Abstract
Background
The U.S. Food and Drug Administration has prohibited epidural steroid injection (ESI) with particulate steroids. Thus, this study aimed to compare the efficacy and safety of ESI with two nonparticulate steroids, dexamethasone and betamethasone.
Methods
The eligible patients (n = 600) who received ESI (0 week) with dexamethasone (ESI-dexa) or betamethasone (ESI-beta) had follow-up visits at 2, 4, and 8 weeks with a phone interview at 12 weeks. The primary endpoint was the proportion of effective responders without pain or who were much improved at 2 weeks. The secondary endpoints were the proportion of crossover injections at 2 weeks; changes in the visual analog scale (VAS) and disability index scores at 2, 4, and 8 weeks; the number of additional ESIs in 12 weeks; the number of participants having spinal surgery, as well as the incidence of adverse events over the 12 weeks.
Results
The proportion of effective responders at 2 weeks was not different between ESI-beta (72/216, 33.3%) and ESI-dexa (63/200, 31.5%; P = 0.670). Adverse events were more common with ESI-dexa (40/200, 20.0%) than with ESI-beta (24/216, 11.1%; P = 0.012). VAS scores decreased more with ESI-beta than with ESI-dexa at 2 weeks (difference, 0.35; P = 0.023) and 4 weeks (difference, 0.42; P = 0.011). The disability score improved significantly more with ESI-beta compared with ESI-dexa at 2 weeks (difference, 3.37; P = 0.009), 4 weeks (difference, 4.01; P = 0.002), and 8 weeks (difference, 3.54; P = 0.007).
Epidural steroid injection (ESI) is a popular non-surgical option used worldwide to manage spinal pain [1]. The efficacy of ESI as well as the safety issues associated with it, including a wide range of complications from minor skin problems to serious adverse events such as death, have been previously reported [2–5]. Traditionally, particulate steroids such as methylprednisolone acetate and triamcinolone acetonide have been used for ESI; therefore, a majority of previous studies investigated the clinical effectiveness of ESI as well as the adverse events associated with particulate steroids [2–5]. However, as shown in a microscopic study, all particulate steroids could aggregate and lead to embolic events such as spinal cord infarction if injected intravascularly, regardless of their molecular size [6–9]. Therefore, some researchers have suggested that only nonparticulate water-soluble steroids such as dexamethasone should be used for ESI, especially for the cervical spine [10–13]. However, the efficacy of the nonparticulate steroids compared with that of particulate steroids remains controversial [14,15], although some studies have demonstrated that the nonparticulate steroid dexamethasone showed similar effectiveness to particulate steroids, without a statistically significant difference [16–19].
Clinicians have concerns about the relatively low effectiveness of nonparticulate steroids compared to that of particulate steroids in daily clinical practice [20–23], because nonparticulate steroids have a tendency to be washed out of the target area owing to their water solubility [7,24]. Despite many debates, the Food and Drug Administration (FDA) announced the following regulation on April 23, 2014: “FDA requires label changes to warn of rare but serious neurologic problems after epidural corticosteroid injections for pain.” [25,26]. This action may affect not only the United States, but also our country, Korea, and the Ministry of Food and Drug Safety, Korea, has since, in line with the FDA regulation, prohibited ESI using the particulate steroid, triamcinolone acetonide. Thus, only nonparticulate water-soluble steroids can be used for ESI in clinical practice in Korea. The two most common nonparticulate steroids used for ESI are dexamethasone disodium phosphate and betamethasone disodium phosphate. To the best of our knowledge, no study has compared the effectiveness of ESI using these two drugs in patients with spinal pain.
Therefore, in this randomized prospective study, we aimed to compare the efficacy and safety of ESI using two nonparticulate steroids, dexamethasone disodium phosphate and betamethasone disodium phosphate.
This prospective study was approved by the institutional review board of Seoul National University Bundang Hospital (Protocol number: B-1304-199-001).
The eligibility criteria for inclusion in the study were as follows: an age of 19 years or more, sustained cervical or lumbar pain with a visual analog scale (VAS) pain intensity score of 5 or more irresponsive to conservative treatment, and referral for ESI from orthopedic surgeons or neurosurgeons of the spine specialty center of Seoul National University Bundang Hospital. The exclusion criteria were as follows: absolute or relative ESI contraindications (uncontrolled coagulopathy, uncontrolled diabetes mellitus, an active systemic or local infection, and contrast medium or steroid allergy); a history of adverse events after ESI; pregnancy or nursing; participation in other studies using ESI; and ESI within 2 weeks prior to study inclusion. A research worker (J.M.C.) met candidate patients to determine whether they fulfilled inclusion or exclusion criteria and recorded their responses on a sheet of inclusion/exclusion criteria before the interview. Thereafter, one of three intervention staff conducted the study in detail on potential subjects who met the inclusion/exclusion criteria and obtained written informed consent from the patients.
The participants were randomly assigned to receive dexamethasone 10 mg (Dexamethasone Disodium Phosphate Injection, 5 mg/mL, Yuhan, Seoul, Korea; the ESI-dexa group) or betamethasone 8 mg (Betamethasone Disodium Phosphate Injection, 5.2 mg/mL with betamethasone 4 mg/mL, Daewon Pharm., Seoul, Korea; the ESI-beta group) that were equivalent. ESI was performed on the enrolment day by one of three intervention staff having at least 2 years of experience in spine interventions (the three staff had 2, 6, and 11 years of specialized experience in spine interventions), who determined the injection method or the target spinal level during the interview, based on clinical manifestations and magnetic resonance imaging or computed tomography of the spine. All interventions were performed simultaneously (week 0, baseline) under fluoroscopic guidance, by using an interlaminar, transforaminal, or caudal approach for the cervical or lumbar spine. At 2 weeks, a crossover injection using another drug, but at the same site and by the same method as the first ESI, was permitted when the degree of subjective satisfaction in pain relief slightly improved or was unchanged, or when pain was aggravated, based on the International Spine Intervention Society (ISIS) guidelines published in 2009.
All participants were encouraged to visit the spine center at the 2-, 4-, and 8-week follow-up. They were asked about the degree of subjective satisfaction in pain relief (to be rated on a 5-point scale: no pain, much improved, slightly improved, not changed, aggravated), the pain intensity using the VAS, and any adverse event. They were also asked to answer a questionnaire, either the Oswestry disability index or neck disability index, by the research worker (J.M.C.). At 12 weeks, the research worker (J.M.C.) conducted phone-call interviews with all participants to inquire about the number of additional ESIs received, whether they underwent spine surgery, or developed any adverse events.
