Abstract
Background
This study aimed to determine the impact of complex decongestive therapy applications on upper extremity function in breast cancer patients who developed adhesive capsulitis after lymphedema.
Methods
Thirty patients who developed adhesive capsulitis due to lymphedema were divided into two groups as study (n = 15) and control (n = 15) groups. Both groups received 20 minutes of exercise five days a week for three weeks using a Biodex isokinetic dynamometer, as well as a hot pack and TENS (Transcutaneous Electrical Nerve Stimulation) treatment to the shoulder joint. The study group received 45 minutes of intensive decongestive therapy along with the adhesive capsulitis treatment. The visual analogue scale was used to assess pain, circumference, and volumetric measurements were used to assess edema, and the Arm, Shoulder, and Hand Problems Questionnaire (DASH Disabilities of the Arm, Shoulder, and Hand) was used to assess upper extremity functionality. The shoulder range of motion was evaluated.
Results
Both groups had improvements in pain (P < 0.001), shoulder joint range of motion (P < 0.001), and upper extremity functionality (P < 0.001) after the treatment. There was a significant decrease in circumference and volumetric measurements in the study group (P < 0.001). However, no differences were seen in measurements in the control group.
Conclusions
The results showed that complex decongestive therapy was beneficial in reducing lymphedema in breast cancer patients who acquired adhesive capsulitis due to lymphedema. Consequently, the authors believe that supplementing conventional physiotherapy with complex decongestive therapy will benefit patients.
Lymphedema is a chronic disorder caused by a buildup of protein-rich tissue fluid in the intercellular space due to a developing region or injury to the lymphatic system [1]. While advancements in the field of radiotherapy, which plays a critical role in cancer treatment today, reduce mortality and increase disease-related life expectancy in the majority of breast cancer cases, they also increase the risk of developing a severe complication such as lymphedema at a rate of 5%–42% following breast cancer treatment [2]. Lymphedema is a chronic condition that requires lifelong care. In the literature, risk factors for lymphedema, approaches to prevent these factors, evaluation methods, and treatment options are still discussed. However, complex decongestive therapy (CDT) is considered the gold standard for the treatment of lymphedema. The therapy concept consists of four basic components: manual lymph drainage (MLD), skin care, compression therapy, and therapeutic exercises. Studies have shown that if patients do not continue complex degongestive therapy or exercise treatment, their lymphedema worsens [3,4].
The most significant problems affecting patients’ everyday activities and often occurring after breast cancer treatment are those involving upper extremity functions [5]. According to Bosompra et al. [6], 13%–15% of women with lymphedema had pain, 35% experienced edema, 36% experienced upper extremity numbness, and 1%–4% experienced difficulty with abduction and flexion motions of the shoulder throughout the day. However, it has been reported that they struggle with daily activities. It has been reported that 87.5% of patients with breast surgery and axillary dissection experience at least one upper extremity-related issue [7]. In addition, upper extremity problems that emerge from breast cancer treatment result in functional impairment, psychological morbidity, and impaired quality of life in patients [8]. Conditions such as pain, numbness in the extremity, and shoulder restriction may arise independently or in conjunction with one another, or as a result of lymphedema [9]. Voogd et al. [10] reported that many patients had restricted shoulder motion and problems with everyday activities after mastectomy for breast cancer. In a study examining shoulder morbidity and the impact of radiotherapy, it was discovered that 17% of patients who had radiotherapy after breast cancer surgery developed shoulder problems, compared to 2% of patients who did not get radiotherapy [11].
Adhesive capsulitis is a condition that results in acute pain and restriction of joint mobility in all planes of the shoulder joint during active and passive motions [12]. Shoulder joint range of motion (ROM) limits associated with essential breast cancer treatment have a detrimental effect on the patient’s quality of life by impairing functionality. For example, in a study comparing the extremities of the operated side to the healthy side of patients after breast cancer surgery, a substantial restriction in shoulder ROM was discovered on the side of the operation [13].
This study aims to examine the effects of CDT techniques on pain and upper extremity results and the efficacy of the treatment in patients with lymphedema secondary to breast cancer who are later diagnosed with adhesive capsulitis.
The randomized-controlled study was conducted between July 2020 and December 2020 at Baskent University Adana Practice and Research Center, Department of Physical Therapy and Rehabilitation. Non-Interventional Research approved the study protocol of the Faculty of Health Sciences, Hasan Kalyoncu University, on December 24, 2019 (Decision no: 2019/129). Informed consent was obtained from the patients. Participants have been allocated into two groups using a research randomizer (www.randomizer.org).
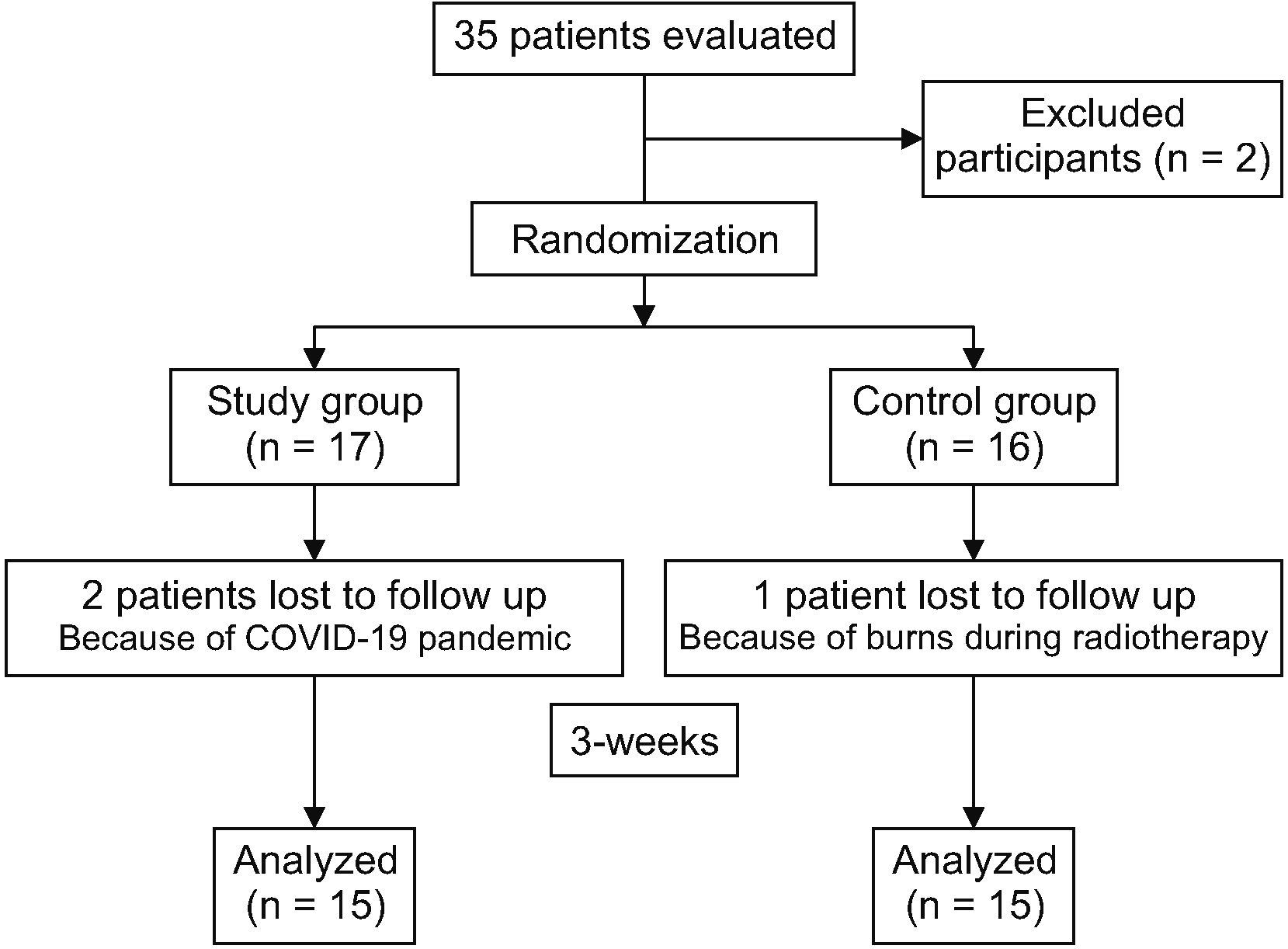
The study included 30 patients aged 30 to 60 with unilateral breast cancer who developed adhesive capsulitis after lymphedema and satisfied the inclusion criteria. Individuals who underwent mastectomy surgery at least one year before were diagnosed with stage II adhesive capsulitis, had the visual analogue scale (VAS) score of 4 or above, and Disabilities of the Arm, Shoulder, and Hand (DASH) score of 40 or greater were included in the study. Individuals with abnormal blood pressure during exercise or CDT, those who did not participate in the study consistently, those who ceased volunteering, and those who had undergone bilateral breast cancer surgery were excluded from the study. A total of 35 patients were evaluated. Two of them were excluded due to not meeting the requirements for inclusion. Due to the COVID-19 epidemic, two patients opted out of volunteering. One patient’s treatment had to be halted due to a burn sustained during radiotherapy. The final study group of 30 subjects (study group = 15, control group = 15) (Fig. 1).
We documented the subjects’ demographic and clinical characteristics. Circumference measurements in the lymphedema and intact extremities, pain, joint ROM in the extremity with adhesive capsulitis, and difficulties with upper extremity use in daily life were all recorded as part of the authors’ evaluation at the start of the treatment program and after three weeks of treatment.
The VAS is important and recommended for evaluation because of its high reliability. The severity of pain was evaluated using a VAS ranging from 0 (no pain) to 10 (intolerable pain). Patients marked the level at which they felt pain. A ruler was used to measure the specified distance from the left end (cm) [14].
