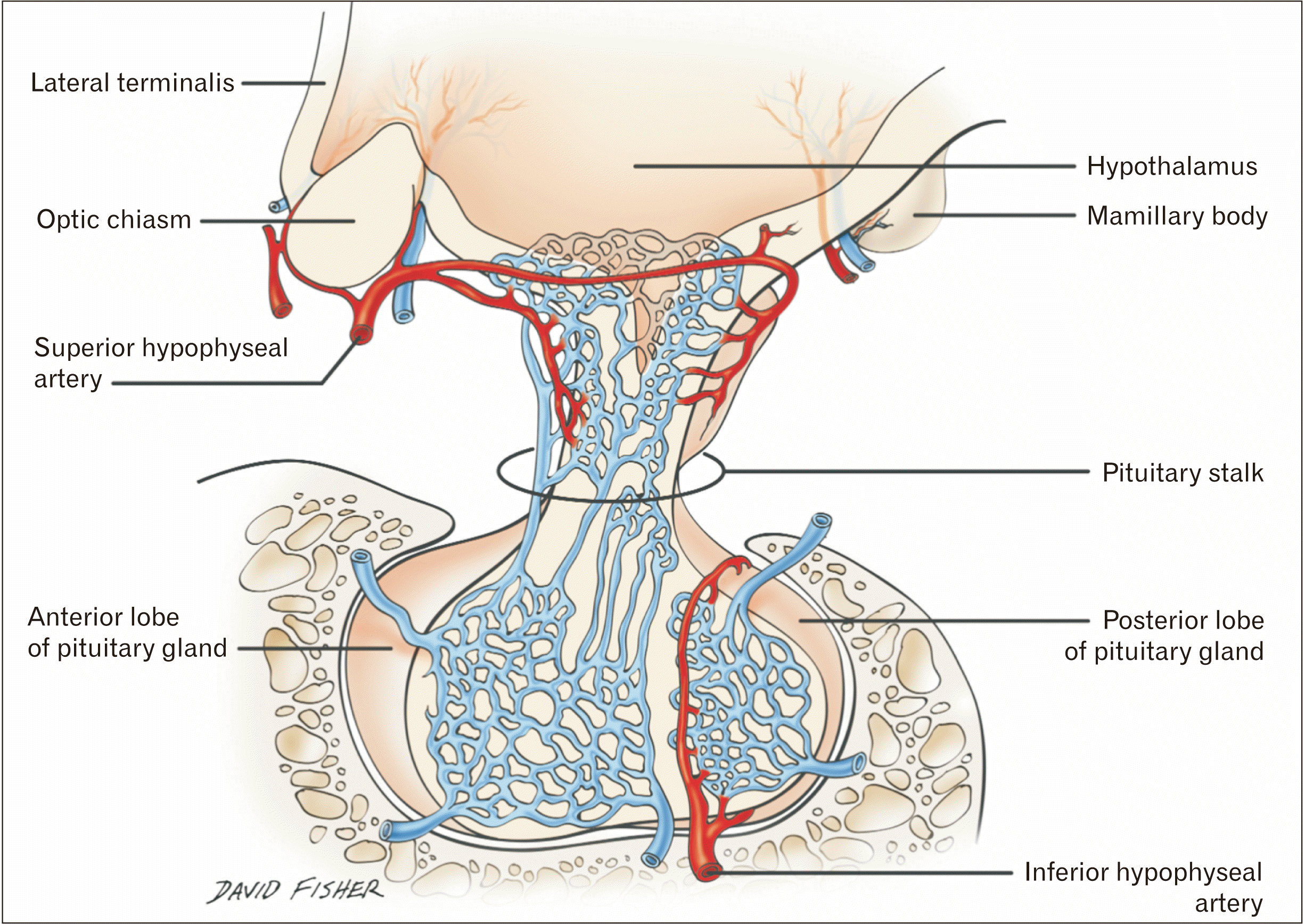 | Fig. 1Schematic drawing of the various vessels supplying the pituitary gland. 
|
Overview of pituitary gland vascular anatomy
The pituitary gland is served by the superior hypophysial (SHA), inferior hypophysial (IHA), capsular, infundibular, and prechiasmal arteries [
1]. The SHA originates medially and bilaterally from the internal carotid artery (ICA), usually near the ophthalmic segment of that vessel, but it has also been described as originating near the clinoid segment. The SHA anastomoses with its contralateral counterpart anterior to the infundibulum and provides arterial branches to the optic nerve, optic chiasm, median eminence, infundibulum, and adenohypophysis. Descending branches from it join the infundibular and prechiasmal arteries giving rise to the circuminfundibular anastomoses [
1]. The IHA originates from the cavernous segment of the ICA and crosses medially within the cavernous sinus to supply the posterior lobe of the pituitary gland. It anastomoses with its contralateral counterpart in the interlobar tissue of the gland, giving rise to interlobar arteries supplying the area between the anterior and posterior portions of the pituitary gland. Descending branches of the SHA anastomose with these interlobar branches, effectively joining the two circulations.
The capsular arteries also originate from the cavernous portion of the ICA. The inferior capsular artery originates from the inferomedial aspect of the cavernous ICA and travels toward the gland in the inferior aspect of the medial cavernous sinus wall. It follows a similar path to the IHA and goes on to anastomose with that vessel, supplying the anterior portion of the gland and the sella tucica [
1]. The anterior capsular artery originates more anteriorly from the cavernous ICA and supplies the lateral aspect of the sella turcica. Infundibular arteries, when present, commonly originate from the posterior communicating artery and supply the pituitary stalk and posterior lobe. Prechiasmal arteries, when present, originate from the proximal ophthalmic artery and supply anterior portions of the optic chiasm and pituitary gland stalk. In some cases, the dorsal meningeal artery can provide a branch to the posterior pituitary gland [
2].
