1. Takemura M, Sugiyo S, Moritani M, Kobayashi M, Yonehara N. 2006; Mechanisms of orofacial pain control in the central nervous system. Arch Histol Cytol. 69:79–100. DOI:
10.1679/aohc.69.79. PMID:
16819148.

5. Voisin T, Rouet-Benzineb P, Reuter N, Laburthe M. 2003; Orexins and their receptors: structural aspects and role in peripheral tissues. Cell Mol Life Sci. 60:72–87. DOI:
10.1007/s000180300005. PMID:
12613659.

6. DiMicco JA, Samuels BC, Zaretskaia MV, Zaretsky DV. 2002; The dorsomedial hypothalamus and the response to stress: part renaissance, part revolution. Pharmacol Biochem Behav. 71:469–80. DOI:
10.1016/S0091-3057(01)00689-X. PMID:
11830181.
7. Lungwitz EA, Molosh A, Johnson PL, Harvey BP, Dirks RC, Dietrich A, et al. 2012; Orexin-A induces anxiety-like behavior through interactions with glutamatergic receptors in the bed nucleus of the stria terminalis of rats. Physiol Behav. 107:726–32. DOI:
10.1016/j.physbeh.2012.05.019. PMID:
22652097. PMCID:
PMC3482273.

8. Yeoh JW, Campbell EJ, James MH, Graham BA, Dayas CV. 2014; Orexin antagonists for neuropsychiatric disease: progress and potential pitfalls. Front Neurosci. 8:36. DOI:
10.3389/fnins.2014.00036. PMID:
24616658. PMCID:
PMC3934415.

9. Darwinkel A, Stanić D, Booth LC, May CN, Lawrence AJ, Yao ST. 2014; Distribution of orexin-1 receptor-green fluorescent protein- (OX1-GFP) expressing neurons in the mouse brain stem and pons: co-localization with tyrosine hydroxylase and neuronal nitric oxide synthase. Neuroscience. 278:253–64. DOI:
10.1016/j.neuroscience.2014.08.027. PMID:
25168728.

10. Raoof M, Esmaeili-Mahani S, Nourzadeh M, Raoof R, Abbasnejad M, Amirkhosravi L, et al. 2015; Noxious stimulation of the rat tooth pulp may impair learning and memory through the induction of hippocampal apoptosis. J Oral Facial Pain Headache. 29:390–7. DOI:
10.11607/ofph.1452. PMID:
26485387.

11. Chuang YC, Yoshimura N, Huang CC, Wu M, Chiang PH, Chancellor MB. 2008; Intraprostatic botulinum toxin a injection inhibits cyclooxygenase-2 expression and suppresses prostatic pain on capsaicin induced prostatitis model in rat. J Urol. 180:742–8. DOI:
10.1016/j.juro.2007.07.120. PMID:
18554636.

12. Lee KM, Kang BS, Lee HL, Son SJ, Hwang SH, Kim DS, et al. 2004; Spinal NF-kB activation induces COX-2 upregulation and contributes to inflammatory pain hypersensitivity. Eur J Neurosci. 19:3375–81. DOI:
10.1111/j.0953-816X.2004.03441.x. PMID:
15217394.

13. Rahbar I, Abbasnejad M, Haghani J, Raoof M, Kooshki R, Esmaeili-Mahani S. 2019; The effect of central administration of alpha-pinene on capsaicin-induced dental pulp nociception. Int Endod J. 52:307–17. DOI:
10.1111/iej.13006. PMID:
30152861.

17. Webster MJ, Herman MM, Kleinman JE, Shannon Weickert C. 2006; BDNF and trkB mRNA expression in the hippocampus and temporal cortex during the human lifespan. Gene Expr Patterns. 6:941–51. DOI:
10.1016/j.modgep.2006.03.009. PMID:
16713371.

18. Raoof M, Ashrafganjoui E, Kooshki R, Abbasnejad M, Haghani J, Amanpour S, et al. 2018; Effect of chronic stress on capsaicin-induced dental nociception in a model of pulpitis in rats. Arch Oral Biol. 85:154–9. DOI:
10.1016/j.archoralbio.2017.10.012. PMID:
29073563.