The primary endpoint with regard to the efficacy of each steroid was the proportion of the effective responders who were defined as participants with effective pain relief and showing subjective satisfaction in pain relief of “no pain” or “much improved” at 2 weeks. The secondary endpoints were the proportion of participants with crossover injection at 2 weeks; changes in the VAS and disability index scores at 2, 4, and 8 weeks; the number of additional ESIs in 12 weeks; the number of participants having spinal surgery in 12 weeks; and incidence of adverse events in 12 weeks. All the data were gathered by the research worker (J.M.C.); the intervention staff and participants were blinded to these data until the end of the study.
We hypothesized that the ESI-beta group would show a better therapeutic effect than that shown by the ESI-dexa group when the proportion of the effective responders at 2 weeks in the ESI-beta group was 10% higher than that in the ESI-dexa group. Based on previous literature, the proportion of patients with satisfactory pain reduction in the ESI-dexa group was expected to be 70% [10]. To detect a difference of 10% or more between the proportions of effective responders at 2 weeks, with a two-sided 5% significance level and a power of 80%, a sample size of 300 patients per group was necessary, given an anticipated dropout rate of 20%.
A nurse (J.Y.Y.) decided that the ESI-dexa group would be designated as “1” whereas the other group was designated as “2”, arbitrarily. This information was not provided to the participants or anyone else involved until the end of the study. A research worker (J.S.K.) prepared a computer-generated randomization list with a block size of 6; the treatment groups were designated as “1” and “2” in this list, instead of being designated by the steroid used. This randomization sequence was concealed, numbered, and sealed in opaque envelopes. The research worker (J.S.K.) directly gave these envelopes to a nurse (H.S.S.). The nurse (H.S.S.) assigned the corresponding numbered envelopes to the patients consecutively according to the randomization list, then sealed and gave these envelopes to another independent nurse (J.Y.Y.) who knew which number corresponded to which steroid. Thereafter, the nurse (J.Y.Y.) opened the envelopes alone and prepared injection syringes according to the given number during a study session in a room away from the participants and the intervention staff.
The prepared syringe was carried out of the room and to the place of injection by the previous nurse (H.S.S.). Because both betamethasone and dexamethasone appeared colorless and were odorless when added to syringes in equivalent doses in the same volume, 2 mL, the solutions could not be distinguished from each other, so double-blinding, for both the patients and the intervention staff, was possible. The envelopes containing the randomization list were resealed after each study session and stored by the nurse (J.Y.Y.). The process of allocation was designed to be complex because the glass ampoule of dexamethasone was colorless but that of betamethasone was brown, and it would have been impossible to ensure double-blinding when the steroids were in the ampoules. Moreover, it was also impossible to prepare syringes filled with drugs before the study session, because there was a possibility of the drug being contaminated once taken out of the ampoule.
Statistical analysis was performed for demographic data using a t-test between the ESI-dexa and ESI-beta groups, as well as on an intention-to-treat basis for primary and secondary outcomes, involving participants who were available at the 2-week follow-up. Missing data were dealt with by using the last observation carried forward method, therefore, there was no censored data during the follow-up period. For a binary outcome, a two-sample test of proportions and a chi-square test were used. Continuous outcomes, as well as VAS and disability scores, were analyzed with the t-test and the linear mixed model using the delta method [27], in which VAS and disability scores were considered dependent variables and the follow-up period and the drugs, as explanatory ones. Statistical analyses were performed using statistics software (STATA/SE 10.1; Stata Corp LP, College Station, TX). A P value of less than 0.05 was considered to indicate a significant difference.
Eligible patients were recruited from October 21, 2013, to January 21, 2015, and the study ended on April 15, 2015, with the last patient’s (600th patient) phone interview at 12 weeks. Three hundred patients were randomized to either ESI-dexa or ESI-beta group and allocated a drug; all patients visited the clinic and received the allocated injection at the time of randomization (baseline). Thereafter, follow-ups were conducted during visits at 2, 4, and 8 weeks, and a phone interview was conducted at 12 weeks. This sequence is shown in a flow diagram as Fig. 1. There was no protocol deviation during the study period. Baseline demographic and clinical characteristics for each group are listed in Table 1 with no difference between the two groups.
The proportion of effective responders at 2 weeks, which was the primary endpoint, was higher in the ESI-beta group (72/216, 33.3%) than in the ESI-dexa group (63/200, 31.5%), with the intergroup difference in proportions being non-significant (P = 0.670). With regard to the secondary endpoints, intergroup differences in the proportion of participants with crossover injection (125 in ESI-dexa, 141 in ESI-beta; P = 0.551) and the number of participants who underwent surgery (16 in ESI-dexa, 13 in ESI-beta; P = 0.428) were not significant, whereas adverse events were more common in the ESI-dexa group (40/200, 20.0%) than in the ESI-beta group (24/216, 11.1%) (P = 0.012; Table 2). The following adverse events were frequently encountered in the two groups: facial flushing (eight cases in ESI-dexa; six in ESI-beta), fever (nine in ESI-dexa; three in ESI-beta), urticaria (six in ESI-dexa; five in ESI-beta), insomnia (six in ESI-dexa; three in ESI-beta), itching (five in ESI-dexa; three in ESI-beta), and dizziness (five in ESI-dexa; two in ESI-beta). All adverse events were minor, and there was no major event that would necessitate hospital admission nor was any event life-threatening.
The VAS scores at 2 and 4 weeks were significantly more decreased in the ESI-beta group compared to those in the ESI-dexa group, but the differences were small (difference, 0.35 at 2 weeks and 0.42 at 4 weeks; P = 0.023 at 2 weeks and 0.011 at 4 weeks). At all follow-up visits, the disability score in the ESI-beta group was significantly better than that in the ESI-dexa group (difference, 3.37 at 2 weeks, 4.01 at 4 weeks, and 3.54 at 8 weeks; P = 0.009 at 2 weeks, 0.002 at 4 weeks, and 0.007 at 8 weeks). The requirement for additional ESI treatments was higher in the ESI-dexa group (0.45) than in the ESI-beta (0.38), but the intergroup difference was not significant (P = 0.308). The results are summarized in Table 3.