Here, we detail our anatomical findings regarding a newly described blood supply and drainage of the pituitary gland and discuss the potential surgical and clinical applications.
Go to :

Materials and Methods
Using a surgical microscope (Zeiss, Oberkochen, Germany) 20 latex injected adult cadaveric heads were dissected. Red latex was used for the arteries and blue latex for the veins. With an oscillating bone saw, the calvaria was removed. Next, the dura mater was opened with dissecting scissors and the brains removed. The dorsum sella was approached from posterior and the pituitary gland and its capsule were slowly elevated from posteriorly to anteriorly using microdissection techniques. Any vessels that were found to penetrate the sella turcica and travel to the pituitary gland were documented and measured using microcalipers (MItutoyo, Kawasaki, Japan). Additionally, 25 adult human skulls were evaluated for the presence, size, and sites of bony foramina in the floor of the sella turcica.
Go to :

Results
Trans-sellar vessels were identified in 13 of 20 specimens (65.0%). The number of vessels in these 13 specimens ranged from 1 to 4 (mean 1.5) and the vessels consisted of usually a mixture of veins and arteries. The mean diameter of these vessels was 0.3 mm and the mean length from the sella turcica to the pituitary gland was 2.3 mm (2.1 to 3.7 mm). These vessels were concentrated in the most concave part of the sella turcica (
Figs. 2–
4).
 | Fig. 2Lateral view of the pituitary gland noting the trans-sellar blood vessels (arrow) traveling along the septum of sphenoidal sinuses. 
|
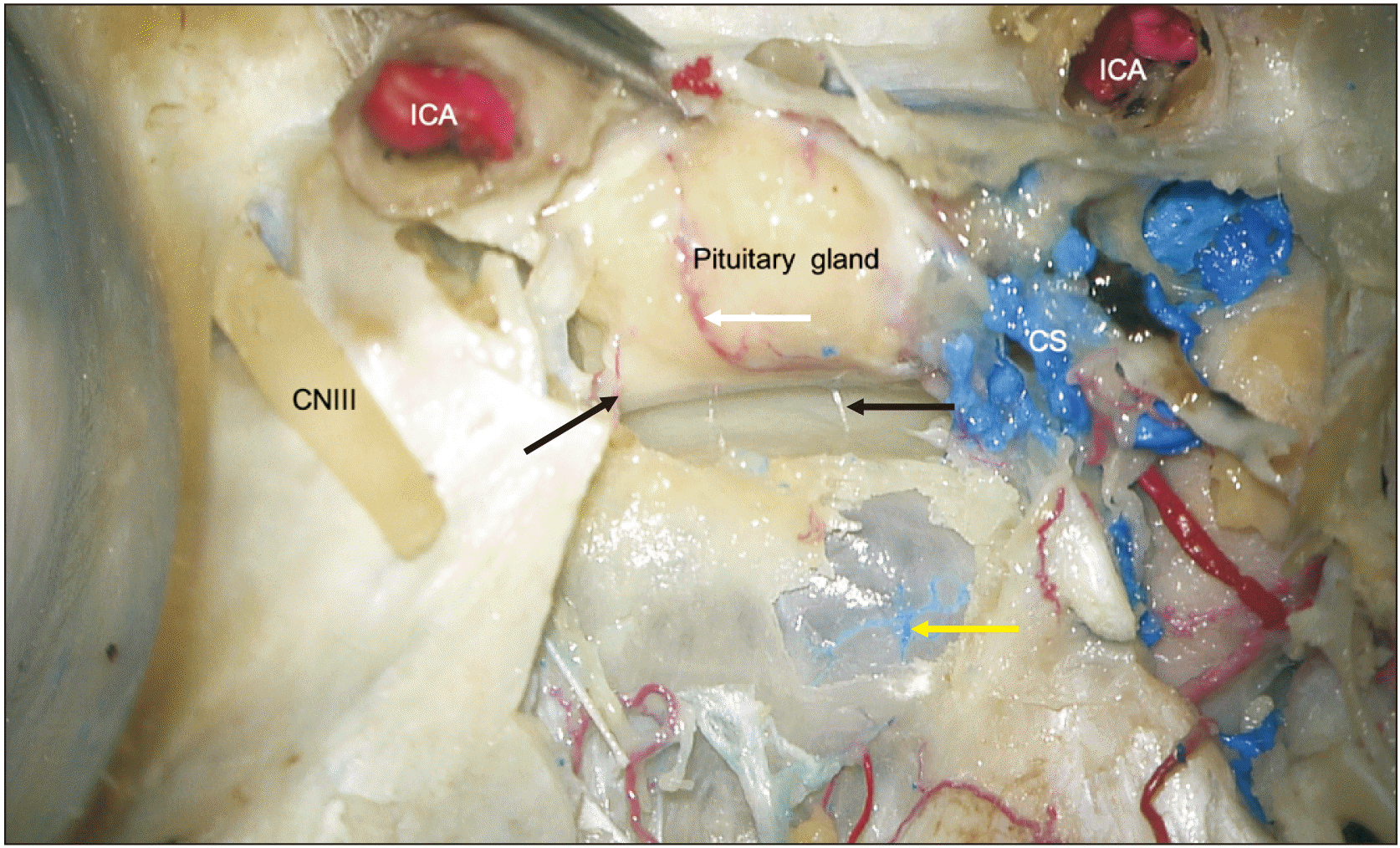 | Fig. 3Posterior view of the cadaveric specimen with the pituitary gland lifted up and pulled forward. The left black arrow notes a trans-sellar artery, which is off the midline. The right black arrow notes a trans-sellar vein with minimal latex within it but when traced inferiorly, this vessel arose from veins of the mucosa of the septum of sphenoidal sinuses. Inferiorly, the thin wall over the sinus is removed showing the intact mucosa and veins (yellow arrow) in its posterior aspect. These veins were confluent with the veins of the mucosa of the septum. For reference, note the transected internal carotid arteries (ICA), oculomotor nerve (CNIII), and cavernous sinus (CS). The white arrow marks the inferior hypophyseal artery. 
