1. Thuret S, Moon LD, Gage FH. 2006; Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 7:628–43. Erratum in: Nat Rev Neurosci 2006; 7: 902. DOI:
10.1038/nrn1955. PMID:
16858391.

2. Fakhri S, Dargahi L, Abbaszadeh F, Jorjani M. 2019; Effects of astaxanthin on sensory-motor function in a compression model of spinal cord injury: Involvement of ERK and AKT signalling pathway. Eur J Pain. 23:750–64. DOI:
10.1002/ejp.1342. PMID:
30427581.

3. Fakhri S, Dargahi L, Abbaszadeh F, Jorjani M. 2018; Astaxanthin attenuates neuroinflammation contributed to the neuropathic pain and motor dysfunction following compression spinal cord injury. Brain Res Bull. 143:217–24. DOI:
10.1016/j.brainresbull.2018.09.011. PMID:
30243665.

4. Naseem M, Parvez S. 2014; Role of melatonin in traumatic brain injury and spinal cord injury. ScientificWorldJournal. 2014:586270. DOI:
10.1155/2014/586270. PMID:
25587567. PMCID:
PMC4283270.

5. Esposito E, Genovese T, Caminiti R, Bramanti P, Meli R, Cuzzocrea S. 2008; Melatonin regulates matrix metalloproteinases after traumatic experimental spinal cord injury. J Pineal Res. 45:149–56. DOI:
10.1111/j.1600-079X.2008.00569.x. PMID:
18298463.

6. Jia Z, Zhu H, Li J, Wang X, Misra H, Li Y. 2012; Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal Cord. 50:264–74. DOI:
10.1038/sc.2011.111. PMID:
21987065.

7. Faden AI, Wu J, Stoica BA, Loane DJ. 2016; Progressive inflammation-mediated neurodegeneration after traumatic brain or spinal cord injury. Br J Pharmacol. 173:681–91. DOI:
10.1111/bph.13179. PMID:
25939377. PMCID:
PMC4742301.

9. Backonja MM, Irving G, Argoff C. 2006; Rational multidrug therapy in the treatment of neuropathic pain. Curr Pain Headache Rep. 10:34–8. DOI:
10.1007/s11916-006-0007-1. PMID:
16499828.

10. Hare JT, Elliott DP. 2003; Grapefruit juice and potential drug interactions. Consult Pharm. 18:466–72. PMID:
16563062.
11. Palma-Duran SA, Caire-Juvera G, Robles-Burgeño Mdel R, Ortega-Vélez MI, Gutiérrez-Coronado Mde L, Almada Mdel C, et al. 2015; Serum levels of phytoestrogens as biomarkers of intake in Mexican women. Int J Food Sci Nutr. 66:819–25. DOI:
10.3109/09637486.2015.1092019. PMID:
26417700.

12. Pinho-Ribeiro FA, Zarpelon AC, Fattori V, Manchope MF, Mizokami SS, Casagrande R, et al. 2016; Naringenin reduces inflammatory pain in mice. Neuropharmacology. 105:508–19. DOI:
10.1016/j.neuropharm.2016.02.019. PMID:
26907804.

13. Hu CY, Zhao YT. 2014; Analgesic effects of naringenin in rats with spinal nerve ligation-induced neuropathic pain. Biomed Rep. 2:569–73. DOI:
10.3892/br.2014.267. PMID:
24944810. PMCID:
PMC4051469.

14. Al-Rejaie SS, Aleisa AM, Abuohashish HM, Parmar MY, Ola MS, Al-Hosaini AA, et al. 2015; Naringenin neutralises oxidative stress and nerve growth factor discrepancy in experimental diabetic neuropathy. Neurol Res. 37:924–33. DOI:
10.1179/1743132815Y.0000000079. PMID:
26187552.

15. Kaulaskar S, Bhutada P, Rahigude A, Jain D, Harle U. 2012; Effects of naringenin on allodynia and hyperalgesia in rats with chronic constriction injury-induced neuropathic pain. Zhong Xi Yi Jie He Xue Bao. 10:1482–9. DOI:
10.3736/jcim20121223. PMID:
23257145.

16. Pinho-Ribeiro FA, Zarpelon AC, Mizokami SS, Borghi SM, Bordignon J, Silva RL, et al. 2016; The citrus flavonone naringenin reduces lipopolysaccharide-induced inflammatory pain and leukocyte recruitment by inhibiting NF-κB activation. J Nutr Biochem. 33:8–14. DOI:
10.1016/j.jnutbio.2016.03.013. PMID:
27260463.