The rapid questionnaire of arm-shoulder-hand disability (Q-DASH) is a self-report instrument with Turkish validity and reliability that assesses physical functionality and symptoms in patients with upper extremity problems. It has eleven subjects drawn from the DASH survey. At least 10 of 11 questions must be answered in order to calculate the Q-DASH score. Each title contains 5 answer options, the score of the scale is calculated from the title scores. Additionally, a high score suggests a significant level of impairment [15].
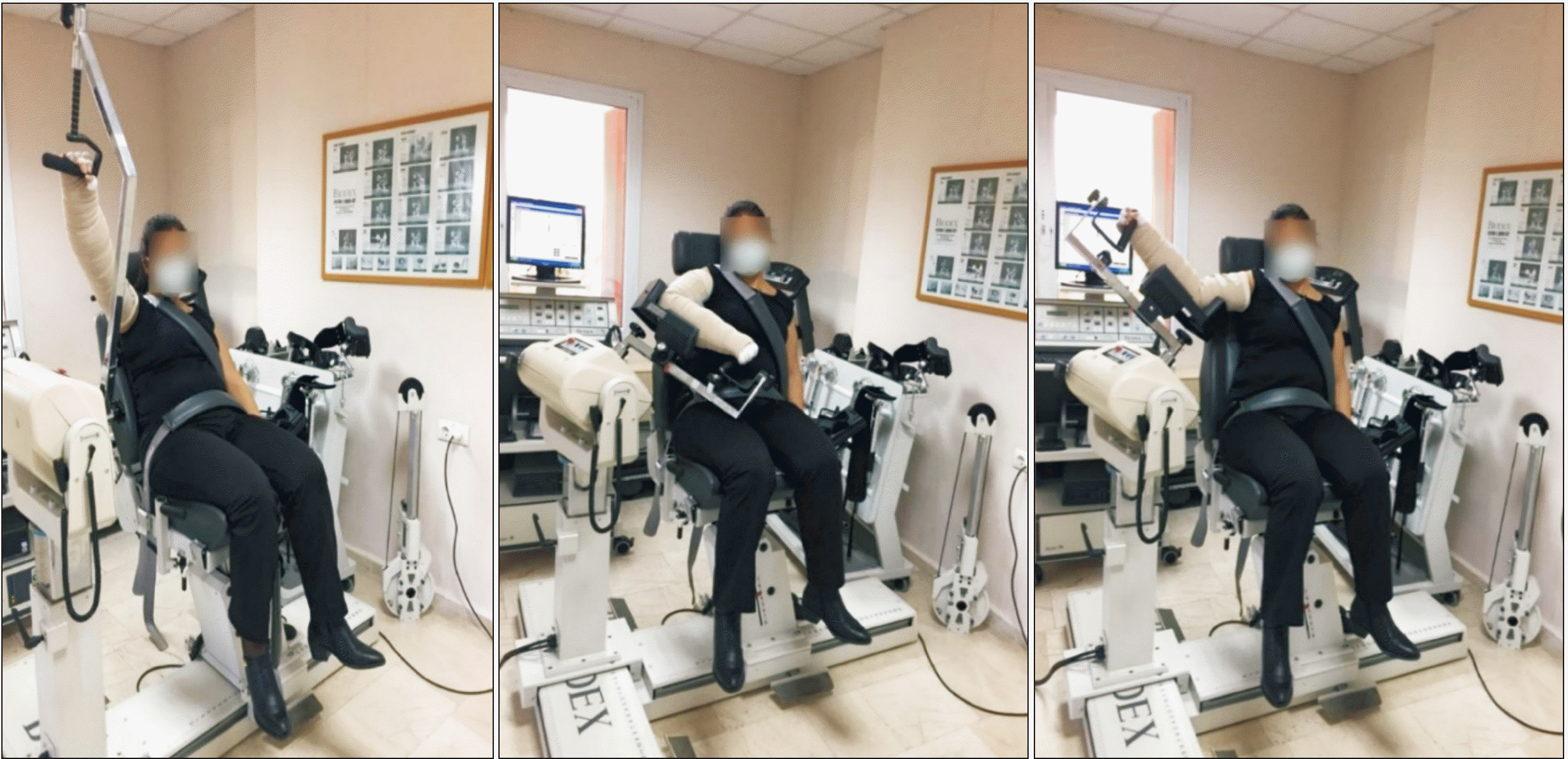
Shoulder motions were made passively on an isokinetic dynamometer manufactured by Biodex (Biodex Medical Systems, Shirley, NY). Using the isokinetic dynamometer is an accurate, highly reliable method. It allows quantitative measurement of the musculoskeletal system in terms of performance. The ROM of the joint can also be evaluated with goniometry. However, the goniometric measurement may not give precise, reliable, and clear results. Therefore, the authors evaluated the ROM with this objective method. The dynamometer adapts to the patient’s resistance during movement due to reasons such as pain and contracture. In this way, the risk of muscle pain and injury after exercise is very low [16,17]. This system includes isokinetic, isometric, isotonic, passive, and reactive eccentric exercise modes, as well as procedures for measuring proprioceptive sensory information. Concentric speeds range between 30° and 500° per second, whereas eccentric speeds range between 10° and 300° per second. The maximum torque values for concentric contraction are 500 ft-lbs, and for eccentric contraction are 300 ft-lbs. The range of rotation is 330 degrees. The Biodex isokinetic system can measure concentric activity at fast speeds and can be configured to strengthen diagonal planes. Additionally, it generates a thorough report after an isokinetic examination. Previous investigations have shown the Biodex system’s reliability and validity [18]. The patients were seated with a 90° seatback angle during the measurement. The patients were instructed to grasp the handles on the side of the chair throughout the motions. Bands were used to support the patient’s pelvis and trunk. The patient’s shoulder, elbow, and wrist were stabilized with the help of an adjustable lever arm and support device with pads. The dynamometer arm’s axis was aligned with the humeral head. The torque concerning gravity was established and verified using the computer’s software at 90°, 120°, 150°, and 180°.
Circumference measurement is a manual technique for determining the volume of the limb. It is a method that utilizes intermittent measurements of the extremity by choosing specific reference locations. A non-stretchable tape measure is used to make the measurement. It may be used to assess treatment progression before and after MLD [19]. The volumetric measurements were taken on the patients without jewelry or compression clothing on the extremity, in a container filled with room temperature water that did not overflow, with the subject in full extension from distal to proximal, and the arm sank to the axilla level. The volume of the arm was determined in milliliters by collecting the spilling water in a graduated cup [20].
For the treatment of adhesive capsulitis in both groups, for 3 weeks, 5 days a week, (a) 20 minutes of heat was applied to the shoulder joint with a hot pack, (b) 20 minutes of exercise was performed with the Biodex isokinetic exercise system, and (c) 20 minutes conventional TENS (Transcutaneous Electrical Nerve Stimulation) as applied to the shoulder joint and the painful points around it. Planned evaluations were made before and after the 3-week adhesive capsulitis treatment program [21,22].
For adhesive capsulitis treatment, 45 minutes of CDT was applied to the study group’s patients. Neck and abdominal drainage were performed using this application as part of the MLD treatment. Ventral and dorsal axillo-inguinal/axillo-axillary anastomoses were created, as well as dorsal axillo-inguinal/axillo-axillary anastomoses. Finally, MLD was finished with the application of arm drainage. Simple neck drainage and abdominal drainage were performed on the patient while he was supine with his legs flexed. Intense grips were used in conjunction with breathing on the significant lymph nodes in the abdominal region. Ventral and dorsal axillo-axillary and axillo-inguinal anastomosis paths were created, and lymph fluid was drained based on the surgery location of the patient. In patients with intact skin integrity, the thinned skin was taken care of with the help of pH-neutral, water-based, and low-fat creams. Wound care was performed on patients with compromised skin integrity using suitable medicinal creams.
Compression treatment for lymphedema was applied with the use of an inelastic, multilayered bandage. When multilayer bandaging is applied vigorously at a submaximal tension (e.g., 60 mmHg), edema develops and declines [23]. Bandaging was performed by placing a 100% cotton stockinet (under the plaster stocking) on the patient’s upper extremity, wrapping the fingers with elastomole (a finger bandage), wrapping organic cotton to absorb pressure, and wrapping short tension bandages from distal to proximal from high to low pressure.
Both groups performed passive shoulder flexion, extension, abduction, internal, and external rotation exercises using the Biodex isokinetic system. Patients in the study group were operated on while wearing a compression bandage. Thus, it was intended to improve the compression bandage’s treatment response (Fig. 2). Both groups received ROM exercises for the upper extremities and pumping exercises as a home exercise program. According to the patients’ testimony, they were followed whether they did it during the day or not.
Four minutes of flexion, 4 minutes of extension, 4 minutes of abduction, 4 minutes of internal rotation, and 4 minutes of external rotation were programmed into the isokinetic system as exercises. Each movement was performed passively at a 90° angle per second, with a 2-minute rest period.
Type I error (alpha) was taken as 0.05. The power of the test (1-beta) was found as 0.8 when the minimum sample size required for a significant difference in the resting VAS score was calculated as 10 in each group based on a previous study [24]. IBM SPSS Statistics 23.0 (IBM Co., Armonk, NY) was used for statistical analysis. Numerical data were expressed as arithmetic mean and standard deviation. For non-numerical data, frequency values were calculated as percentages (%). The Mann–Whitney U-test was used to compare non-parametric and non-normally distributed data across groups (pain, normal ROM, volumetric measurements, DASH scores). The independent sample t-test was used to compare parametric data (age, circumference measures) among groups. In intergroup comparisons, the paired t-test was used where the data were normally distributed and the Wilcoxon signed-rank test was utilized when the data were not normally distributed. A P value ≤ 0.05 was considered statistically significant.
The mean age of the patients was 46.3 ± 9.2 years. The body mass index (BMI) of the control group and the study group were 28.8 ± 5.7 kg/m2 and 28.5 ± 5.2 kg/m2 respectively. As shown in Table 1, the age and BMI findings of the groups were similar. The dominant sides of most patients were the right side. It was determined that the affected side was the left side with a 60% ratio. While 40% of the patients did not have a comorbid disease (diabetes, hypertension, hypotension), 60% did. It was determined that the most crucial accompanying complaint of 93.3% of the patients, who had 50% mastectomy and 50% lumpectomy, was pain.