|
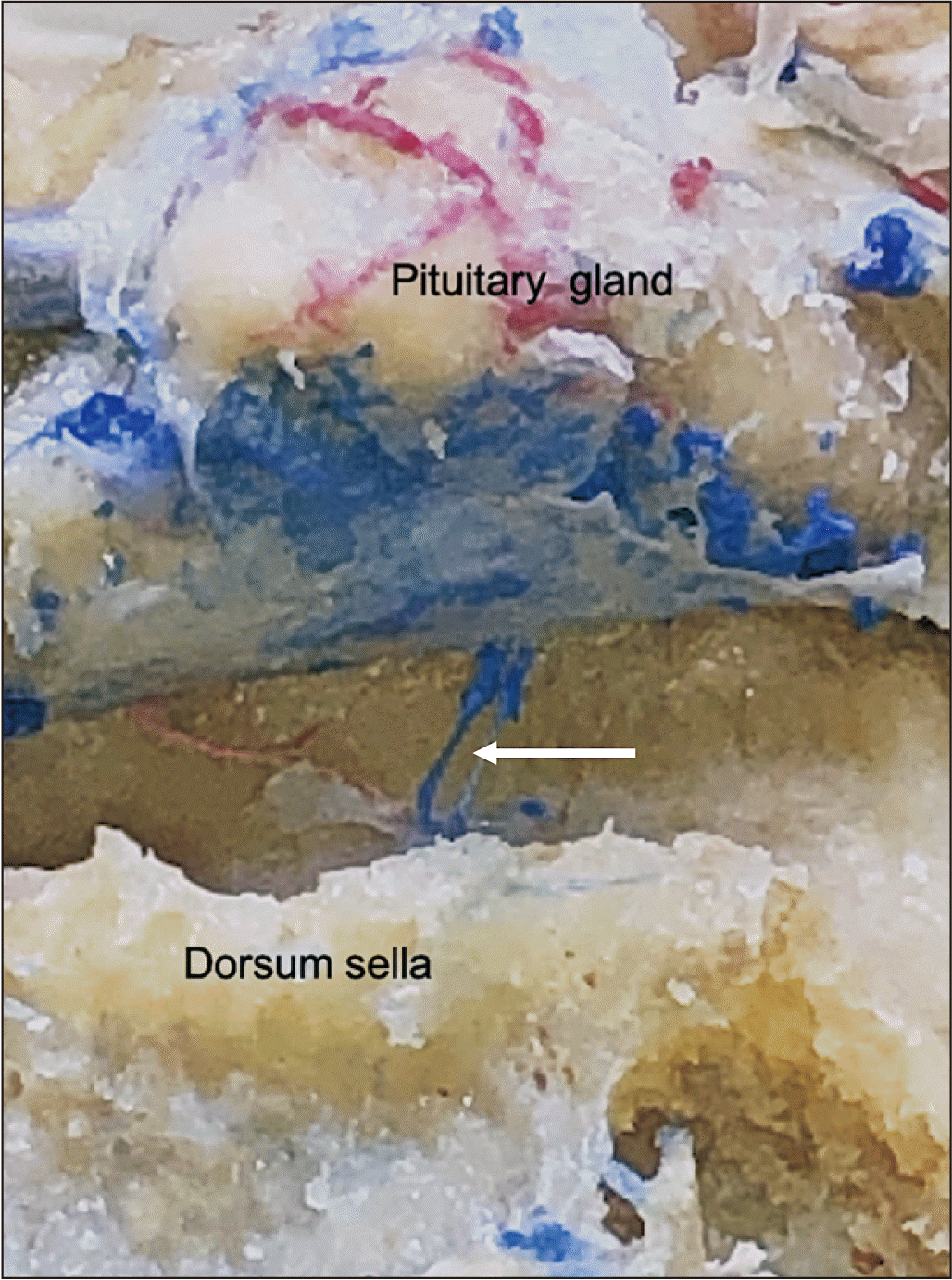 | Fig. 4Posterior cadaveric view noting a midline trans-sellar vein (arrow) with the pituitary gland lifted up and pulled anteriorly. 