20. Inada M, Matsumoto C, Uematsu S, Akira S, Miyaura C. 2006; Membrane-bound prostaglandin E synthase-1-mediated prostaglandin E2 production by osteoblast plays a critical role in lipopolysaccharide-induced bone loss associated with inflammation. J Immunol. 177:1879–85. DOI:
10.4049/jimmunol.177.3.1879. PMID:
16849500.

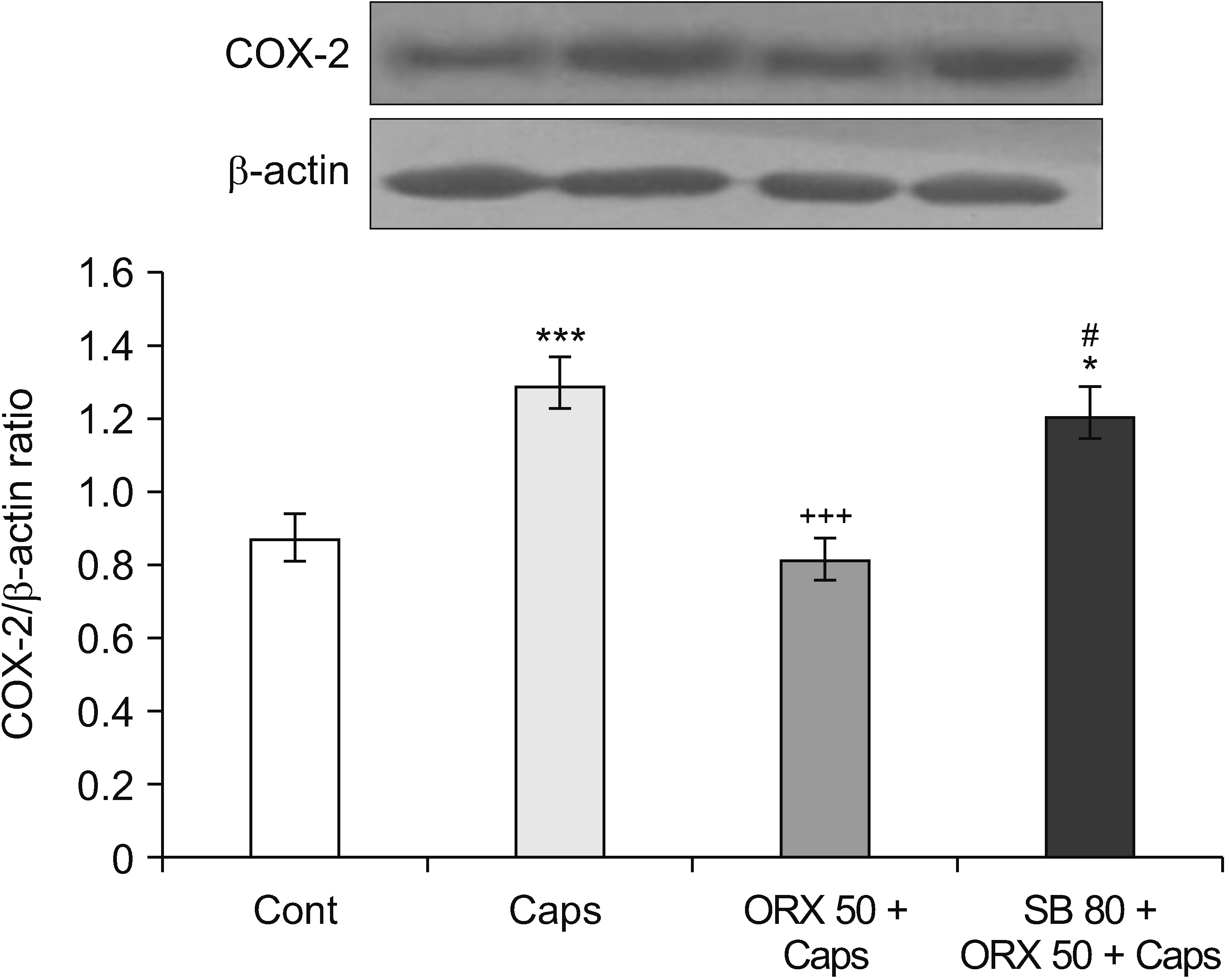