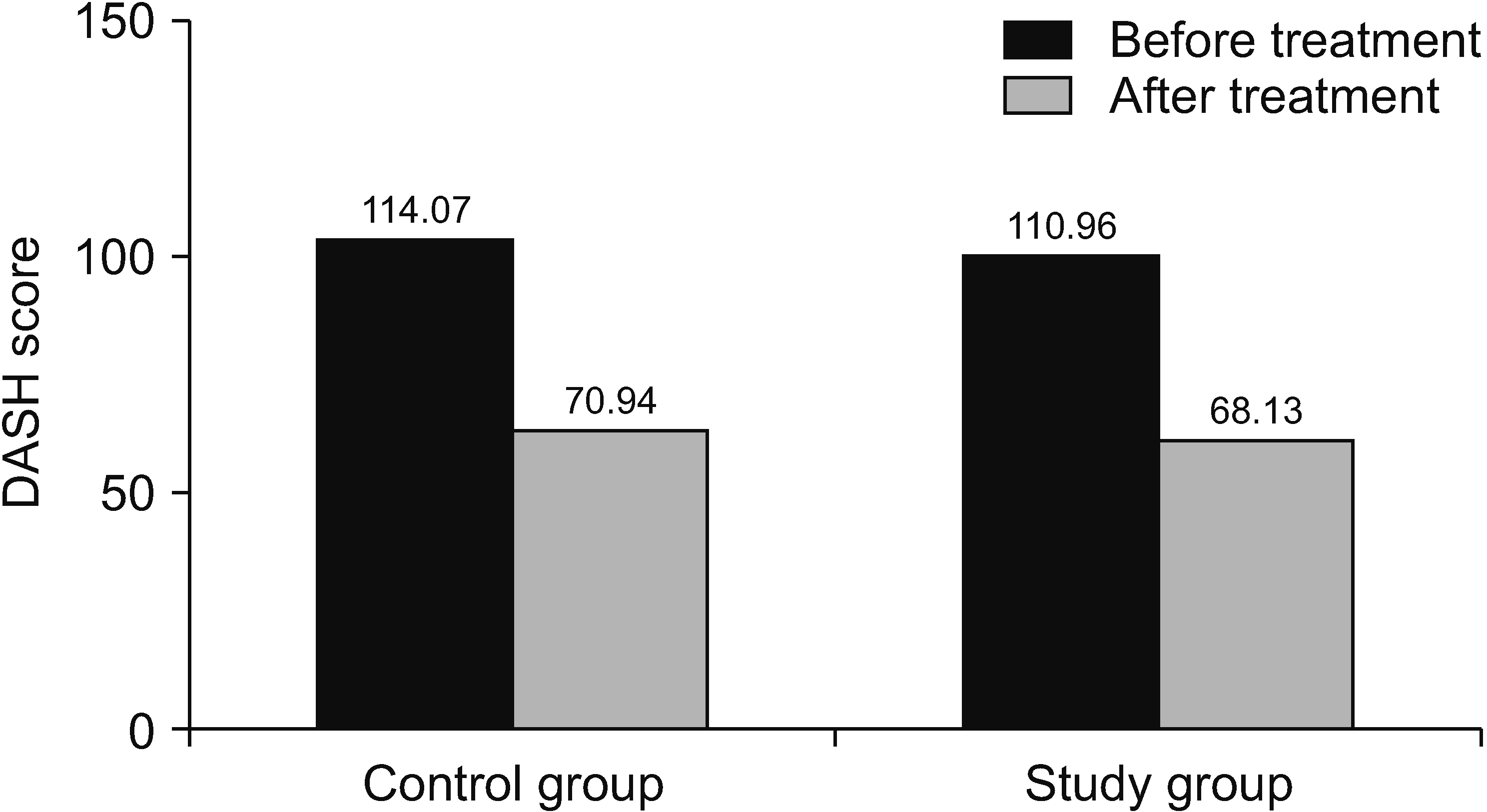
In the DASH questionnaire in which upper extremity problems were evaluated, there was a significant decrease in the scores of both groups (Fig. 4). The ROM assessment values showed increases in both groups when compared to pre-treatment values in all aspects (P = 0.520). Initial assessments were given in Table 3.
There was no significant change in the volumetric measurements of the control group when compared to pre-treatment values (P = 0.655). However, there was a significant improvement in the study group’s post-treatment volumetric measurement values (P = 0.001). There was no significant difference in the post-treatment values of the individuals in the control group when the findings of the edema assessment using circumference measurements were compared to the pre-treatment values. All circumference measures of participants in the study group showed significant improvements when compared to pre-treatment values (Table 4).
The DASH scores were compared between the groups after the treatment and it was seen that both groups had similar scores. It was established that both groups improved similarly in terms of pain when the values for night pain, activity pain, and rest pain were compared between the pre- and post-treatment values. Table 2 summarizes the results.
In the pre-treatment values of the groups, there was no difference in any circumference measurements except the elbow circumference. Wrist and forearm circumference measurements across the groups revealed an improvement in favor of the study group (P = 0.005, P = 0.035, respectively), although elbow and arm circumference measurements after treatment revealed no difference between the groups (P = 0.982, P = 0.967) (Table 2).
When the joint ROM values of the two groups were compared after treatment, no difference in flexion, extension, and abduction movements was seen between the groups (P = 0.166, P = 0.110, P = 0.901, respectively). All findings are given in Table 3. The study group improved more rapidly than the control group in external and internal rotation movements (P = 0.001).
This study investigated the effects of CDT applications on pain and upper extremity function in patients with breast cancer who developed adhesive capsulitis following lymphedema. It was found that the applications and isokinetic exercises performed on this patient group decreased pain, and improved shoulder ROM and functionality.
In the previous studies, higher lymphedema risk in patients with breast cancer was stated as being at around 50 years [25,26]. Haydaroğlu et al. [27] stressed the importance of advanced age as a risk factor for lymphedema. De Vrieze et al. [28] reported that advanced age is a risk factor for lymphedema, owing to reduced physical activity. This study was likewise conducted in the age group identified by De Vrieze et al. [28] as having a higher level of physical activity. The mean age of the patients in this study was 46 years, which is consistent with the literature.
The standard of care for lymphedema is a multimodal decongestive therapy regimen that includes MLD, skincare, compression bandaging, and exercise. The CDT has been shown to improve extremity volume, pain, and quality of life in patients who develop lymphedema due to breast cancer [29]. Kim et al. [30] reported a reduction in lymphedema volume with CDT. The present study found a significant reduction in edema and an increase in functionality in patients who received CDT over pre-treatment, which is consistent with the literature. Increased functionality may be an increase of a reduction in the edema burden on the extremity.
In a study by Yesil et al. [31], which examined the effectiveness of CDT and other treatment modalities in patients with lymphedema, it was reported that therapies coupled with CDT may considerably decrease extremity volume. Although isokinetic exercises performed with the Biodex isokinetic system resulted in significant improvements in pain, functionality, and joint ROM in both the control and study groups in this study, that the significant improvement in edema was found only in the study group emphasizes the inclusion of CDT in the treatment protocol.
Oh et al. [32] investigated the effectiveness of compression bandages in patients who developed lymphedema due to breast cancer and found a significant improvement in DASH scores after CDT. In line with the study of Oh et al. [32] the authors found significant improvements in DASH scores in the study group after 3 weeks of CDT. Smoot et al. [2] evaluated the shoulder functions of patients with and without lymphedema in the post-breast cancer period using the DASH questionnaire and found that the DASH score was significantly higher in the lymphedema group than in the non-lymphedema group. In the study by Park et al. [33], the impact of CDT on upper extremity function in lymphedema was evaluated using the DASH questionnaire. According to the DASH questionnaire scores, the lymphedema patient’s upper extremity functions were impaired with increasing age. After CDT, circumference measurements of limbs with lymphedema decreased significantly [33]. However, significant improvements in both groups’ DASH scores were seen in this study. This boost in shoulder functionality may have been achieved by raising ROM values and significantly reducing VAS scores.
Magens discovered that the volumetric measurement employed in the diagnosis and follow-up of lymphedema had an extremely high degree of reliability [34]. Morgan et al. [35] reported that after four weeks of CDT treatment, the volume decreased by more than 50% in the first two weeks in 78 female patients who had lymphedema owing to breast cancer. In addition, volumetric analysis was compared to volumetric measurement utilizing the circumference measurement technique in patients with and without lymphedema after breast cancer surgery in a study by Meijer et al. [36]. They reported that the water displacement technique was the gold standard for evaluating lymphedema. In addition, Johansson et al. [37] reported in their randomized controlled study on the efficacy of compression garments in patients having breast cancer surgery that a 7% decrease in volumetric measurements taken in all patients during the first two weeks improved upper extremity functioning. Cormier et al. [38] discovered that even a 5% reduction in volume improved upper extremity functionality and a decrease in lymphedema symptoms in their prospective study. In this study, the authors used volumetric measurement to determine volume changes while adhering to cleanliness standards. The findings indicate that volumetric decrease is associated with an increase in functionality in both groups.
In their literature review on quality of life questionnaires in patients with breast cancer-related lymphedema, the VAS is critical in assessing the person since it has a broad range of scores, is extensively used in pain evaluation, and is subjective [38]. Ha et al. [39] compared patients with mastectomy-induced lymphedema and ROM limitation in the study group (MLD and exercise) to patients in the control group (MLD). While both groups saw a significant drop in the VAS score, the MLD and exercise groups experienced a higher decline. The present study also utilized VAS to assess pain, since it has been used before and has a low error rate.