17. Manchope MF, Calixto-Campos C, Coelho-Silva L, Zarpelon AC, Pinho-Ribeiro FA, Georgetti SR, et al. 2016; Naringenin inhibits superoxide anion-induced inflammatory pain: role of oxidative stress, cytokines, Nrf-2 and the NO-cGMP-PKG-KATP channel signaling pathway. PLoS One. 11:e0153015. DOI:
10.1371/journal.pone.0153015. PMID:
27045367. PMCID:
PMC4821586.
18. Fakhri S, Kiani A, Jalili C, Abbaszadeh F, Piri S, Farzaei MH, et al. 2021; Intrathecal administration of melatonin ameliorates the neuroinflammation- mediated sensory and motor dysfunction in a rat model of compression spinal cord injury. Curr Mol Pharmacol. 14:646–57. DOI:
10.2174/1874467213666201230101811. PMID:
33380311.

19. Mestre C, Pélissier T, Fialip J, Wilcox G, Eschalier A. 1994; A method to perform direct transcutaneous intrathecal injection in rats. J Pharmacol Toxicol Methods. 32:197–200. DOI:
10.1016/1056-8719(94)90087-6. PMID:
7881133.

20. Kauppila T. 2000; Cold exposure enhances tactile allodynia transiently in mononeuropathic rats. Exp Neurol. 161:740–4. DOI:
10.1006/exnr.1999.7287. PMID:
10686093.

21. Hama AT, Plum AW, Sagen J. 2010; Antinociceptive effect of ambroxol in rats with neuropathic spinal cord injury pain. Pharmacol Biochem Behav. 97:249–55. DOI:
10.1016/j.pbb.2010.08.006. PMID:
20732348. PMCID:
PMC2956774.

22. Janicki P, Libich J. 1979; Detection of antagonist activity for narcotic analgesics in mouse hot-plate test. Pharmacol Biochem Behav. 10:623–6. DOI:
10.1016/0091-3057(79)90244-2. PMID:
461487.

23. Basso DM, Beattie MS, Bresnahan JC. 1995; A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 12:1–21. DOI:
10.1089/neu.1995.12.1. PMID:
7783230.

24. Samini F, Samarghandian S, Borji A, Mohammadi G, bakaian M. 2013; Curcumin pretreatment attenuates brain lesion size and improves neurological function following traumatic brain injury in the rat. Pharmacol Biochem Behav. 110:238–44. DOI:
10.1016/j.pbb.2013.07.019. PMID:
23932920.

25. Rahman I, Kode A, Biswas SK. 2006; Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 1:3159–65. DOI:
10.1038/nprot.2006.378. PMID:
17406579.

27. Masoudi A, Dargahi L, Abbaszadeh F, Pourgholami MH, Asgari A, Manoochehri M, et al. 2017; Neuroprotective effects of astaxanthin in a rat model of spinal cord injury. Behav Brain Res. 329:104–10. DOI:
10.1016/j.bbr.2017.04.026. PMID:
28442361.

28. Nouri Z, Fakhri S, El-Senduny FF, Sanadgol N, Abd-ElGhani GE, Farzaei MH, et al. 2019; On the neuroprotective effects of naringenin: pharmacological targets, signaling pathways, molecular mechanisms, and clinical perspective. Biomolecules. 9:690. DOI:
10.3390/biom9110690. PMID:
31684142. PMCID:
PMC6920995.

29. Mao L, Wang H, Qiao L, Wang X. 2010; Disruption of Nrf2 enhances the upregulation of nuclear factor-kappaB activity, tumor necrosis factor-α, and matrix metalloproteinase-9 after spinal cord injury in mice. Mediators Inflamm. 2010:238321. DOI:
10.1155/2010/238321. PMID:
20862369. PMCID:
PMC2938451.
31. Stetler-Stevenson WG. 1996; Dynamics of matrix turnover during pathologic remodeling of the extracellular matrix. Am J Pathol. 148:1345–50. PMID:
8623905. PMCID:
PMC1861572.
32. Shibata N, Ohnuma T, Higashi S, Usui C, Ohkubo T, Kitajima A, et al. 2005; Genetic association between matrix metalloproteinase MMP-9 and MMP-3 polymorphisms and Japanese sporadic Alzheimer's disease. Neurobiol Aging. 26:1011–4. DOI:
10.1016/j.neurobiolaging.2004.09.004. PMID:
15748780.