|
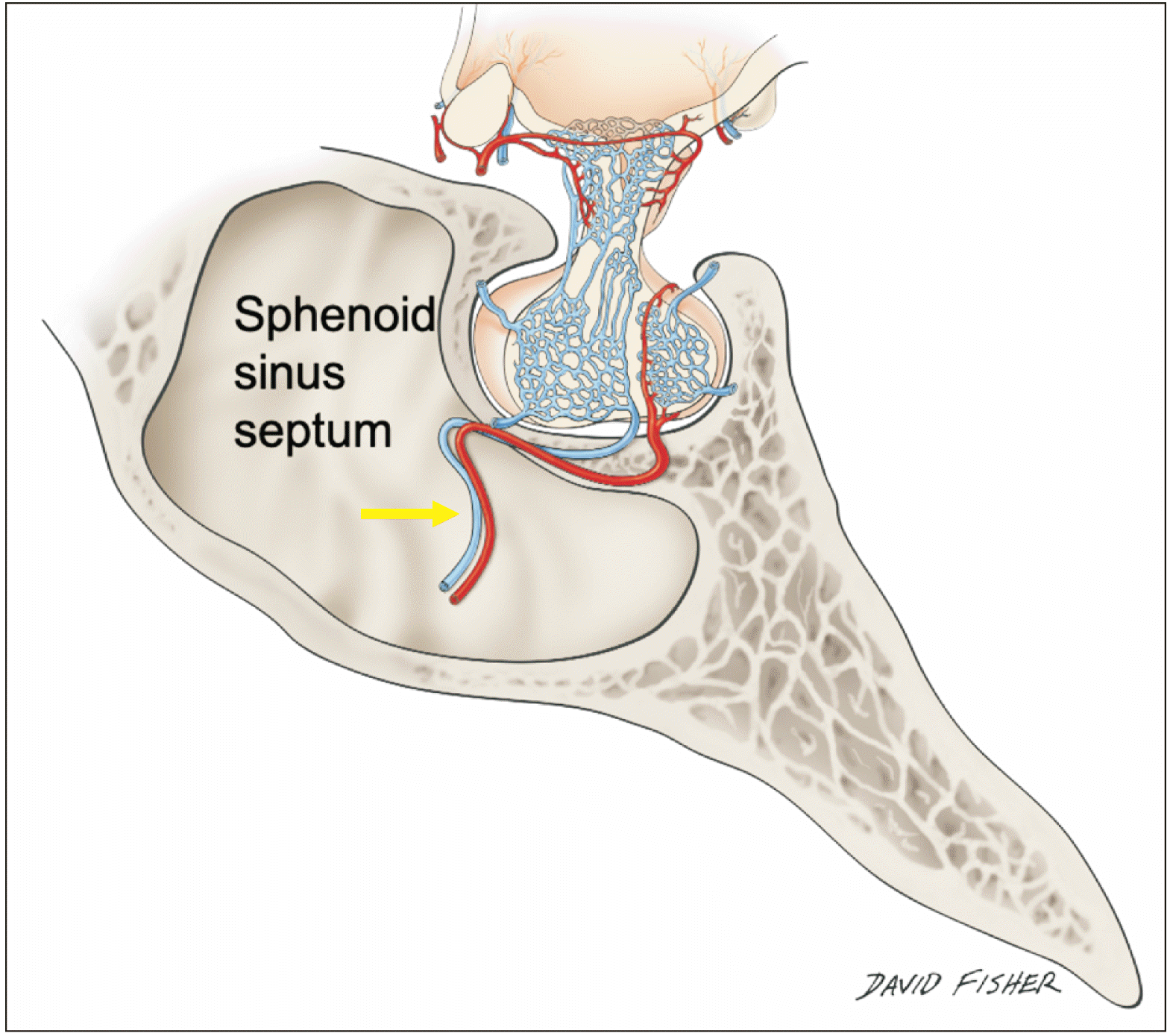
All sphenoidal sinuses with trans-sellar vessels were of the sellar type that is the sphenoidal sinus was well developed and there were no conchal types where the region below the sella was completely ossified without an air cavity or presellar types where the sphenoidal sinus had a moderately sized air cavity with no sellar indentation. However, six of seven specimens without trans-sellar vessels were presellar in nature and one was conchal. Due to their small size we were unable to localize the exact source of these very small vessels, but all but one coursed along the septum of sphenoidal sinuses (
Figs. 5,
6). The other was a small artery that was off the midline and in the lateral region of the sella turcica (
Fig. 3).
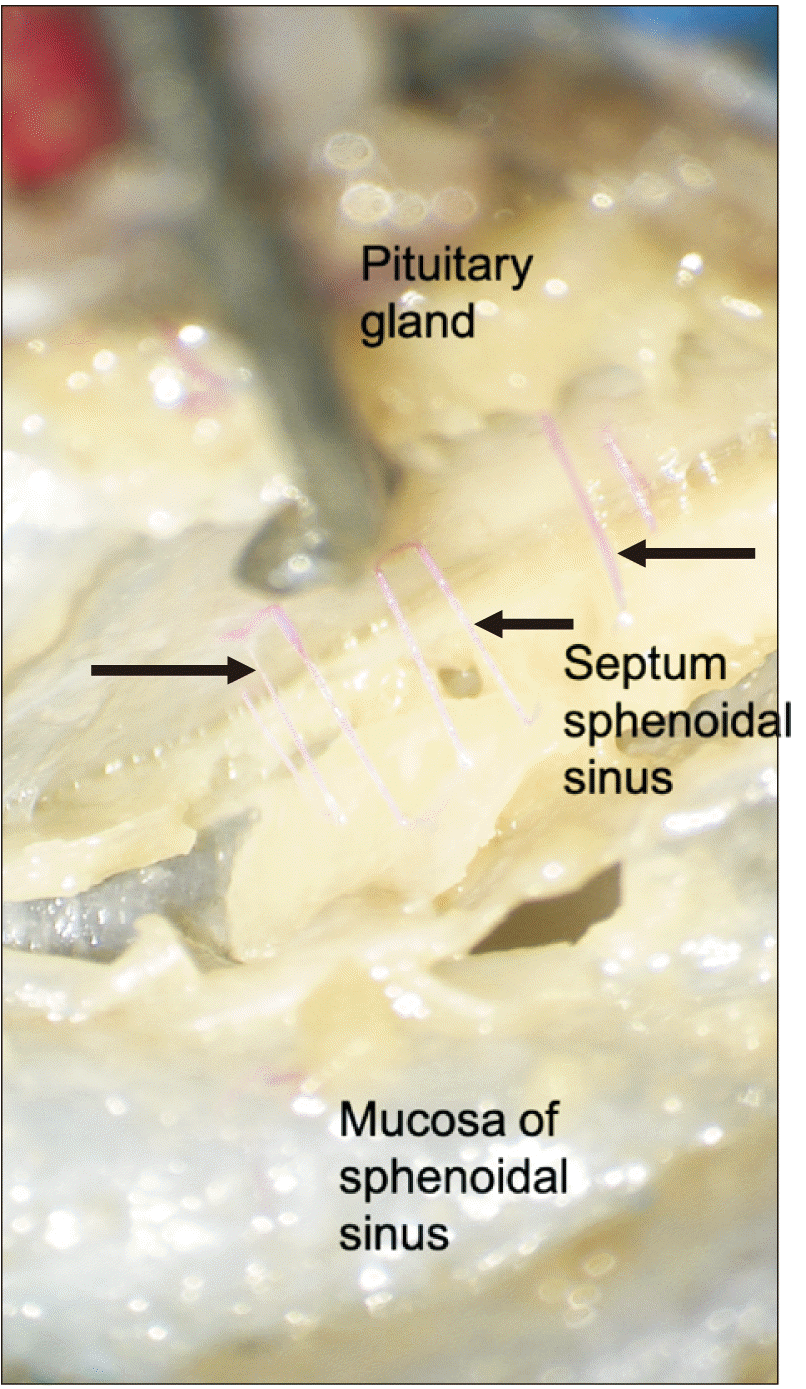 | Fig. 5Medial view with the pituitary gland lift up and noting trans-sellar arteries (arrows) traveling along the septum of sphenoidal sinuses. 