In a study conducted by Sezgin Ozcan et al. [40], they examined the effects of CDT on upper extremity functioning, pain intensity, and quality of life. They discovered that VAS scores reduced significantly after CDT. In addition, Mobarakeh et al. [41] identified significant decreases in VAS severity in their study, which sought to assess the effectiveness of CDT and the minimal number of sessions necessary to significantly reduce pain in patients with breast cancer-related lymphedema. In their two-group study, Melam et al. [42] found significant improvement in both groups’ VAS scores at the 4th and 6th weeks after treatment using CDT and MLD solely. In addition, the researchers reported that the CDT group saw a more significant reduction in pain than the group treated with MLD alone. In contrast to the general literature, the authors evaluated the VAS score in three distinct ways in this study: at rest, during exercise, and at night. During the post-treatment examination, it was noticed that both groups had a significant reduction in pain during activity, rest, and sleep.
Upper extremity functionality diminishes, and certain limits occur due to physiological issues such as pain, muscular weakness, and lymphedema after breast cancer surgery [43]. Limitations in shoulder ROM after breast cancer surgery resulted in a loss of functionality and became issues affecting the patient’s quality of life [8]. According to Beaulac et al. [44], ROM problems, which effect upper extremity functions, are caused by lymphedema. When the studies were analyzed, it was shown that most shoulder ROM was in the abduction direction and that ROM restriction was related to upper extremity impairment [2]. Levangie and Drouin [45] evaluated the long-term effects of breast cancer treatment on shoulder function and discovered that axillary lymph node removal and radiotherapy influenced the pectoral muscles. They discovered that this condition significantly limits shoulder flexion and abduction directions. Smoot et al. [2] evaluated shoulder ROM in their study and found no reduction in abduction or flexion ROM, as well as no significant difference in external and internal rotation angular values. According to the literature, there were primarily restrictions in the present study’s flexion and abduction components of the pre-treatment values. While both groups improved significantly after treatment, the study group improved significantly more in exterior and internal rotation movements. When shoulder movements are compared internally, it is clear that internal and exterior rotation movements are more sophisticated and harder to execute [46]. The authors believe that the reduction of lymphedema caused by CDT treatment improved internal and exterior rotation movements in the study group.
Isokinetic dynamometers are very reliable instruments that provide doctors with measurement data [47]. Abd Elhameed et al. [48] examined changes in impacted and unaffected shoulder movements in various age groups after unilateral mastectomy using the isokinetic system. According to Fong et al. [49], the reduction in flexion, adduction, and internal rotation after breast cancer surgery may be related to nerve injury to the pectoral and latissimus dorsi muscles, which are responsible for these movements. Additionally, they claimed that it might result from manipulation of the pectoral muscles and possibly harm the pectoral nerves during mastectomy. In their study, Fong et al. [49] found a reduction of strength in the flexion, extension, abduction, as well as internal and exterior rotation directions after breast cancer surgery; however, no significant difference in the ROM assessments was found. This study assessed limitations in shoulder movements in the flexion, extension, abduction, as well as internal and external rotation directions using joint ROM tests performed prior to treatment. Passive shoulder ROM exercises in both directions was performed using the isokinetic system, which responds to the shoulder joint’s mechanical qualities. Following treatment, biodex isokinetic dynamometer studies revealed a significant improvement in the limitations. Liszka and Samborski [22] demonstrated in their study that dynamic shoulder assessment findings in patients who had undergone breast cancer surgery showed a decline in the features of the muscle groups responsible for shoulder joint function. Following treatment with the Biodex isokinetic system, a significant improvement in the internal and exterior rotation ROM was seen. After treatment with exercises performed in the Biodex isokinetic system, the authors saw a significant improvement in all elements of ROM assessments. The study group achieved a more significant increase in internal and external rotation using isokinetic exercises coupled with CDT, consistent with Liszka and Samborski [22].
As a result, the authors discovered that CDT and isokinetic exercises enhance pain, ROM, and function. For example, the authors found that the number of studies evaluating the isokinetic properties of the shoulders in patients who develop lymphedema after breast cancer treatment is highly restricted. In this study, passive exercises were performed with the Biodex isokinetic system at a constant angular velocity of 90 degrees. The aim was to provide a more objective method to exercise at the “same speed and time” for everyone. This study is unique in that it uses original and objective assessments, and it may serve as a model for future research in this area.
The current study has some limitations. First, it was a single-center study with a small sample size. Second, the authors could not determine exactly when the limitation developed in the shoulder joint ROM after surgery in the participants of the study group. Third, because of the COVID-19 pandemic there was a small volunteer patient group who wanted to receive treatment. The fourth one is related to the pharmalogical treatment differences of patients. They had been receiving different doses of different medicines. The DASH questionnaire was not developed specifically for the shoulder problems after breast cancer treatment. It is a general questionnare for shoulder functionality and symptoms in patients with upper extremity problems.
In conclusion, this study emphasizes the relevance of isokinetic exercises in preventing joint limitations in breast cancer patients by demonstrating that upper extremity dysfunction may be connected with breast cancer and its treatment. Exercises performed in an isokinetic system might also be advised to motivate patients by requiring their active involvement. According to the study’s findings, CDT applications effectively reduce lymphedema in patients with breast cancer who develop adhesive capsulitis due to lymphedema. Therefore, the authors believe that including CDT in addition to standard physiotherapy will be beneficial.
Notes
DATA AVAILABILITY
Data files are available from Harvard Dataverse: https://doi.org/10.7910/DVN/13PPLB.
REFERENCES
1. Badger C, Preston N, Seers K, Mortimer P. 2004; Physical therapies for reducing and controlling lymphoedema of the limbs. Cochrane Database Syst Rev. (4):CD003141. DOI: 10.1002/14651858.CD003141.pub2. PMCID: PMC8407243. PMID: 15495042.