33. Lorenzl S, Albers DS, Narr S, Chirichigno J, Beal MF. 2002; Expression of MMP-2, MMP-9, and MMP-1 and their endogenous counterregulators TIMP-1 and TIMP-2 in postmortem brain tissue of Parkinson's disease. Exp Neurol. 178:13–20. DOI:
10.1006/exnr.2002.8019. PMID:
12460604.

34. Beuche W, Yushchenko M, Mäder M, Maliszewska M, Felgenhauer K, Weber F. 2000; Matrix metalloproteinase-9 is elevated in serum of patients with amyotrophic lateral sclerosis. Neuroreport. 11:3419–22. DOI:
10.1097/00001756-200011090-00003. PMID:
11095490.

35. Noble LJ, Donovan F, Igarashi T, Goussev S, Werb Z. 2002; Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J Neurosci. 22:7526–35. DOI:
10.1523/JNEUROSCI.22-17-07526.2002. PMID:
12196576. PMCID:
PMC2792199.

37. Wells JE, Rice TK, Nuttall RK, Edwards DR, Zekki H, Rivest S, et al. 2003; An adverse role for matrix metalloproteinase 12 after spinal cord injury in mice. J Neurosci. 23:10107–15. DOI:
10.1523/JNEUROSCI.23-31-10107.2003. PMID:
14602826. PMCID:
PMC6740867.

38. Zhang H, Chang M, Hansen CN, Basso DM, Noble-Haeusslein LJ. 2011; Role of matrix metalloproteinases and therapeutic benefits of their inhibition in spinal cord injury. Neurotherapeutics. 8:206–20. DOI:
10.1007/s13311-011-0038-0. PMID:
21455784. PMCID:
PMC3077748.

39. Piao MS, Lee JK, Jang JW, Hur H, Lee SS, Xiao L, et al. 2014; Melatonin improves functional outcome via inhibition of matrix metalloproteinases-9 after photothrombotic spinal cord injury in rats. Acta Neurochir (Wien). 156:2173–82. DOI:
10.1007/s00701-014-2119-4. PMID:
24879621.

40. Jang JW, Lee JK, Kim SH. 2011; Activation of matrix metalloproteinases-9 after photothrombotic spinal cord injury model in rats. J Korean Neurosurg Soc. 50:288–92. DOI:
10.3340/jkns.2011.50.4.288. PMID:
22200008. PMCID:
PMC3243829.

41. Bai X, Zhang X, Chen L, Zhang J, Zhang L, Zhao X, et al. 2014; Protective effect of naringenin in experimental ischemic stroke: down-regulated NOD2, RIP2, NF-κB, MMP-9 and up-regulated claudin-5 expression. Neurochem Res. 39:1405–15. DOI:
10.1007/s11064-014-1326-y. PMID:
24842554.

42. Hsu JY, McKeon R, Goussev S, Werb Z, Lee JU, Trivedi A, et al. 2006; Matrix metalloproteinase-2 facilitates wound healing events that promote functional recovery after spinal cord injury. J Neurosci. 26:9841–50. DOI:
10.1523/JNEUROSCI.1993-06.2006. PMID:
17005848. PMCID:
PMC2659718.

43. Shi LB, Tang PF, Zhang W, Zhao YP, Zhang LC, Zhang H. 2016; Naringenin inhibits spinal cord injury-induced activation of neutrophils through miR-223. Gene. 592:128–33. DOI:
10.1016/j.gene.2016.07.037. PMID:
27432064.

44. Puspitasari V, Gunawan PY, Wiradarma HD, Hartoyo V. 2019; Glial fibrillary acidic protein serum level as a predictor of clinical outcome in ischemic stroke. Open Access Maced J Med Sci. 7:1471–4. DOI:
10.3889/oamjms.2019.326. PMID:
31198457. PMCID:
PMC6542382.

45. Khajevand-Khazaei MR, Ziaee P, Motevalizadeh SA, Rohani M, Afshin-Majd S, Baluchnejadmojarad T, et al. 2018; Naringenin ameliorates learning and memory impairment following systemic lipopolysaccharide challenge in the rat. Eur J Pharmacol. 826:114–22. DOI:
10.1016/j.ejphar.2018.03.001. PMID:
29518393.

46. Krishna Chandran AM, Christina H, Das S, Mumbrekar KD, Satish Rao BS. 2019; Neuroprotective role of naringenin against methylmercury induced cognitive impairment and mitochondrial damage in a mouse model. Environ Toxicol Pharmacol. 71:103224. DOI:
10.1016/j.etap.2019.103224. PMID:
31376681.