Using the linear mixed model, the VAS score in ESI-beta was expected to be about 0.31 points lower than that of ESI-dexa on average (95% confidence interval, –0.60 and –0.01) during the 8 weeks, which would be statistically significant (P = 0.045), when the interaction effect between drug and follow-up period was not considered. The change in pain intensity over time is shown in Table 4 and Fig. 2. On the contrary, the disability score was predicted to be about 2.45 points lower in ESI-beta (95% confidence interval, –5.02 and 0.13) than that of ESI-dexa along the 8-week follow-up period, however, it was not statistically significant (P = 0.063), There was no interaction effect between drug and follow-up period. The change of the disability score over time is demonstrated in Table 5 and Fig. 3. Regardless of whether having a crossover injection or not, the linear decrease of the pain intensity was found to be greater in ESI-beta (0.29 without crossover injection and –0.21 with crossover injection) than in ESI-dexa, however, this was not statistically significant (P = 0.333 without crossover injection and 0.328 with crossover injection). The disability score also decreased more in ESI-beta (–0.05 without crossover injection and –2.39 with crossover injection), without statistical significance (P = 0.983 without crossover injection and 0.194 with crossover injection).
In 2014, the U.S. FDA warned about serious adverse events that might occur after epidural injection of corticosteroids including triamcinolone acetonide [25,26]. Therefore, ESI has been classified as an off-label use with a potential risk since then. Because serious adverse events in the central nervous system have not yet been reported for ESI with water-soluble nonparticulate steroids, dexamethasone or pure, soluble betamethasone may be the drug of choice for ESI. Both dexamethasone sodium phosphate and betamethasone sodium phosphate are long-acting corticosteroids, have similar anti-inflammatory potency (compared to hydrocortisone, dexamethasone has 30-fold potency and betamethasone has 25- to 40-fold potency), and the same duration of action (36–54 hour) without mineralocorticoid activity [28]. With regard to hypothalamus-pituitary-adrenal (HPA) suppression, dexamethasone is 17 times more potent than hydrocortisone, whereas betamethasone does not show HPA suppression [28].
Concerning the risk of major complications caused by aggregation and embolic events due to particulate steroids, many previous studies have used dexamethasone as the nonparticulate steroid in clinical evaluations of the effectiveness of nonparticulate steroids relative to that of particulate steroids [10,11,13,16–23]. Most of these studies concluded that nonparticulate steroids are similar or non-inferior to particulate steroid in terms of clinical effectiveness [10,11,13,19–21]. A few authors have warned that patients may need more additional nonparticulate steroid injections compared to those that might be required after particulate steroid injection because of a shorter period of symptom relief after nonparticulate steroid injection [17,22,23]. Some studies showed that nonparticulate steroids may be superior to particulate steroids in terms of functional improvement, i.e., improvement in the disability score [16,18]. It is impossible to compare previous results with those of this study because no previous study used two nonparticulate steroids. In this study, the ESI-beta group showed a significantly greater improvement in pain intensity (VAS score) at 2 and 4 weeks than that shown by the ESI-dexa group, although the intergroup difference in VAS scores was small. The disability score also improved more significantly in the ESI-beta group. All adverse events were minor and no major complication was noted; these findings were similar to those of a previous study [3]. However, adverse events occurred more frequently in the ESI-dexa group, which may be related to the greater HPA suppression potency of dexamethasone.
We hypothesized that the proportion of effective responders would be 70% and considered that a difference of 10% in this parameter between the two groups at 2 weeks after ESI would be necessary to prove the superior efficacy of one steroid compared to that of another. Unfortunately, the proportion of effective responders at 2 weeks was 31.5% in the ESI-dexa group and 33.3% in the ESI-beta group, which were much smaller than those reported previously [14,15]. In addition, there was a high crossover injection rate of 62.0% in the ESI-dexa groups and 64.8% in the ESI-beta group. Because the possibility of a crossover injection was open to the enrolled patients, many patients might have wanted to receive ESI with another steroid. This may explain the low proportion of responders at 2 weeks and the high crossover injection rate in both the groups.
This study has some limitations. First of all, it was difficult to avoid the carry-over effect in this study, which is any effect from a previous experimental treatment that carries over into a period after the experiment has been terminated and the subjects are no longer experiencing the treatment. Ideally, to avoid the carry-over effect, it would have been required to conduct a cross-over injection after a sufficient time elapsed after the reaction was completely evaluated following the first injection. However, if applied as such in actual clinical practice, there is a high possibility that many patients will drop-out during the study, so it would be difficult to proceed with the study. Therefore, in this study, the authors chose a method that allowed cross-over injection after 2 weeks at the level following the 2009 ISIS guideline. Despite these limitations, we think that, through this study, the clinical efficacy of nonparticulate steroids compared to particulate steroids could be elucidated to some extent. Second, our study design afforded the opportunity of crossover injection, which may provide more therapeutic opportunities to the patients; however, this made the interpretation of the results difficult. In particular, it is likely that we may have underestimated the proportion of effective responders at 2 weeks. Third, the baseline disability score was significantly different between the two groups, which could have influenced the subsequent scores during the study period. Fourth, this study was performed at a single tertiary medical center and was not a multicenter study. Thus, there may be a selection bias.
In conclusion, although the proportion of effective responders was not satisfactory in both the ESI-dexa and ESI-beta groups, betamethasone disodium phosphate would be a more appropriate nonparticulate steroid for ESI compared to dexamethasone disodium phosphate.
ACKNOWLEDGMENTS
We are grateful to An-joo Lee (A.J.L), Hye-Sun Shin (H.S.S.), Ji Young Yoon (J.Y.Y.), Jungmin Choi (J.M.C.), and Jin Sun Kwan (J.S.K) for their contributions to our study and to So-yeon Ahn and Young Mi Park for statistical analyses.
Notes
REFERENCES
1. Manchikanti L, Boswell MV, Datta S, Fellows B, Abdi S, Singh V, et al. 2009; Comprehensive review of therapeutic interventions in managing chronic spinal pain. Pain Physician. 12:E123–98. DOI: 10.36076/ppj.2009/12/E123. PMID: 19668281.
2. Shah RV. 2014; Paraplegia following thoracic and lumbar transforaminal epidural steroid injections: how relevant are particulate steroids? Pain Pract. 14:297–300. DOI: 10.1111/papr.12110. PMID: 24152137.