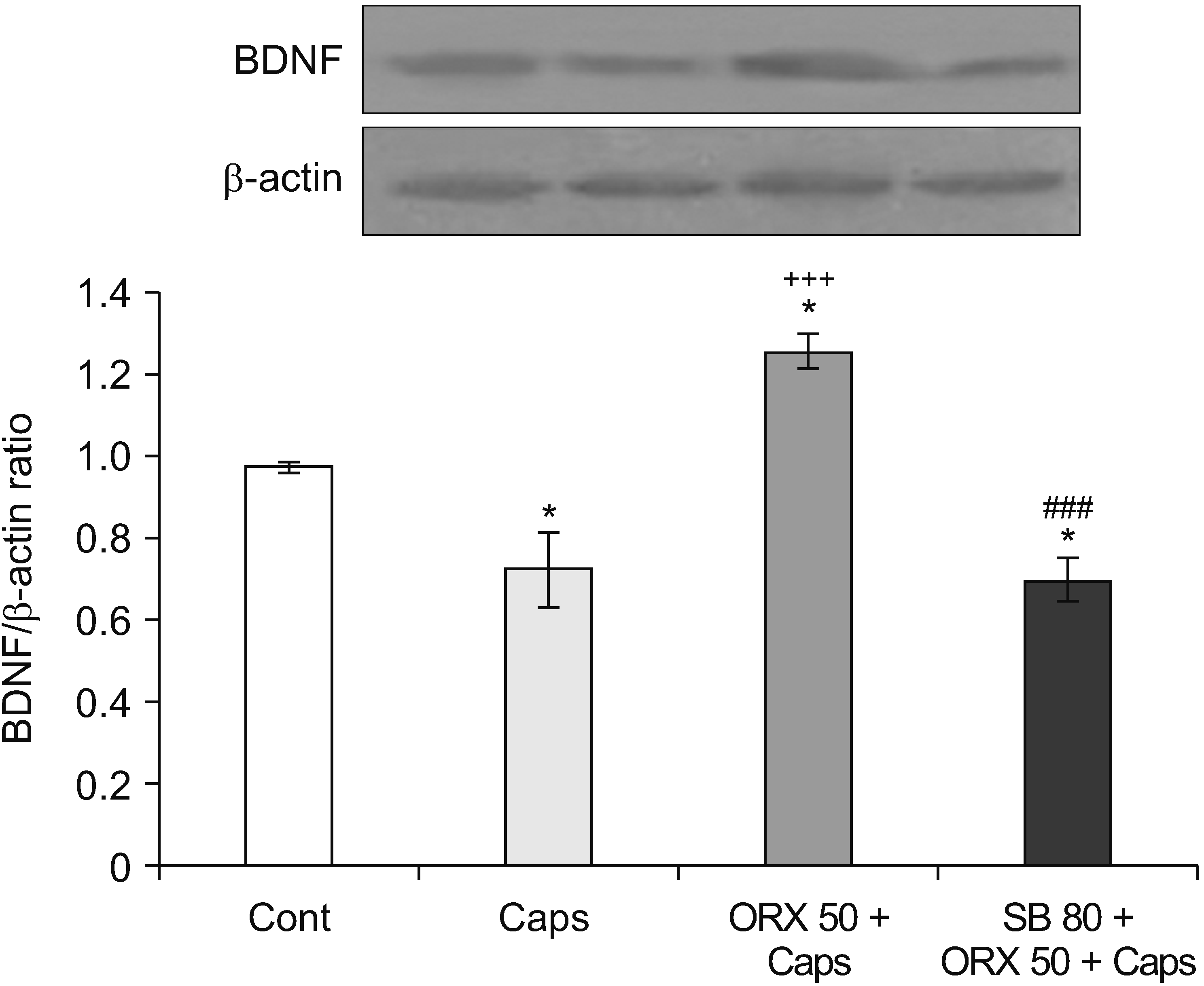
21. Kooshki R, Abbasnejad M, Esmaeili Mahani S, Raoof M, Moeini Aghtaei MM, Dabiri S. 2018; Orexin-A inhibits capsaicin-induced changes in cyclooxygenase-2 and brain-derived neurotrophic factor expression in trigeminal nucleus caudalis of rats. Korean J Pain. 31:174–82. DOI:
10.3344/kjp.2018.31.3.174. PMID:
30013731. PMCID:
PMC6037813.