47. Chtourou Y, Fetoui H, Gdoura R. 2014; Protective effects of naringenin on iron-overload-induced cerebral cortex neurotoxicity correlated with oxidative stress. Biol Trace Elem Res. 158:376–83. DOI:
10.1007/s12011-014-9948-0. PMID:
24682942.

48. Muthaiah VP, Venkitasamy L, Michael FM, Chandrasekar K, Venkatachalam S. 2013; Neuroprotective role of naringenin on carbaryl induced neurotoxicity in mouse neuroblastoma cells. J Pharmacol Pharmacother. 4:192–7. DOI:
10.4103/0976-500X.114599. PMID:
23960424. PMCID:
PMC3746302.

50. Giangregorio L, McCartney N. 2006; Bone loss and muscle atrophy in spinal cord injury: epidemiology, fracture prediction, and rehabilitation strategies. J Spinal Cord Med. 29:489–500. DOI:
10.1080/10790268.2006.11753898. PMID:
17274487. PMCID:
PMC1949032.

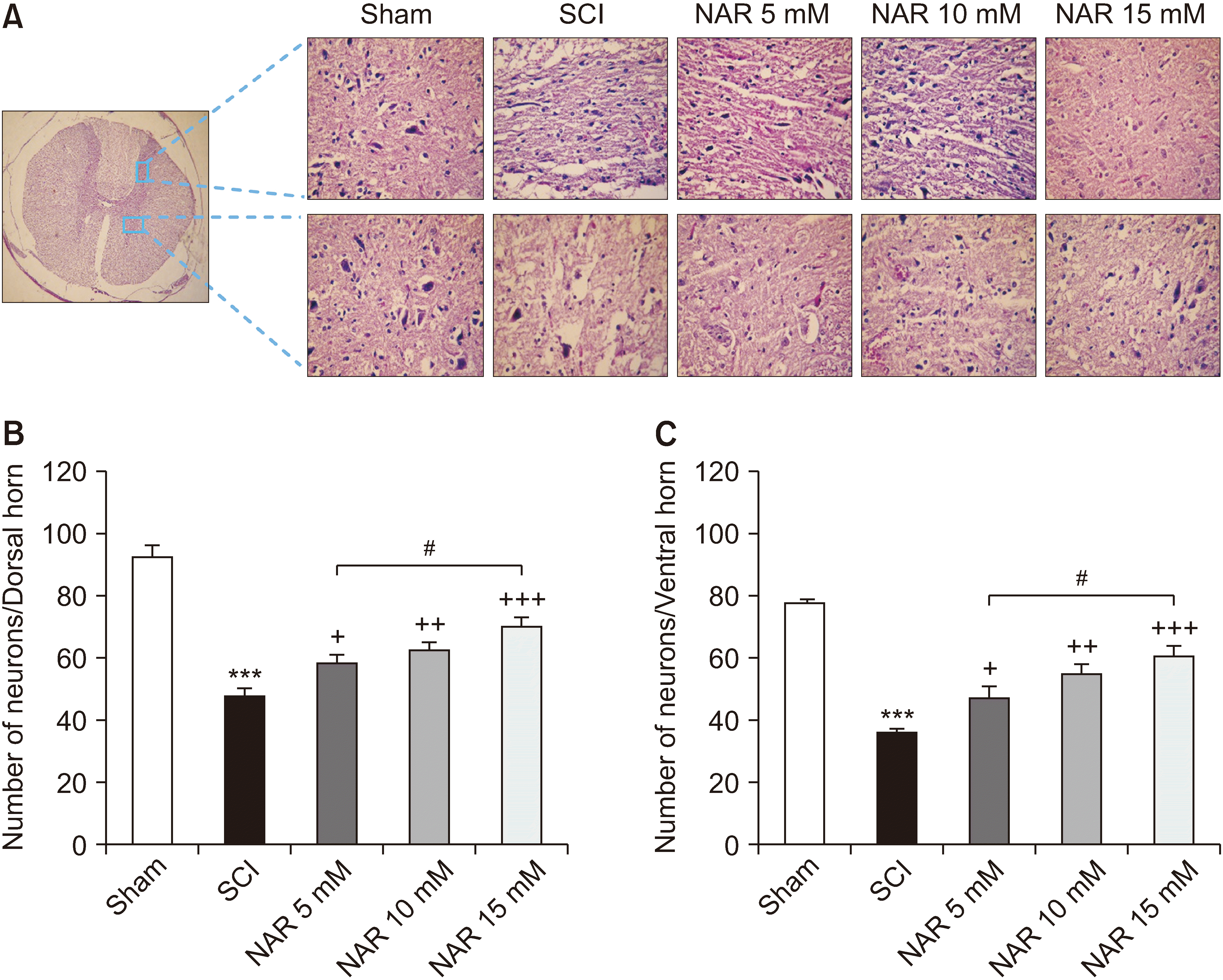
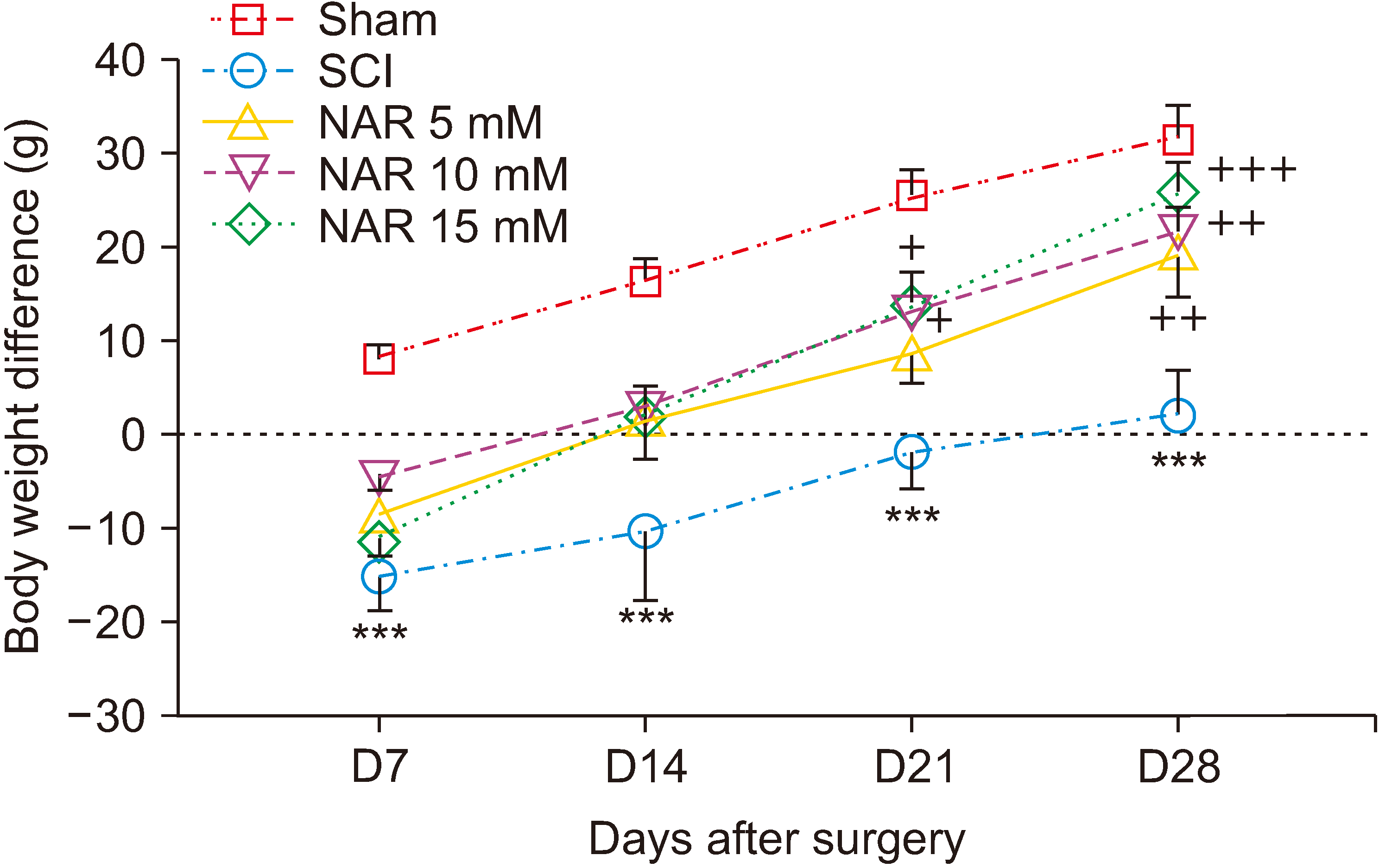




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download