3. El Abd O, Amadera J, Pimentel DC, Gomba L. 2015; Immediate and acute adverse effects following transforaminal epidural steroid injections with dexamethasone. Pain Physician. 18:277–86. DOI: 10.36076/ppj.2015/18/277. PMID: 26000671.
4. Gharibo CG, Fakhry M, Diwan S, Kaye AD. 2016; Conus medullaris infarction after a right l4 transforaminal epidural steroid injection using dexamethasone. Pain Physician. 19:E1211–4. DOI: 10.36076/ppj/2016.19.E1211. PMID: 27906952.
5. Laemmel E, Segal N, Mirshahi M, Azzazene D, Le Marchand S, Wybier M, et al. 2016; Deleterious effects of intra-arterial administration of particulate steroids on microvascular perfusion in a mouse model. Radiology. 279:731–40. DOI: 10.1148/radiol.2015142746. PMID: 26761719.

6. Derby R, Lee SH, Date ES, Lee JH, Lee CH. 2008; Size and aggregation of corticosteroids used for epidural injections. Pain Med. 9:227–34. DOI: 10.1111/j.1526-4637.2007.00341.x. PMID: 18298706.

7. MacMahon PJ, Eustace SJ, Kavanagh EC. 2009; Injectable corticosteroid and local anesthetic preparations: a review for radiologists. Radiology. 252:647–61. DOI: 10.1148/radiol.2523081929. PMID: 19717750.