22. Ahn DK, Choi HS, Yeo SP, Woo YW, Lee MK, Yang GY, et al. 2007; Blockade of central cyclooxygenase (COX) pathways enhances the cannabinoid-induced antinociceptive effects on inflammatory temporomandibular joint (TMJ) nociception. Pain. 132:23–32. DOI:
10.1016/j.pain.2007.01.015. PMID:
17321048.

24. Zhou Y, Long H, Ye N, Liao L, Yang X, Jian F, et al. 2016; The effect of capsaicin on expression patterns of CGRP in trigeminal ganglion and trigeminal nucleus caudalis following experimental tooth movement in rats. J Appl Oral Sci. 24:597–606. DOI:
10.1590/1678-775720160150. PMID:
28076465. PMCID:
PMC5161258.

25. Hay C, de Belleroche J. 1997; Carrageenan-induced hyperalgesia is associated with increased cyclo-oxygenase-2 expression in spinal cord. Neuroreport. 8:1249–51. DOI:
10.1097/00001756-199703240-00038. PMID:
9175123.

26. Carlson JD, Maire JJ, Martenson ME, Heinricher MM. 2007; Sensitization of pain-modulating neurons in the rostral ventromedial medulla after peripheral nerve injury. J Neurosci. 27:13222–31. DOI:
10.1523/JNEUROSCI.3715-07.2007. PMID:
18045916. PMCID:
PMC6673414.

27. Sanoja R, Tortorici V, Fernandez C, Price TJ, Cervero F. 2010; Role of RVM neurons in capsaicin-evoked visceral nociception and referred hyperalgesia. Eur J Pain. 14:120.e1–9. DOI:
10.1016/j.ejpain.2009.04.006. PMID:
19443247. PMCID:
PMC2914578.

28. Kooshki R, Abbasnejad M, Esmaeili-Mahani S, Raoof M. 2016; The role of trigeminal nucleus caudalis orexin 1 receptors in orofacial pain transmission and in orofacial pain-induced learning and memory impairment in rats. Physiol Behav. 157:20–7. DOI:
10.1016/j.physbeh.2016.01.031. PMID:
26821188.

29. Azhdari-Zarmehri H, Semnanian S, Fathollahi Y, Pakdel FG. 2014; Tail flick modification of orexin-a induced changes of electrophysiological parameters in the rostral ventromedial medulla. Cell J. 16:131–40. PMID:
24567942. PMCID:
PMC4072083.
31. Messal N, Fernandez N, Dayot S, Gratio V, Nicole P, Prochasson C, et al. 2018; Ectopic expression of OX1R in ulcerative colitis mediates anti-inflammatory effect of orexin-A. Biochim Biophys Acta Mol Basis Dis. 1864:3618–28. DOI:
10.1016/j.bbadis.2018.08.023. PMID:
30251681.

33. Raoof R, Esmaeili-Mahani S, Abbasnejad M, Raoof M, Sheibani V, Kooshki R, et al. 2015; Changes in hippocampal orexin 1 receptor expression involved in tooth pain-induced learning and memory impairment in rats. Neuropeptides. 50:9–16. DOI:
10.1016/j.npep.2015.03.002. PMID:
25817882.

34. Buldyrev I, Tanner NM, Hsieh HY, Dodd EG, Nguyen LT, Balkowiec A. 2006; Calcitonin gene-related peptide enhances release of native brain-derived neurotrophic factor from trigeminal ganglion neurons. J Neurochem. 99:1338–50. DOI:
10.1111/j.1471-4159.2006.04161.x. PMID:
17064360. PMCID:
PMC2440676.

35. Trang T, Beggs S, Salter MW. 2011; Brain-derived neurotrophic factor from microglia: a molecular substrate for neuropathic pain. Neuron Glia Biol. 7:99–108. DOI:
10.1017/S1740925X12000087. PMID:
22613083. PMCID:
PMC3748035.

37. Duric V, McCarson KE. 2006; Persistent pain produces stress-like alterations in hippocampal neurogenesis and gene expression. J Pain. 7:544–55. DOI:
10.1016/j.jpain.2006.01.458. PMID:
16885011.