2. Smoot B, Wong J, Cooper B, Wanek L, Topp K, Byl N, et al. 2010; Upper extremity impairments in women with or without lymphedema following breast cancer treatment. J Cancer Surviv. 4:167–78. DOI: 10.1007/s11764-010-0118-x. PMID: 20373044. PMCID: PMC2882040.

3. Bakar Y, Berdici B, Şahin N, Pala ÖO. 2014; Lymphedema after breast cancer and its treatment. J Breast Health. 10:6–14. Turkish. DOI: 10.5152/tjbh.2014.1651.
4. Özdemir ÖÇ, Yildirim B, Köse E. 2020; Alternative treatment methods in lymphedema. Izmir Democr Univ Health Sci J. 3:1–7. Turkish. DOI: 10.5152/tjbh.2014.1651.
5. Hidding JT, Beurskens CH, van der Wees PJ, van Laarhoven HW, Nijhuis-van der Sanden MW. 2014; Treatment related impairments in arm and shoulder in patients with breast cancer: a systematic review. PLoS One. 9:e96748. DOI: 10.1371/journal.pone.0096748. PMID: 24816774. PMCID: PMC4016041. PMID: a65e04e692b14b38b242177fad5cb8e8.

6. Bosompra K, Ashikaga T, O'Brien PJ, Nelson L, Skelly J. 2002; Swelling, numbness, pain, and their relationship to arm function among breast cancer survivors: a disablement process model perspective. Breast J. 8:338–48. DOI: 10.1046/j.1524-4741.2002.08603.x. PMID: 12390356.