8. MacMahon PJ, Shelly MJ, Scholz D, Eustace SJ, Kavanagh EC. 2011; Injectable corticosteroid preparations: an embolic risk assessment by static and dynamic microscopic analysis. AJNR Am J Neuroradiol. 32:1830–5. DOI: 10.3174/ajnr.A2656. PMID: 21940803. PMCID: PMC7965993.

9. Benzon HT, Chew TL, McCarthy RJ, Benzon HA, Walega DR. 2007; Comparison of the particle sizes of different steroids and the effect of dilution: a review of the relative neurotoxicities of the steroids. Anesthesiology. 106:331–8. DOI: 10.1097/00000542-200702000-00022. PMID: 17264728.
10. Lee JW, Park KW, Chung SK, Yeom JS, Kim KJ, Kim HJ, et al. 2009; Cervical transforaminal epidural steroid injection for the management of cervical radiculopathy: a comparative study of particulate versus non-particulate steroids. Skeletal Radiol. 38:1077–82. DOI: 10.1007/s00256-009-0735-5. PMID: 19543892.

11. Dreyfuss P, Baker R, Bogduk N. 2006; Comparative effectiveness of cervical transforaminal injections with particulate and nonparticulate corticosteroid preparations for cervical radicular pain. Pain Med. 7:237–42. DOI: 10.1111/j.1526-4637.2006.00162.x. PMID: 16712623.

12. Ahadian FM, McGreevy K, Schulteis G. 2011; Lumbar transforaminal epidural dexamethasone: a prospective, randomized, double-blind, dose-response trial. Reg Anesth Pain Med. 36:572–8. DOI: 10.1097/AAP.0b013e318232e843. PMID: 22005659.
13. Shakir A, Ma V, Mehta B. 2013; Comparison of pain score reduction using triamcinolone vs. dexamethasone in cervical transforaminal epidural steroid injections. Am J Phys Med Rehabil. 92:768–75. DOI: 10.1097/PHM.0b013e318282c9f2. PMID: 23370580.

14. Mehta P, Syrop I, Singh JR, Kirschner J. 2017; Systematic review of the efficacy of particulate versus nonparticulate corticosteroids in epidural injections. PM R. 9:502–12. DOI: 10.1016/j.pmrj.2016.11.008. PMID: 27915069.

15. Feeley IH, Healy EF, Noel J, Kiely PJ, Murphy TM. 2017; Particulate and non-particulate steroids in spinal epidurals: a systematic review and meta-analysis. Eur Spine J. 26:336–44. DOI: 10.1007/s00586-016-4437-0. PMID: 26873103.
16. El-Yahchouchi C, Geske JR, Carter RE, Diehn FE, Wald JT, Murthy NS, et al. 2013; The noninferiority of the nonparticulate steroid dexamethasone vs the particulate steroids betamethasone and triamcinolone in lumbar transforaminal epidural steroid injections. Pain Med. 14:1650–7. DOI: 10.1111/pme.12214. PMID: 23899304.

17. Kennedy DJ, Plastaras C, Casey E, Visco CJ, Rittenberg JD, Conrad B, et al. 2014; Comparative effectiveness of lumbar transforaminal epidural steroid injections with particulate versus nonparticulate corticosteroids for lumbar radicular pain due to intervertebral disc herniation: a prospective, randomized, double-blind trial. Pain Med. 15:548–55. DOI: 10.1111/pme.12325. PMID: 24393129.

18. Denis I, Claveau G, Filiatrault M, Fugère F, Fortin L. 2015; Randomized double-blind controlled trial comparing the effectiveness of lumbar transforaminal epidural injections of particulate and nonparticulate corticosteroids for lumbosacral radicular pain. Pain Med. 16:1697–708. DOI: 10.1111/pme.12846. PMID: 26095339.

19. McCormick ZL, Cushman D, Marshall B, Caldwell M, Patel J, Ghannad L, et al. 2016; Pain reduction and repeat injections after transforaminal epidural injection with particulate versus nonparticulate steroid for the treatment of chronic painful lumbosacral radiculopathy. PM R. 8:1039–45. DOI: 10.1016/j.pmrj.2016.03.011. PMID: 27060648.