|
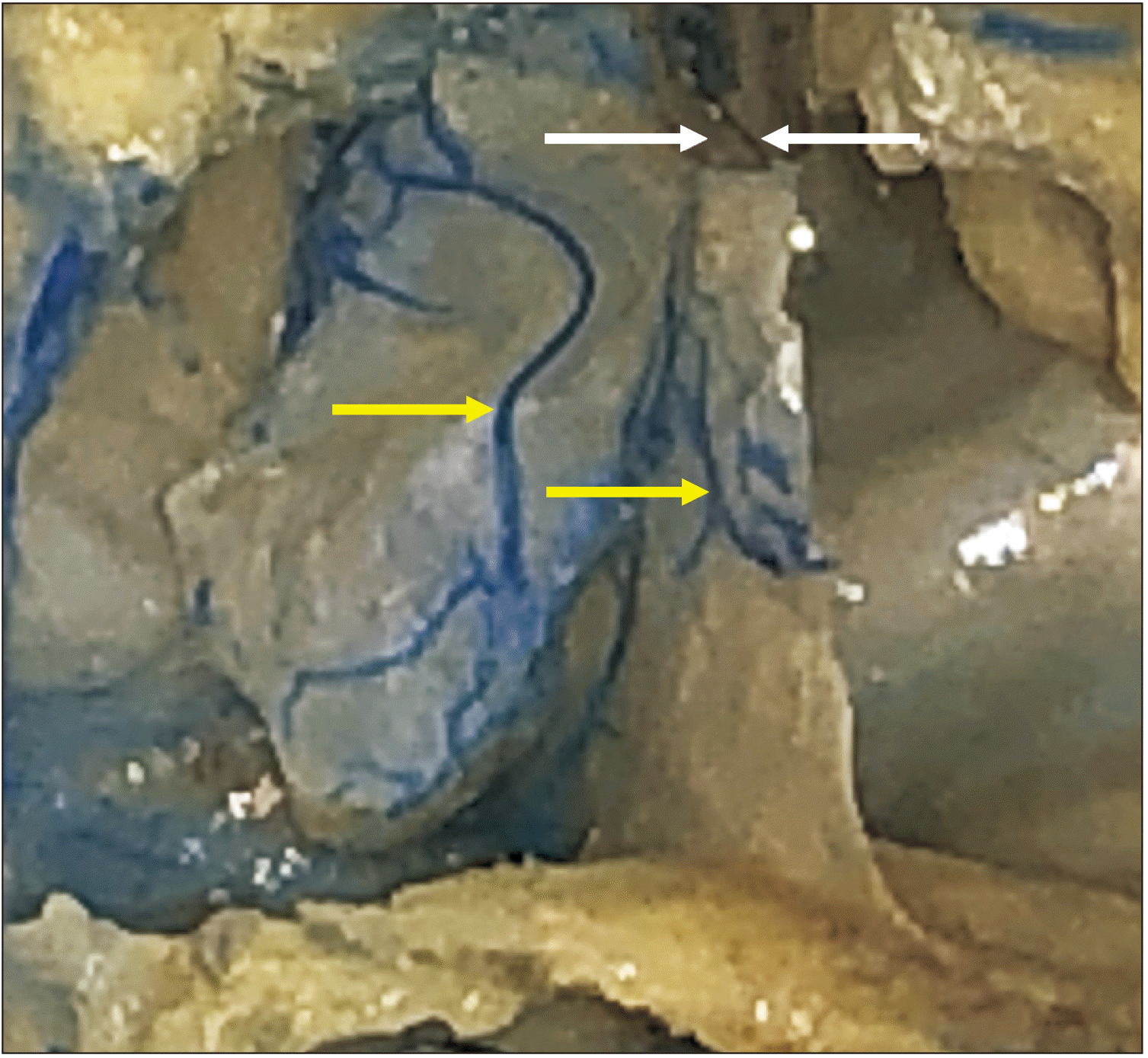 | Fig. 6Cadaveric dissection after removal of the posterior wall of the sphenoidal sinus. The left yellow arrow marks a large vein running in the mucosa of the sphenoidal sinus and the right yellow arrow marks a vein on the septum of sinuses. The left white arrow marks the sella turcica. The right white arrow shows a trans-sellar vein arising from the vessels in the septum of sphenoidal sinuses. 
|
In bony specimens, the number of trans-sellar foramina ranged from 0 to 24 (mean 4). The diameter of these foramina ranged from 0.3 to 0.6 mm (mean 0.4) in size (
Figs. 7,
8). These were usually concentrated near the center part of the sella turcia and had no regular pattern. In agreement with the cadaveric findings, all sphenoidal sinuses with trans-sellar vessels were of the sellar type and there were no conchal or presellar types (
Fig. 9).