7. Selçuk B, Dalyan M, İnanır M, Akyüz M, Çakcı A. 2001; Musculoskeletal problems of upper extremities in patients with mastectomy and axillary dissection. Turk J Phys Med Rehabil. 47:38–46. Turkish. DOI: 10.1046/j.1524-4741.2002.08603.x.
8. Rietman JS, Dijkstra PU, Debreczeni R, Geertzen JH, Robinson DP, De Vries J. 2004; Impairments, disabilities and health related quality of life after treatment for breast cancer: a follow-up study 2.7 years after surgery. Disabil Rehabil. 26:78–84. DOI: 10.1080/09638280310001629642. PMID: 14668143.

9. McCredie MR, Dite GS, Porter L, Maskiell J, Giles GG, Phillips KA, et al. 2001; Prevalence of self-reported arm morbidity following treatment for breast cancer in the Australian Breast Cancer Family Study. Breast. 10:515–22. DOI: 10.1054/brst.2000.0291. PMID: 14965632.

10. Voogd AC, Ververs JM, Vingerhoets AJ, Roumen RM, Coebergh JW, Crommelin MA. 2003; Lymphoedema and reduced shoulder function as indicators of quality of life after axillary lymph node dissection for invasive breast cancer. Br J Surg. 90:76–81. DOI: 10.1002/bjs.4010. PMID: 12520579.

11. Højris I, Andersen J, Overgaard M, Overgaard J. 2000; Late treatment-related morbidity in breast cancer patients randomized to postmastectomy radiotherapy and systemic treatment versus systemic treatment alone. Acta Oncol. 39:355–72. DOI: 10.1080/028418600750013131. PMID: 10987233.

12. Hardwick DH, Beebe JA, McDonnell MK, Lang CE. 2006; A comparison of serratus anterior muscle activation during a wall slide exercise and other traditional exercises. J Orthop Sports Phys Ther. 36:903–10. DOI: 10.2519/jospt.2006.2306. PMID: 17193867.

13. Lauridsen MC, Overgaard M, Overgaard J, Hessov IB, Cristiansen P. 2008; Shoulder disability and late symptoms following surgery for early breast cancer. Acta Oncol. 47:569–75. DOI: 10.1080/02841860801986627. PMID: 18465324.

14. Gummesson C, Atroshi I, Ekdahl C. 2003; The disabilities of the arm, shoulder and hand (DASH) outcome questionnaire: longitudinal construct validity and measuring self-rated health change after surgery. BMC Musculoskelet Disord. 4:11. DOI: 10.1186/1471-2474-4-11. PMID: 12809562. PMCID: PMC165599.

15. Frisiello S, Gazaille A, O'Halloran J, Palmer ML, Waugh D. 1994; Test-retest reliability of eccentric peak torque values for shoulder medial and lateral rotation using the Biodex isokinetic dynamometer. J Orthop Sports Phys Ther. 19:341–4. DOI: 10.2519/jospt.1994.19.6.341. PMID: 8025574.

16. Habets B, Staal JB, Tijssen M, van Cingel R. 2018; Intrarater reliability of the Humac NORM isokinetic dynamometer for strength measurements of the knee and shoulder muscles. BMC Res Notes. 11:15. DOI: 10.1186/s13104-018-3128-9. PMID: 29321059. PMCID: PMC5764011.

17. Palmer TB, Blinch J, Farrow AC, Agu-Udemba CC, Mitchell EA. 2020; Real-time measurement of isometric peak torque and rate of torque development using a novel strength testing device: a validity and reliability study. Physiol Meas. 41:115005. DOI: 10.1088/1361-6579/abc40b. PMID: 33091881.

18. Bérard A, Kurz X, Zuccarelli F, Ducros JJ, Abenhaim L. 1998; Reliability study of the Leg-O-Meter, an improved tape measure device, in patients with chronic venous insufficiency of the leg. VEINES Group.(Venous Insufficiency Epidemiologic and Economic Study). Angiology. 49:169–73. DOI: 10.1177/000331979804900301. PMID: 9523538.
19. Sander AP, Hajer NM, Hemenway K, Miller AC. 2002; Upper-extremity volume measurements in women with lymphedema: a comparison of measurements obtained via water displacement with geometrically determined volume. Phys Ther. 82:1201–12. DOI: 10.1093/ptj/82.12.1201. PMID: 12444879.

20. French RM. 2011. The complete guide to lymph drainage massage. 2nd ed. Cengage Learning;Clifton Park: https://www.worldcat.org/title/complete-guide-to-lymph-drainage-massage/oclc/1086247292?referer=br&ht=edition. DOI: 10.1093/ptj/82.12.1201.
21. Calis M, Demir H, Ulker S, Kirnap M, Duygulu F, Calis HT. 2006; Is intraarticular sodium hyaluronate injection an alternative treatment in patients with adhesive capsulitis? Rheumatol Int. 26:536–40. DOI: 10.1007/s00296-005-0022-2. PMID: 16091920.

22. Liszka M, Samborski W. 2018; Assessment of biomechanical parameters of the shoulder joint at the operated side versus non-operated side in patients treated surgically for breast cancer. Rep Pract Oncol Radiother. 23:378–83. DOI: 10.1016/j.rpor.2018.07.001. PMID: 30127678. PMCID: PMC6097393.

23. Kayıhan H, Dolunay N. 1992. Heat-light and hydrotherapy in physiotherapy. Hacettepe University;Ankara: Turkish. DOI: 10.1007/s00296-005-0022-2.
24. Malicka I, Marciniak D. 2015; The effects of complete decongestive therapy on the extent of secondary lymphoedema in women after breast cancer treatment. Fizjoterapia. 23:3–8. Polish. DOI: 10.1515/physio-2015-0001.
25. De Vrieze T, Gebruers N, Nevelsteen I, Tjalma WAA, Thomis S, De Groef A, et al. 2020; Physical activity level and age contribute to functioning problems in patients with breast cancer-related lymphedema: a multicentre cross-sectional study. Support Care Cancer. 28:5717–31. DOI: 10.1007/s00520-020-05375-3. PMID: 32193692.

26. Geller BM, Vacek PM, O'Brien P, Secker-Walker RH. 2003; Factors associated with arm swelling after breast cancer surgery. J Womens Health (Larchmt). 12:921–30. DOI: 10.1089/154099903770948159. PMID: 14670172.

27. Haydaroğlu A, Dubova S, Özsaran Z, Bölükbaşı Y, Yılmaz R, Kapkaç M, et al. 2005; Breast cancer in Ege University "evaluation of 3897 cases". J Breast Health. 1:6–11. Turkish. DOI: 10.1089/154099903770948159.
28. De Vrieze T, Gebruers N, Devoogdt N. 2018; Concerning quality of life questionnaires in breast cancer-related lymphedema patients: review of the literature by Cornelissen et al. Lymphat Res Biol. 16:421–2. DOI: 10.1089/lrb.2018.0024. PMID: 30130161.

29. Didem K, Ufuk YS, Serdar S, Zümre A. 2005; The comparison of two different physiotherapy methods in treatment of lymphedema after breast surgery. Breast Cancer Res Treat. 93:49–54. DOI: 10.1007/s10549-005-3781-2. PMID: 16184458.