20. Park CH, Lee SH, Kim BI. 2010; Comparison of the effectiveness of lumbar transforaminal epidural injection with particulate and nonparticulate corticosteroids in lumbar radiating pain. Pain Med. 11:1654–8. DOI: 10.1111/j.1526-4637.2010.00941.x. PMID: 20807343.

21. Kim D, Brown J. 2011; Efficacy and safety of lumbar epidural dexamethasone versus methylprednisolone in the treatment of lumbar radiculopathy: a comparison of soluble versus particulate steroids. Clin J Pain. 27:518–22. DOI: 10.1097/AJP.0b013e31820c53e0. PMID: 21562412.
22. Kim JY, Lee JW, Lee GY, Lee E, Yoon CJ, Kang HS. 2016; Comparative effectiveness of lumbar epidural steroid injections using particulate vs. non-particulate steroid: an intra-individual comparative study. Skeletal Radiol. 45:169–76. DOI: 10.1007/s00256-015-2277-3. PMID: 26537154.

23. McCormick Z, Kennedy DJ, Garvan C, Rivers E, Temme K, Margolis S, et al. 2015; Comparison of pain score reduction using triamcinolone vs. betamethasone in transforaminal epidural steroid injections for lumbosacral radicular pain. Am J Phys Med Rehabil. 94:1058–64. DOI: 10.1097/PHM.0000000000000296. PMID: 25888660.