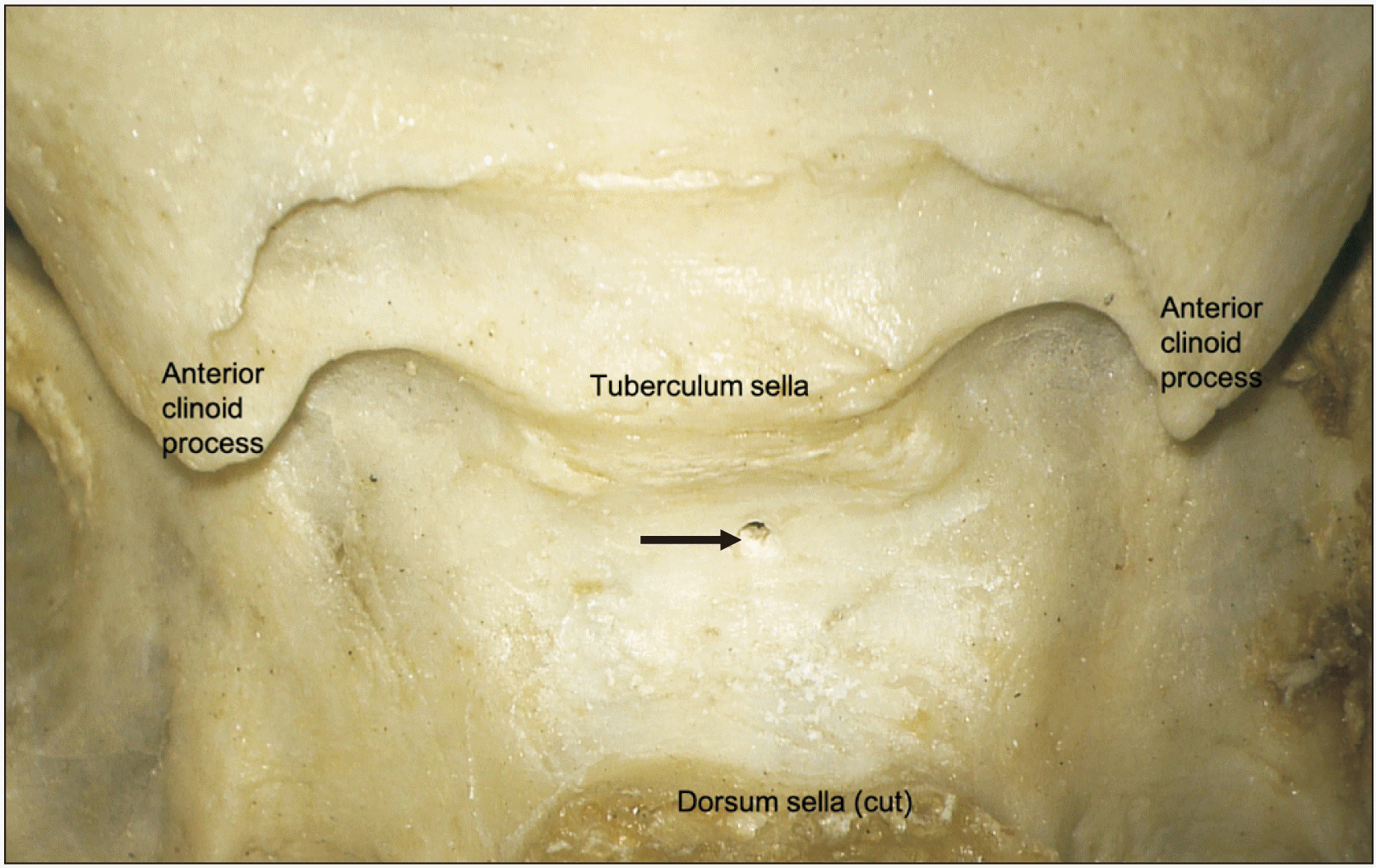 | Fig. 7Superior view of a dry bone specimen noting a single trans-sellar foramen in the sella turcica (arrow). 