30. Kim SJ, Yi CH, Kwon OY. 2007; Effect of complex decongestive therapy on edema and the quality of life in breast cancer patients with unilateral leymphedema. Lymphology. 40:143–51. PMID: 18062617.
31. Yesil H, Eyigor S, Caramat İ, Isık R. 2019; Comparison of four different therapy protocols on extremity volume in breast cancer related lymphedema. Medeni Med J. 34:7–14. https://medeniyetmedicaljournal.org/jvi.aspx?pdir=medeniyet&plng=eng&un=MEDJ-26657. DOI: 10.5222/MMJ.2019.26657.

32. Oh SH, Ryu SH, Jeong HJ, Lee JH, Sim YJ. 2019; Effects of different bandaging methods for treating patients with breast cancer-related lymphedema. Ann Rehabil Med. 43:677–85. DOI: 10.5535/arm.2019.43.6.677. PMID: 31918530. PMCID: PMC6960079. PMID: d0293edd2a6840c3b406eb887773adc6.

33. Park JH, Lee WH, Chung HS. 2008; Incidence and risk factors of breast cancer lymphoedema. J Clin Nurs. 17:1450–9. DOI: 10.1111/j.1365-2702.2007.02187.x. PMID: 18482142.

34. Engler HS, Sweat RD. 1962; Volumetric arm measurements: technique and results. Am Surg. 28:465–8. PMID: 13890278.
35. Morgan RG, Casley-Smith JR, Mason MR, Casley-Smith JR. 1992; Complex physical therapy for the lymphoedematous arm. J Hand Surg Br. 17:437–41. DOI: 10.1016/S0266-7681(05)80270-0. PMID: 1402274.

36. Meijer RS, Rietman JS, Geertzen JH, Bosmans JC, Dijkstra PU. 2004; Validity and intra- and interobserver reliability of an indirect volume measurements in patients with upper extremity lymphedema. Lymphology. 37:127–33. PMID: 15560108.
37. Johansson K, Lie E, Ekdahl C, Lindfeldt J. 1998; A randomized study comparing manual lymph drainage with sequential pneumatic compression for treatment of postoperative arm lymphedema. Lymphology. 31:56–64. PMID: 9664269.
38. Cormier JN, Xing Y, Zaniletti I, Askew RL, Stewart BR, Armer JM. 2009; Minimal limb volume change has a significant impact on breast cancer survivors. Lymphology. 42:161–75. DOI: 10.3410/f.12969957.14320054. PMID: 20218084. PMCID: PMC2882028.
39. Ha KJ, Lee SY, Lee H, Choi SJ. 2017; Synergistic effects of proprioceptive neuromuscular facilitation and manual lymphatic drainage in patients with mastectomy-related lymphedema. Front Physiol. 8:959. DOI: 10.3389/fphys.2017.00959. PMID: 29234287. PMCID: PMC5712373.

40. Sezgin Ozcan D, Dalyan M, Unsal Delialioglu S, Duzlu U, Polat CS, Koseoglu BF. 2018; Complex decongestive therapy enhances upper limb functions in patients with breast cancer-related lymphedema. Lymphat Res Biol. 16:446–52. DOI: 10.1089/lrb.2017.0061. PMID: 29356592.

41. Mobarakeh ZS, Mokhtari-Hesari P, Lotfi-Tokaldany M, Montazeri A, Heidari M, Zekri F. 2019; Combined decongestive therapy and reduction of pain and heaviness in patients with breast cancer-related lymphedema. Support Care Cancer. 27:3805–11. DOI: 10.1007/s00520-019-04681-9. PMID: 30729334.

42. Melam GR, Buragadda S, Alhusaini AA, Arora N. 2016; Effect of complete decongestive therapy and home program on health- related quality of life in post mastectomy lymphedema patients. BMC Womens Health. 16:23. DOI: 10.1186/s12905-016-0303-9. PMID: 27145867. PMCID: PMC4855407.

43. Haddad CA, Saad M, Perez Mdel C, Miranda Júnior F. 2013; Assessment of posture and joint movements of the upper limbs of patients after mastectomy and lymphadenectomy. Einstein (Sao Paulo). 11:426–34. DOI: 10.1590/S1679-45082013000400004. PMID: 24488379. PMCID: PMC4880377.
44. Beaulac SM, McNair LA, Scott TE, LaMorte WW, Kavanah MT. 2002; Lymphedema and quality of life in survivors of early-stage breast cancer. Arch Surg. 137:1253–7. DOI: 10.1001/archsurg.137.11.1253. PMID: 12413312.

45. Levangie PK, Drouin J. 2009; Magnitude of late effects of breast cancer treatments on shoulder function: a systematic review. Breast Cancer Res Treat. 116:1–15. DOI: 10.1007/s10549-008-0246-4. PMID: 19031114.

46. Inman VT, Saunders JBDM, Abbott LC. 1944; Observations on the function of the shoulder joint. J Bone Joint Surg. 26:1–30. https://journals.lww.com/jbjsjournal/Citation/1944/26010/OBSERVATIONS_ON_THE_FUNCTION_OF_THE_SHOULDER.1.aspx.
47. Ellenbecker TS, Cools A. 2010; Rehabilitation of shoulder impingement syndrome and rotator cuff injuries: an evidence-based review. Br J Sports Med. 44:319–27. DOI: 10.1136/bjsm.2009.058875. PMID: 20371557.

48. Abd Elhameed REED, Ibrahim ZM, Nosier AA. 2015; Evaluation of isokientic activities of shoulder muscles post mastectomy in different age groups. J Med Sci Clin Res. 3:5359–69. https://jmscr.igmpublication.org/v3-i4/40jmscr.pdf.
49. Fong SS, Ng SS, Luk WS, Chung JW, Chung LM, Tsang WW, et al. 2013; Shoulder mobility, muscular strength, and quality of life in breast cancer survivors with and without Tai Chi Qigong training. Evid Based Complement Alternat Med. 2013:787169. DOI: 10.1155/2013/787169. PMID: 23710237. PMCID: PMC3655570.