24. Wright JM, Cowper JJ, Page Thomas DP, Knight CG. 1983; The hydrolysis of cortisol 21-esters by a homogenate of inflamed rabbit synovium and by rheumatoid synovial fluid. Clin Exp Rheumatol. 1:137–41. PMID: 6681133.
25. FDA. 2014. FDA Drug Safety Communication: FDA requires label changes to warn of rare but serious neurologic problems after epidural corticosteroid injections for pain [Internet]. FDA;Silver Spring: Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-requires-label-changes-warn-rare-serious-neurologic-problems-after.
26. FDA. 2014. Kenalog-10 (triamcinolone acetonide) injection and Kenalog-40 (triamcinolone acetonide) injection [Internet]. FDA;Silver Spring: Available at: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2014/012041Orig1s042,014901Orig1s042ltr.pdf.
27. Sinco BR, Kieffer E, Spencer MS. 2014. Using the delta method with proc mixed to generate means and confidence intervals from a linear mixed model on the original scale, when the analysis is done on the log scale. Paper AA-04-2014 [Internet]. MWSUG;Ann Arbor: Available at: https://www.mwsug.org/proceedings/2014/AA/MWSUG-2014-AA04.pdf.
28. Nicolaides NC, Pavlaki AN, Maria Alexandra MA, Chrousos GP. Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, editors. 2000. Glucocorticoid therapy and adrenal suppression. Endotext. MDText.com, Inc;South Dartmouth: https://www.ncbi.nlm.nih.gov/books/NBK279156/. DOI: 10.1530/endoabs.51.cme3.
Fig. 1
Trial profile: flow diagram of this study. ESI-dexa: epidural steroid injection with dexamethasone, ESI-beta: epidural steroid injection with betamethasone.
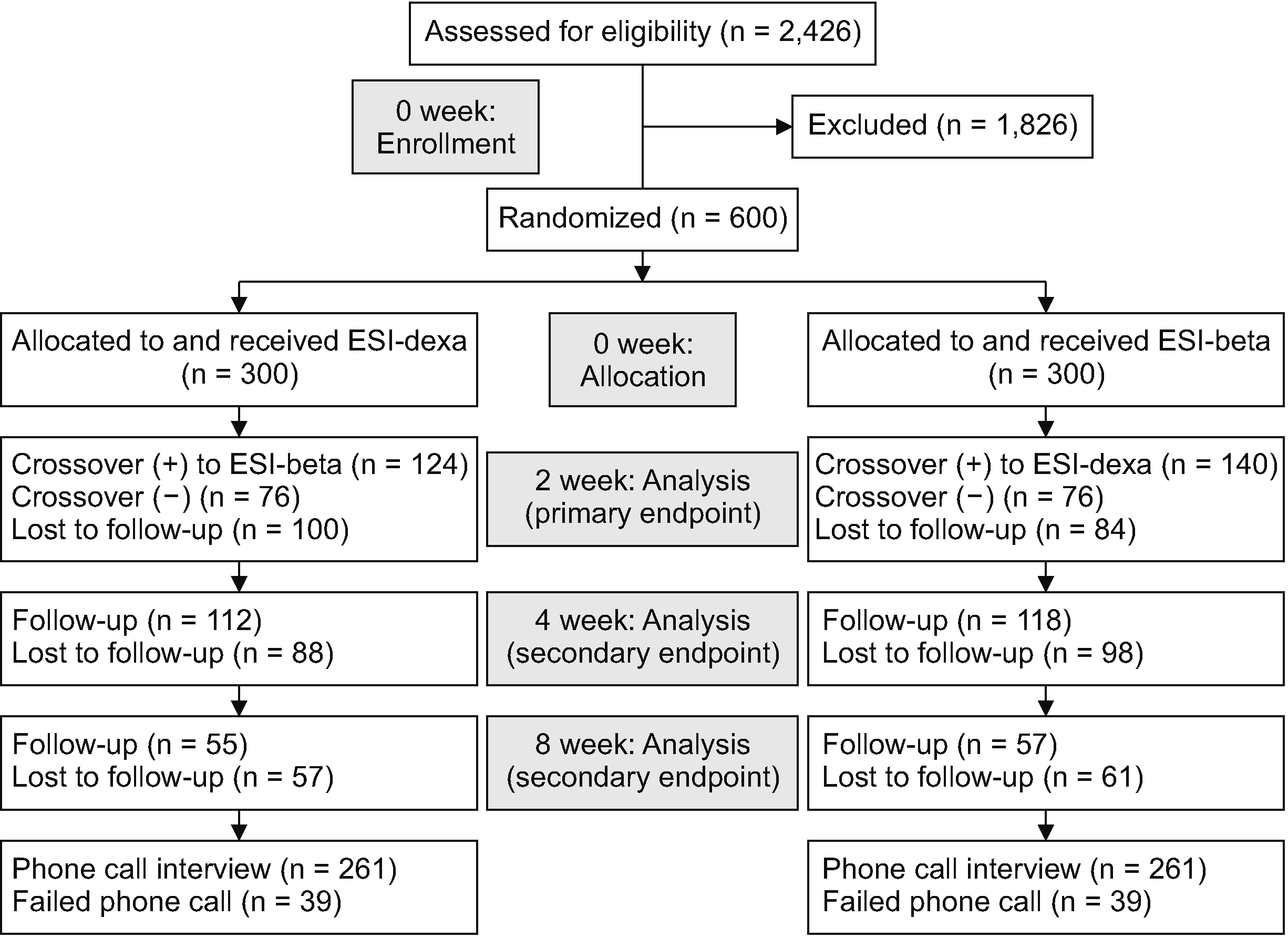
Fig. 2
Change of pain intensity (VAS) along follow-up period (0-, 2-, 4-, and 8-week) with 95% CI using linear mixed model. The error bars indicate 95% CIs. VAS: visual analog scale, CI: confidence interval, ESI-dexa: epidural steroid injection with dexamethasone, ESI-beta: epidural steroid injection with betamethasone.
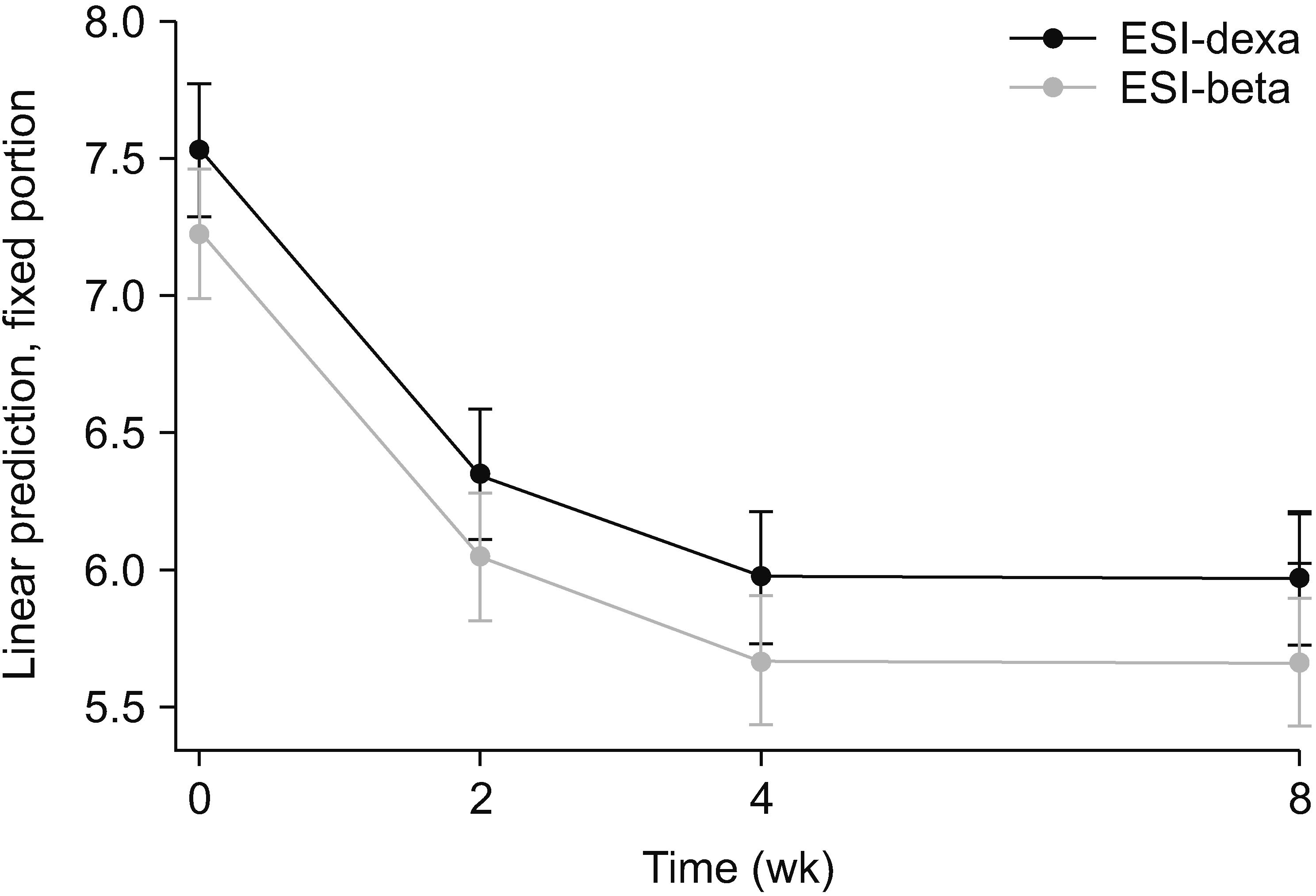
Fig. 3
Change of disability score (ODI or NDI) along follow-up period (0-, 2-, 4-, and 8-week) with 95% CI using linear mixed model. The error bars indicate 95% CIs. ODI: Oswestry disability index, NDI: neck disability index, CI: confidence interval, ESI-dexa: epidural steroid injection with dexamethasone, ESI-beta: epidural steroid injection with betamethasone.