|
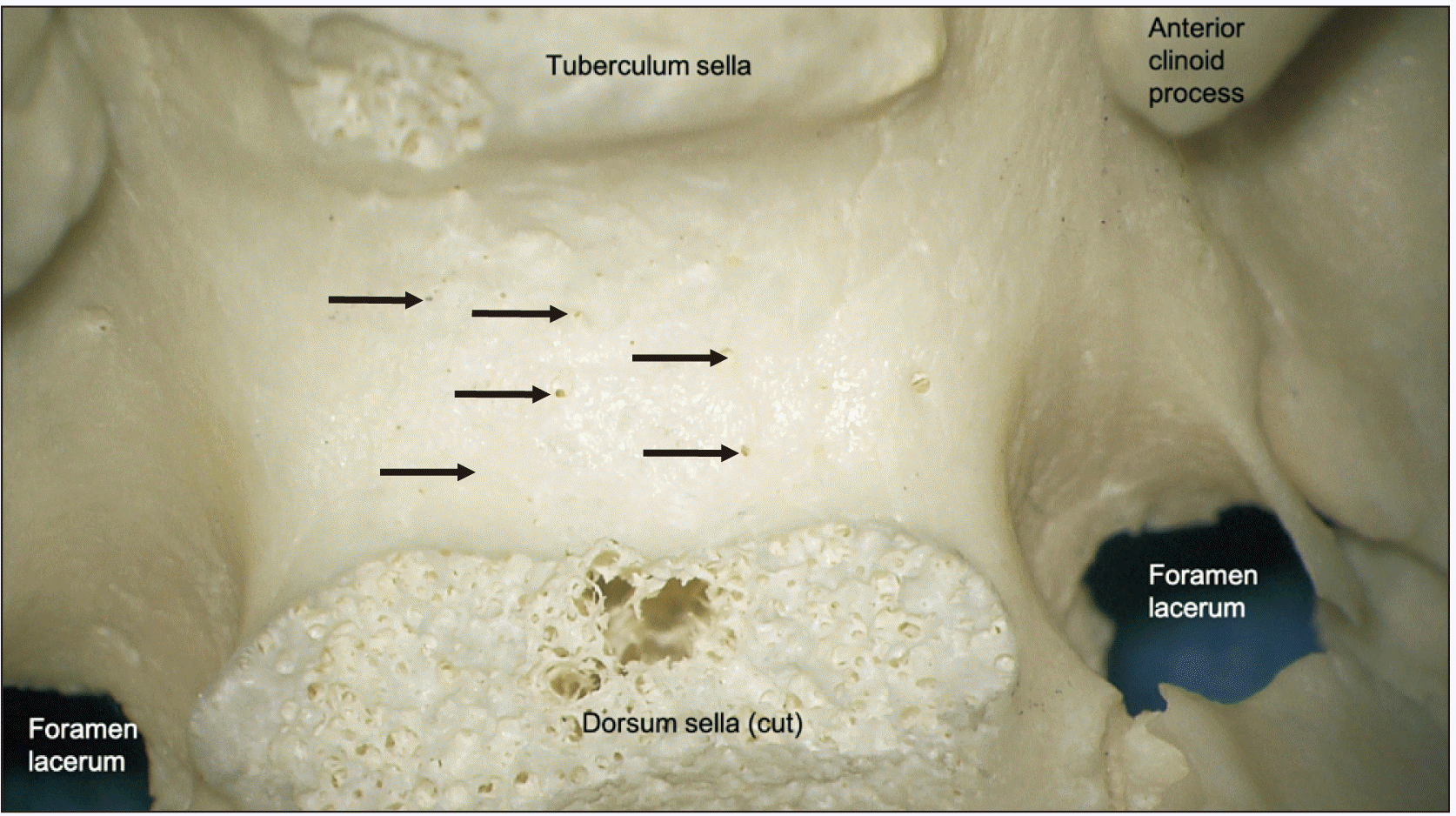 | Fig. 8Superior view of a dry bone specimen noting multiple trans-sellar foramina in the sella turcica (arrows). 
|
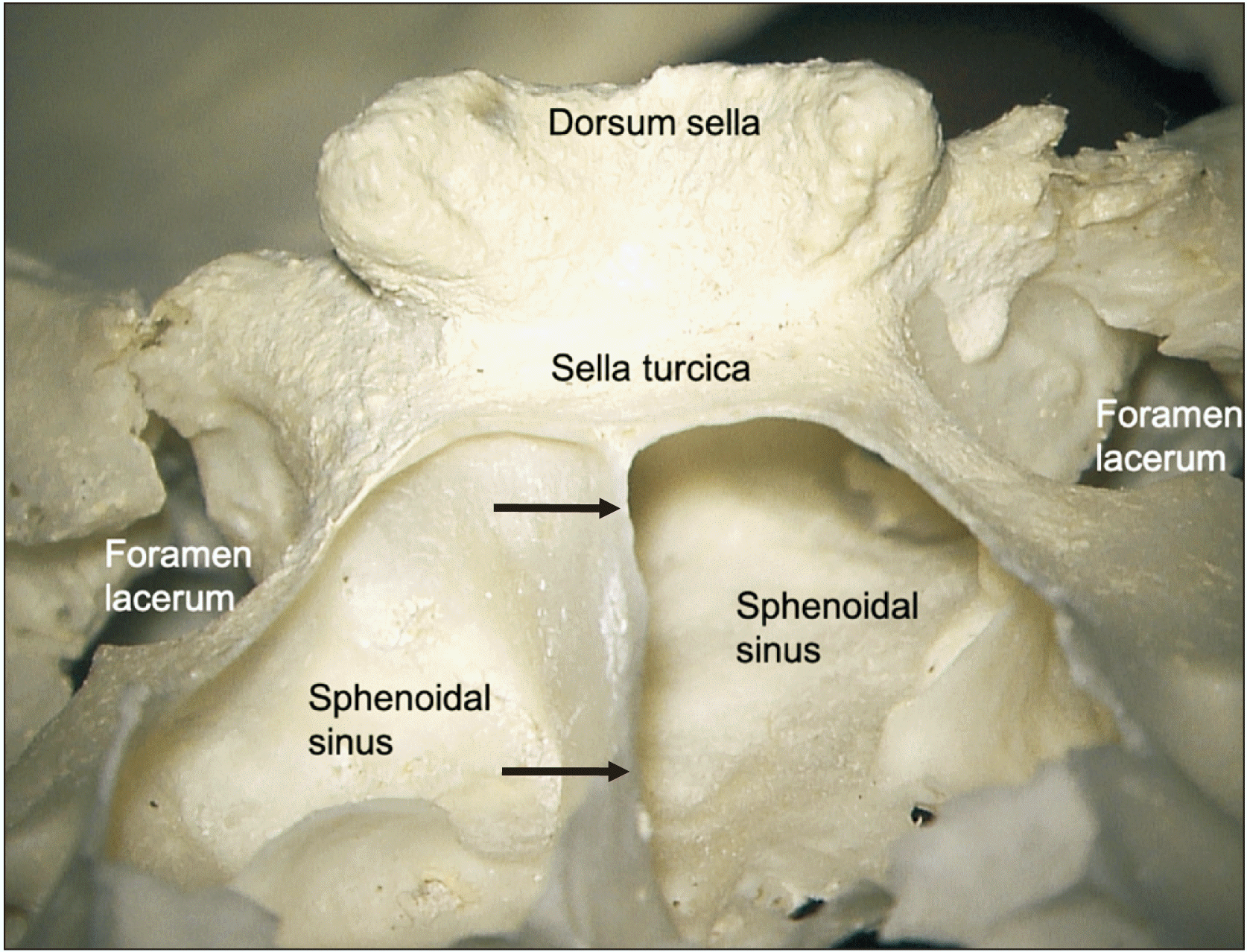 | Fig. 9Anterior view with the sphenoidal sinus opened to show the relationship between its septum (arrows) and the sella turcica. All sphenoidal sinuses with trans-sellar vessels were of the sellar type- that is the sphenoidal sinus was well developed and located inferior to the sella turcica. 