Fig. 3
Healing in pain scores of groups. VAS: visual analogue scale, CG: control group, SG: study group, BT: before treatment, AT: after treatment.
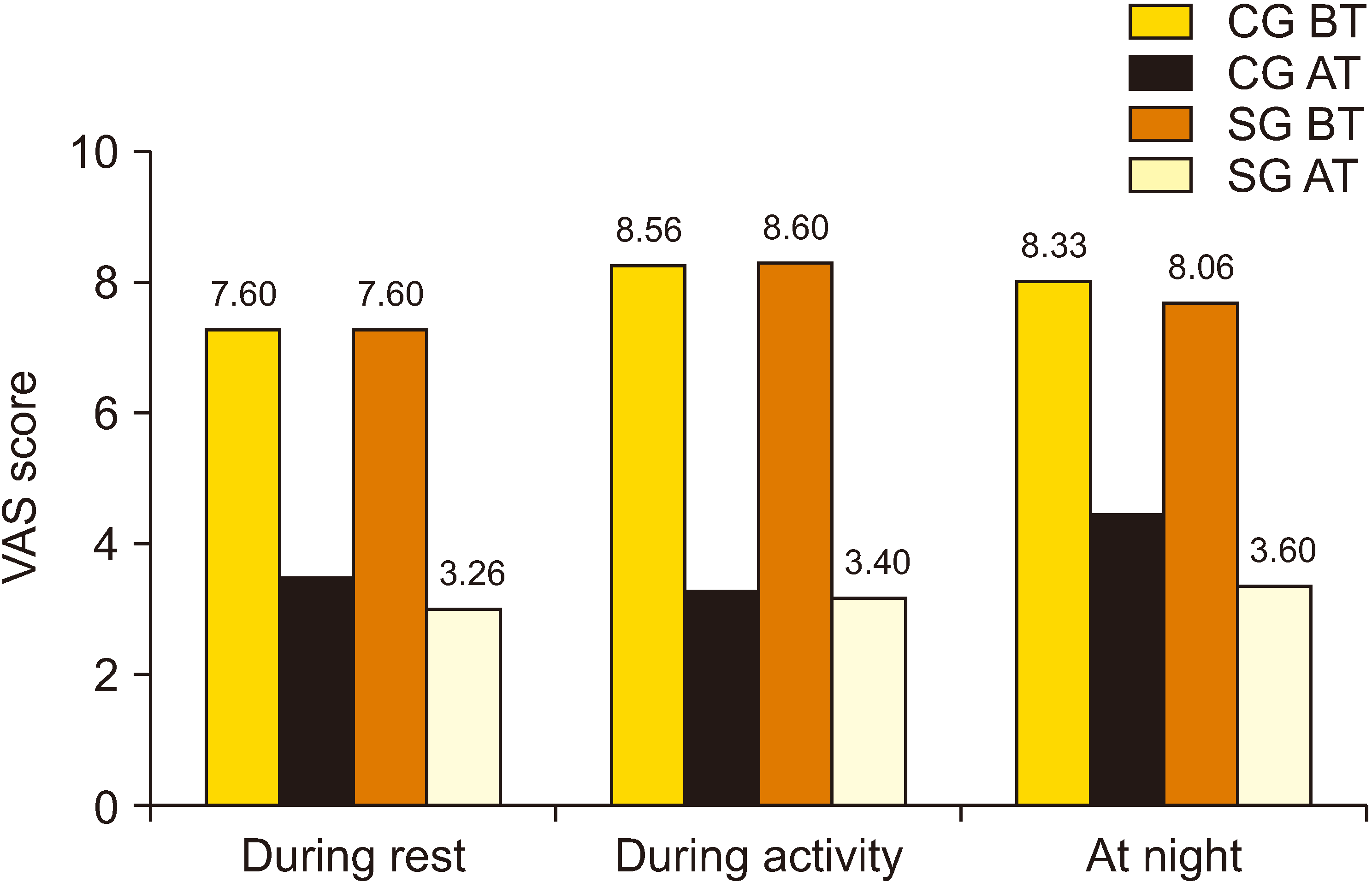
Table 1
Baseline characteristics for both groups
Table 2
Comparison of the groups and intergroups in terms of DASH scores and pain levels before and after treatment
| Score | Control group (n = 15) | Study group (n = 15) | BT | AT | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BT | AT | BT | AT | z | P value | z | P value | ||||
| VAS (R) | 7.6 ± 1.1 | 3.7 ± 1.7 | 7.6 ± 1.5 | 3.2 ± 1.7 | –0.085 | 0.932a | –0.842 | 0.400a | |||
| P value | < 0.001b | < 0.001b | |||||||||
| VAS (A) | 8.4 ± 1.0 | 3.5 ± 2.2 | 8.6 ± 1.3 | 3.4 ± 1.8 | –0.644 | 0.688a | –0.401 | 0.932a | |||
| P value | < 0.001b | < 0.001b | |||||||||
| VAS (N) | 8.3 ± 1.8 | 4.7 ± 2.0 | 8.0 ± 1.9 | 3.6 ± 1.7 | –0.780 | 0.780a | –1.731 | 0.084a | |||
| P value | < 0.001b | < 0.001b | |||||||||
| DASH | 114.0 ± 8.3 | 70.9 ± 9.7 | 110.9 ± 12.2 | 68.1 ± 12.1 | –0.478 | 0.633a | –0.644 | 0.520a | |||
| P value | 0.001b | 0.001b | |||||||||
Table 3
Comparison of the groups and intergroups in terms of joint range of motion
| Motions | Control group (n = 15) | Study group (n = 15) | BT | AT | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BT | AT | BT | AT | Z | P value | Z | P value | ||||
| Flexion | 71.93 ± 16.09 | 158.66 ± 19.31 | 76.13 ± 14.29 | 167.26 ± 14.79 | –0.668 | 0.504 | –1.385 | 0.166 | |||
| P value | 0.001b | 0.001b | |||||||||
| Abduction | 31.86 ± 3.97 | 43.33 ± 2.43 | 25.66 ± 7.50 | 43.00 ± 4.03 | –2.371 | 0.058 | –0.125 | 0.901 | |||
| P value | 0.001b | 0.001b | |||||||||
| Extension | 82.80 ± 11.57 | 167.86 ± 13.61 | 79.26 ± 9.27 | 172.53 ± 12.28 | –1.045 | 0.296 | –1.600 | 0.110 | |||
| P value | 0.001b | 0.001b | |||||||||
| External rotation | 26.40 ± 7.88 | 40.53 ± 2.85 | 26.80 ± 8.81 | 63.86 ± 13.14 | –0.212 | 0.851 | –3.445 | 0.001a | |||
| P value | 0.001b | 0.001b | |||||||||
| Internal rotation | 27.06 ± 8.86 | 44.93 ± 5.39 | 27.66 ± 8.97 | 65.06 ± 13.44 | –0.188 | 0.832 | –4.040 | 0.001a | |||
| P value | 0.001b | 0.001b | |||||||||
Table 4
Comparison of the groups and intergroups in terms of volumetric measurements
| Measurement | Control group (n = 15) | Study group (n = 15) | BT | AT | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BT | AT | BT | AT | z | P value | z | P value | |||||
| Wrist circumference (cm) | 21.66 ± 3.73 | 21.46 ± 3.52 | 19.88 ± 2.74 | 17.95 ± 2.79 | 1.494 | 0.147 | 3.025 | 0.005a | ||||
| P value | 0.189 | 0.001c | ||||||||||
| Forearm circumference (cm) | 27.80 ± 3.72 | 27.60 ± 3.68 | 27.98 ± 3.99 | 24.64 ± 3.61 | –0.132 | 0.896 | 2.218 | 0.035a | ||||
| P value | 0.189 | 0.001c | ||||||||||
| Elbow circumference (cm) | 30.33 ± 4.20 | 30.20 ± 4.07 | 34.35 ± 4.86 | 30.16 ± 4.08 | –2.422 | 0.022a | 0.022 | 0.982 | ||||
| P value | 0.334 | 0.001c | ||||||||||
| Arm circumference (cm) | 33.63 ± 4.63 | 33.40 ± 4.27 | 37.06 ± 5.61 | 33.46 ± 4.46 | –1.826 | 0.079 | –0.042 | 0.967 | ||||
| P value | 0.264 | 0.001c | ||||||||||
| Upper extremity circumference (mL) | 224.26 ± 26.64 | 221.73 ± 26.90 | 338.66 ± 81.29 | 249.80 ± 43.08 | –3.989 | 0.001b | –1.941 | 0.045b | ||||
| P value | 0.655 | 0.001d | ||||||||||




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download