INTRODUCTION
MATERIALS AND METHODS
1. Ethical approval
2. Animals
3. CCI
4. Grouping and treatment
Group A (Control): 10 mL/kg of body weight of normal saline
Group B (Sham): 10 mL/kg of body weight of normal saline
Group C (Ligated control): 10 mL/kg of body weight of normal saline
Group D (Pre-treated acetaminophen): 200 mg/kg of body weight
Group E (Post-treated acetaminophen): 200 mg/kg of body weight
Group F (Pre-treated co-treatment): acetaminophen (200 mg/kg of body weight) + L-carnosine (100 mg/kg of body weight)
Group G (Post-treated co-treatment): acetaminophen (200 mg/kg of body weight) + L-carnosine (100 mg/kg of body weight)
5. Pain behavioral tests
6. Thermal hyperalgesia
7. Mechanical allodynia test
8. Biochemical parameters
9. Histological study
10. Statistical analysis
RESULTS
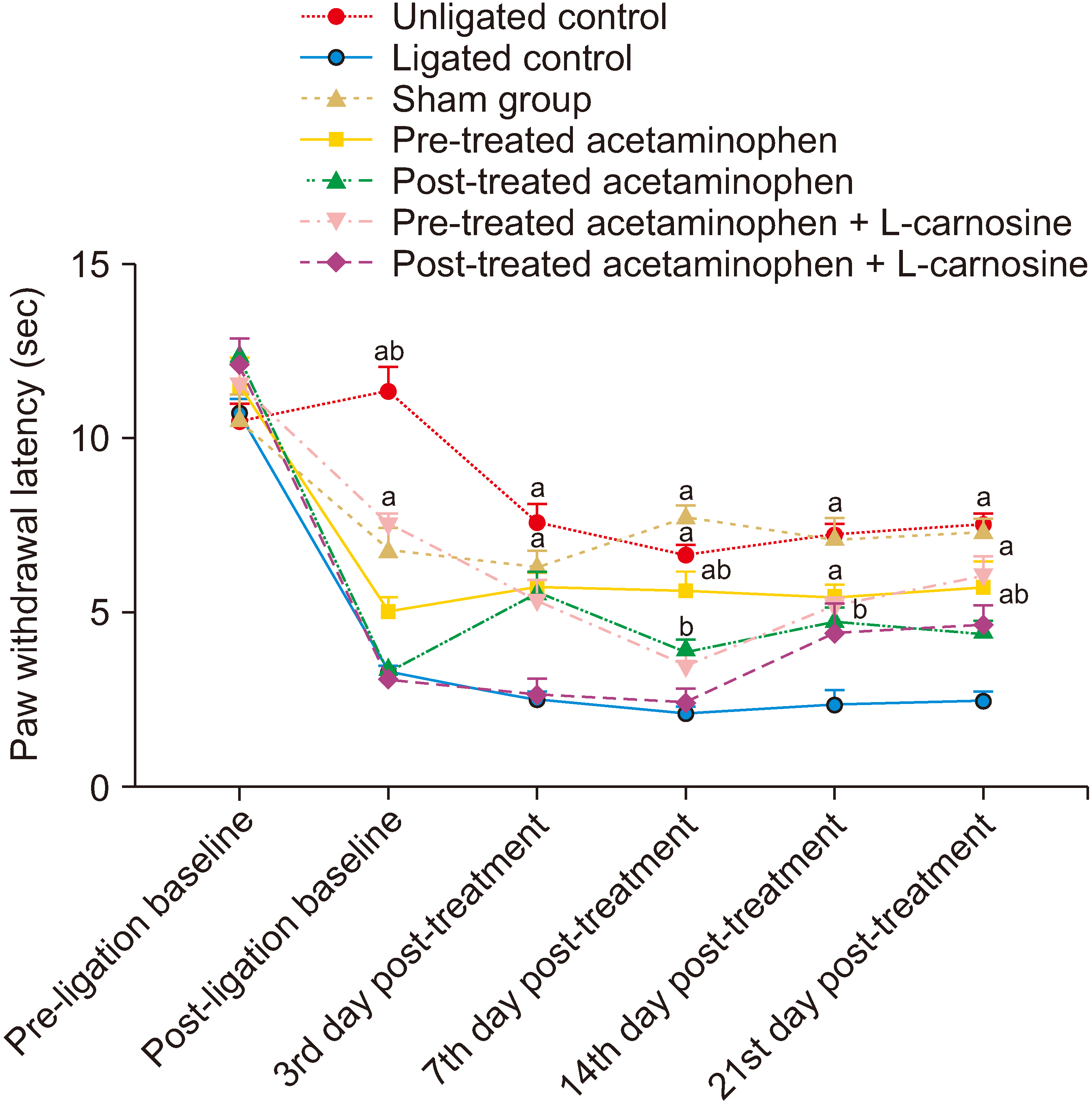
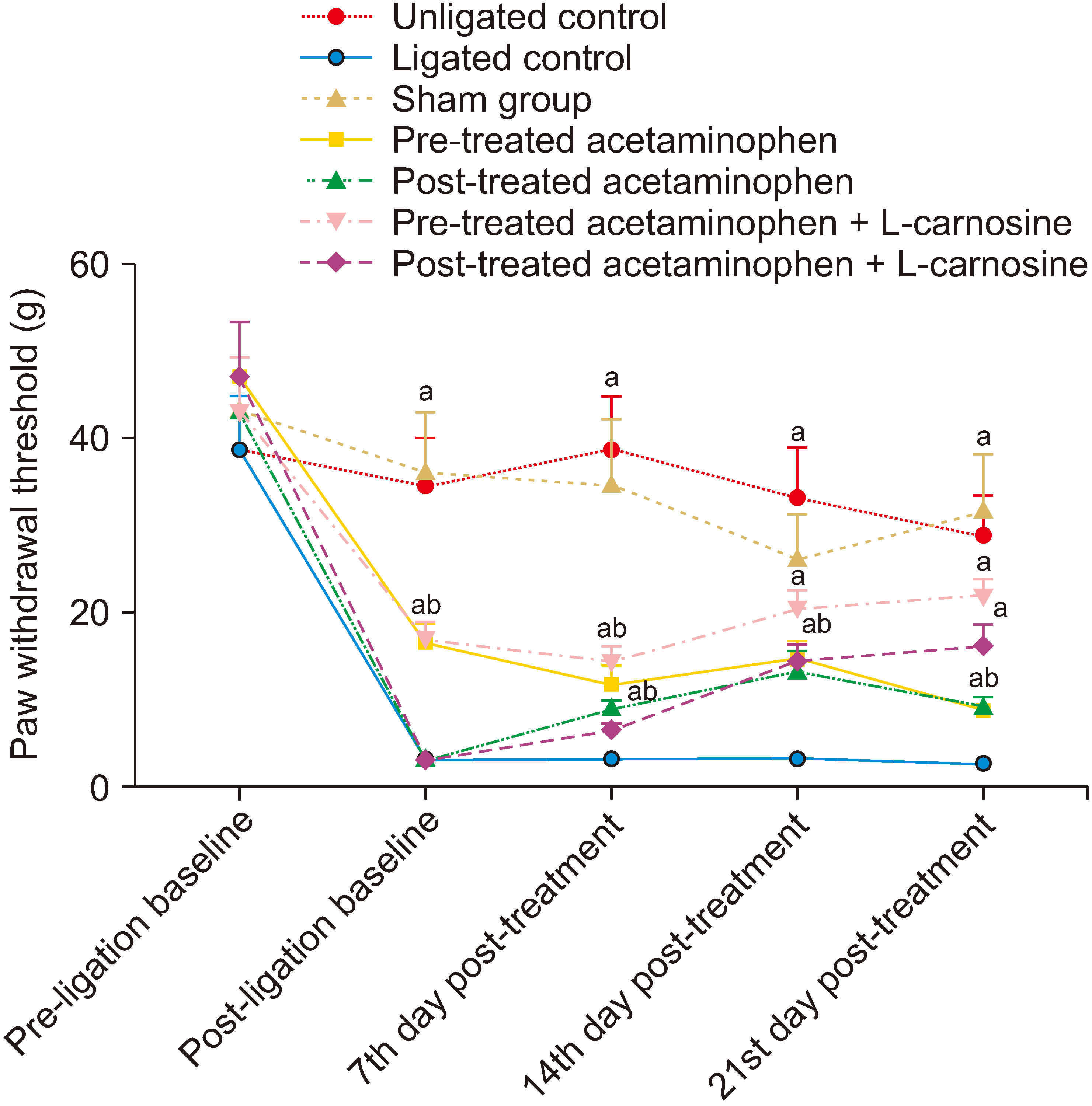
1. Hyperalgesic test
2. Allodynia test
3. Biochemical parameters
 | Fig. 3Acetaminophen + L-carnosine mitigated proinflammatory mediators. Acetaminophen and L-carnosine significantly reduced proinflammatory mediators in the spinal lumbar neurons. (A) Acetaminophen + L-carnosine significantly inhibit tumour necrotic factor-alpha (TNF-α) concentration. (B) Acetaminophen + L-carnosine significantly reduced interleukin-1-beta (IL-1β) concentration. (C) Acetaminophen + L-carnosine significantly attenuated nuclear factor kappa light chain enhancer B cell inhibitor (NF-κB) concentration. (D) Acetaminophen + L-carnosine significantly inhibit calcium (Ca2+) ion concentration. Data were represented as mean ± standard error of the mean (n = 8). aP < 0.05 vs. ligated control, bP < 0.05 vs. sham control. |