38. Aubdool AA, Kodji X, Abdul-Kader N, Heads R, Fernandes ES, Bevan S, et al. 2016; TRPA1 activation leads to neurogenic vasodilatation: involvement of reactive oxygen nitrogen species in addition to CGRP and NO. Br J Pharmacol. 173:2419–33. DOI:
10.1111/bph.13519. PMID:
27189253. PMCID:
PMC4945766.

39. Kapczinski F, Frey BN, Andreazza AC, Kauer-Sant'Anna M, Cunha ÂB, Post RM. 2008; Increased oxidative stress as a mechanism for decreased BDNF levels in acute manic episodes. Braz J Psychiatry. 30:243–5. DOI:
10.1590/S1516-44462008000300011. PMID:
18833425.

40. Akbari E, Naghdi N, Motamedi F. 2007; The selective orexin 1 receptor antagonist SB-334867-A impairs acquisition and consolidation but not retrieval of spatial memory in Morris water maze. Peptides. 28:650–6. DOI:
10.1016/j.peptides.2006.11.002. PMID:
17161886.

41. Choi YS, Cho HY, Hoyt KR, Naegele JR, Obrietan K. 2008; IGF-1 receptor-mediated ERK/MAPK signaling couples status epilepticus to progenitor cell proliferation in the subgranular layer of the dentate gyrus. Glia. 56:791–800. DOI:
10.1002/glia.20653. PMID:
18338791. PMCID:
PMC4152854.

42. Lee S, Yang M, Kim J, Son Y, Kim J, Kang S, et al. 2016; Involvement of BDNF/ERK signaling in spontaneous recovery from trimethyltin-induced hippocampal neurotoxicity in mice. Brain Res Bull. 121:48–58. DOI:
10.1016/j.brainresbull.2016.01.002. PMID:
26772626. PMCID:
PMC4783253.

43. Veyrac A, Gros A, Bruel-Jungerman E, Rochefort C, Kleine Borgmann FB, Jessberger S, et al. 2013; Zif268/egr1 gene controls the selection, maturation and functional integration of adult hippocampal newborn neurons by learning. Proc Natl Acad Sci U S A. 110:7062–7. DOI:
10.1073/pnas.1220558110. PMID:
23569253. PMCID:
PMC3637756.
44. Meyer-Tuve A, Malcangio M, Ebersberger A, Mazario J, Schaible HG. 2001; Effect of brain-derived neurotrophic factor on the release of substance P from rat spinal cord. Neuroreport. 12:21–4. DOI:
10.1097/00001756-200101220-00012. PMID:
11201084.

45. Yang L, Zou B, Xiong X, Pascual C, Xie J, Malik A, et al. 2013; Hypocretin/orexin neurons contribute to hippocampus-dependent social memory and synaptic plasticity in mice. J Neurosci. 33:5275–84. DOI:
10.1523/JNEUROSCI.3200-12.2013. PMID:
23516292. PMCID:
PMC3640412.

46. Marmigère F, Givalois L, Rage F, Arancibia S, Tapia-Arancibia L. 2003; Rapid induction of BDNF expression in the hippocampus during immobilization stress challenge in adult rats. Hippocampus. 13:646–55. DOI:
10.1002/hipo.10109. PMID:
12921353.

47. Wang H, Ye M, Yu L, Wang J, Guo Y, Lei W, et al. 2015; Hippocampal neuronal cyclooxygenase-2 downstream signaling imbalance in a rat model of chronic aluminium gluconate administration. Behav Brain Funct. 11:8. DOI:
10.1186/s12993-015-0054-z. PMID:
25888969. PMCID:
PMC4336726.

48. Arrigo A, Mormina E, Calamuneri A, Gaeta M, Marino S, Milardi D, et al. 2017; Amygdalar and hippocampal connections with brainstem and spinal cord: a diffusion MRI study in human brain. Neuroscience. 343:346–54. DOI:
10.1016/j.neuroscience.2016.12.016. PMID:
28003162.

49. Kooshki R, Abbasnejad M, Esmaeili-Mahani S, Raoof M. 2018; The effect of CA1 administration of orexin-A on hippocampal expression of COX-2 and BDNF in a rat model of orofacial pain. Arq Neuropsiquiatr. 76:603–8. DOI:
10.1590/0004-282x20180099. PMID:
30365624.





 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download