|
Go to :

Discussion
We found small trans-sellar blood vessels supplying or draining the pituitary gland in the majority of cadaveric specimens. Knowledge of complex system of vessels that serve the pituitary gland is important surgically and understanding of the hypothalamo-pituitary axis and its endocrinological interaction with the vascular system is significant physiologically. As these vessels are very small, previous dissection studies might have overlooked these or tore the vessels with elevation of the pituitary gland, especially if not working under magnification or in non-latex injected specimens. Moreover, transsphenoidal approaches to the sella turcica in patients might have difficulty due to both the size of the blood vessels and the pathology of the target.
Due to the small size of these vessels, we could not localize their exact parent artery from inside the sphenoidal sinus. However, the sphenopalatine and posterior ethmoidal arteries are known to supply the sphenoidal sinus. Therefore, these two vessels are the most likely candidates of a source of trans-sellar arteries. The venous drainage of the sphenoidal sinus is often listed as the maxillary vein [
3]. However, some also included the posterior ethmoidal vein as part of the venous drainage [
4]. Thus, the small veins found in our study most likely drain into one or both of these vessels.
Surgical considerations
Pituitary apoplexy after endoscopic sinus surgery have been reported, implying iatrogenic vascular injury. Harju et al. [
5] reported a male patient with an unknown pituitary gland adenoma who underwent a polypectomy from the sphenoidal sinus was performed with a small sphenotomy. A microdebrider was used to remove the polyp and adjacent mucosa. Postoperatively, the patient complained of diplopia. MRI revealed an apoplectic macroadenoma of the pituitary gland which was removed via craniotomy. Fyrmpas et al. [
6] reported a patient with a known macroadenoma who underwent endoscopic ethmoidectomy and polyp removal as a preparatory step for a future trans-sphenoidal resection of the macroadenoma. The patient developed pituitary apoplexy which was treated with emergent endoscopic trans-sphenoidal removal of the tumor.
It is believed that pituitary adenomas are suspectable to hemorrhage and necrosis due to outgrowing their blood supply [
5]. Some also posited that tumor expansion might compress the blood supply to the pituitary against the undersurface of the diaphragma sella [
7]. In regard to our cadaveric findings of a link between the blood supply to the pituitary gland and the sphenoidal sinus, some have appreciated sphenoidal sinus mucosal thickening on MRI in patients with pituitary apoplexy [
8,
9]. In fact, Liu and Couldwell [
9] concluded that higher grades of pituitary apoplexy are correlated with thickened sphenoidal sinus mucosa and that such patients have worse neurological and endocrinological outcomes. Arita et al. [
10] found that the mucosal thickening was predominant just below the sella turcica in patients with pituitary apoplexy. These authors also speculated that the mucosal thickening might be due to venous congestion from obstruction of trans-sellar blood flow as a result of increased intrasellar pressure.
In the present study, trans-sellar vessels were concentrated in the most concave part of the sella turcica and they were found with sellar type of sphenoidal sinuses. Thus, when removing the bone that covers the pituitary gland during transsphenoidal surgery, the trans-sellar vessels might be easily injured due to their small size and location. In addition, repairs of the sphenoidal sinus often require ligation of the sphenopalatine artery [
11]. Such sinus surgery, based on our cadaveric findings, could partially devascularize the pituitary gland when trans-sellar vessels are present (65% in our study). This surgical observation also supports our cadaveric findings. Although, due to their small size, such vessels are unlikely to contribute to significant blood supply to the pituitary gland, they might play a larger role in patients with compromise of other pituitary gland blood supply,
e.g., suprasellar masses that compress the superior hypophyseal arteries. Preoperative radiologic images may provide hints to indicate the presence of the trans-sellar vessels. These results can provide an anatomical guide for transsphenoidal surgery, avoiding surgical complications.
Medical considerations
Arita et al. [
10] hypothesized that the inflammatory reaction of the sphenoidal sinus mucosa seen in some patients with hypophysitis was due to inflammatory substances leaving the pituitary gland via venous flow through an eroded sellar floor. Parenthetically, Tanaka et al. [
12] found that the brain concentration of oxytocin after intranasal administration was much higher than that after intravenous administration. They found that over 95% of oxytocin in the brain was directly transported from nasal cavity. Such a pathway is supported by the trans-sellar blood vessels identified in the present study, especially if the delivery enters the sphenoidal sinus.
Our anatomical study identified vessels traveling along the septum of the sphenoidal sinuses and piercing the sella turcica, supplying and draining the pituitary gland. To our knowledge, no previous sources have studied about these vessels to the gland traversing this route. Based on our findings, a sellar type of sphenoidal sinus might indicate the presence of such trans-sellar vessels. This information might be of use to the skull base surgeon or in better understanding clinical consequences of diseases such as hypophysitis.
Go to :










 PDF
PDF Citation
Citation Print
Print







 XML Download
XML Download