 | Fig. 4Acetaminophen + L-carnosine improved neuroprotection of the neurons against oxidative stress. Combined treatment with acetaminophen and L-carnosine significantly increased antioxidant enzyme activities and reduced lipid peroxidation. (A) Acetaminophen + L-carnosine significantly reduced malondialdehyde (MDA) concentration. (B) Acetaminophen + L-carnosine significantly increased superoxide dismutase (SOD) activities. (C) Acetaminophen + L-carnosine significantly increased glutathione (GSH) concentration. Data were represented as mean ± standard error of the mean (n = 8). aP < 0.05 vs. ligated control, bP < 0.05 vs. sham control. |
4. Histological analysis
1) Sciatic nerve
 | Fig. 5Micrograph showing haematoxyline and eosin stained longitudinal section of the left sciatic nerve. Sciatic nerve ligation induces lost of myelinated (single broken yellow arrow) and unmyelinated (single broken black arrow), shrinkage and lost of nuclei of Schwann cell (single broken red arrow), vacuolarization (black arrow head). Acetaminophen + L-carnosine improved cytoarchitecture of the sciatic nerve by preserving the myelinated (double-broken yellow arrow) and unmyelinated (double-broken black arrow) fibres, nuclei of Schwann cell (red arrow head), regeneration of myelinated neuron (yellow arrow head), and reduces vacuolarization. Red arrow indicate normal Schwann cell, normal myelinated nerve fibre (yellow arrow), and normal unmyelinated nerve fibre (black arrow). (A) Unligated naïve group, (B) Sham group, (C) Ligated naïve group, (D) Post-treated acetaminophen group, (E) Pre-treated acetaminophen group, (F) Post-treated acetaminophen + L-carnosine group, (G) Pre-treated acetaminophen + L-carnosine group. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download