Table 1
Baseline demographic and clinical characteristics of participants
Table 2
Proportion of effective responders, crossover injection, patients who underwent spinal surgery, and adverse events between the dexamethasone and betamethasone groups
| Primary/secondary endpoint | Dexamethasone (n = 200) | Betamethasone (n = 216) |
Risk difference (95% confidence interval) |
Risk ratio (95% confidence interval) |
Odds ratio (95% confidence interval) |
P value |
|---|---|---|---|---|---|---|
| Primary endpoint | ||||||
| Effective responders | 63 (31.5) | 72 (33.3) | –1.83 (–10.83, 7.16) | 1.04 (0.86, 1.26) | 1.09 (0.71, 1.68) | 0.670 |
| Secondary endpoint | ||||||
| Crossover injection | 125 (62.5) | 141 (65.3) | –2.81 (–12.08, 6.45) | 1.06 (0.87, 1.29) | 1.13 (0.74, 1.72) | 0.551 |
| Having spinal surgery | 16 | 13 | 1.98 (–2.93, 6.90) | 0.85 (0.56, 1.29) | 0.74 (0.32, 1.68) | 0.428 |
| Adverse events | 40 (20.0) | 24 (11.1) | 8.89 (1.93, 15.84) | 0.69 (0.49, 0.96) | 0.50 (0.28, 0.89) | 0.012* |
Table 3
Pain intensity, disability score, and the number of additional ESIs
| Time (wk) | Dexamethasone (n = 200) | Betamethasone (n = 216) |
Difference (95% confidence interval) |
P value | |
|---|---|---|---|---|---|
| VAS score | 0 | 7.39 ± 1.38 | 7.35 ± 1.48 | 0.04 (–0.19, 0.27) | 0.731 |
| 2 | 6.71 ± 1.86 | 6.36 ± 1.91 | 0.35 (0.05, 0.65) | 0.023* | |
| 4 | 6.44 ± 1.93 | 6.02 ± 2.10 | 0.42 (0.10, 0.75) | 0.011* | |
| 8 | 6.34 ± 2.07 | 6.05 ± 2.10 | 0.29 (–0.05, 0.62) | 0.093 | |
| Disability score | 0 | 35.36 ± 14.85 | 32.87 ± 14.74 | 2.49 (0.11, 4.86) | 0.040* |
| 2 | 33.25 ± 16.14 | 29.87 ± 15.34 | 3.37 (0.85, 5.90) | 0.009* | |
| 4 | 32.17 ± 16.19 | 28.16 ± 15.57 | 4.01 (1.47, 6.56) | 0.002* | |
| 8 | 31.83 ± 16.05 | 28.29 ± 16.08 | 3.54 (0.97, 6.12) | 0.007* | |
| Additional ESIs | 12 | 0.45 ± 0.72 | 0.38 ± 0.64 | 0.06 (–0.05, 0.17) | 0.308 |
Table 4
Pain intensity (VAS) along follow-up period (0-, 2-, 4-, and 8-week)
Table 5
Disability score (ODI or NDI) along follow-up period (0-, 2-, 4-, and 8-week)




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download