INTRODUCTION
MATERIALS AND METHODS
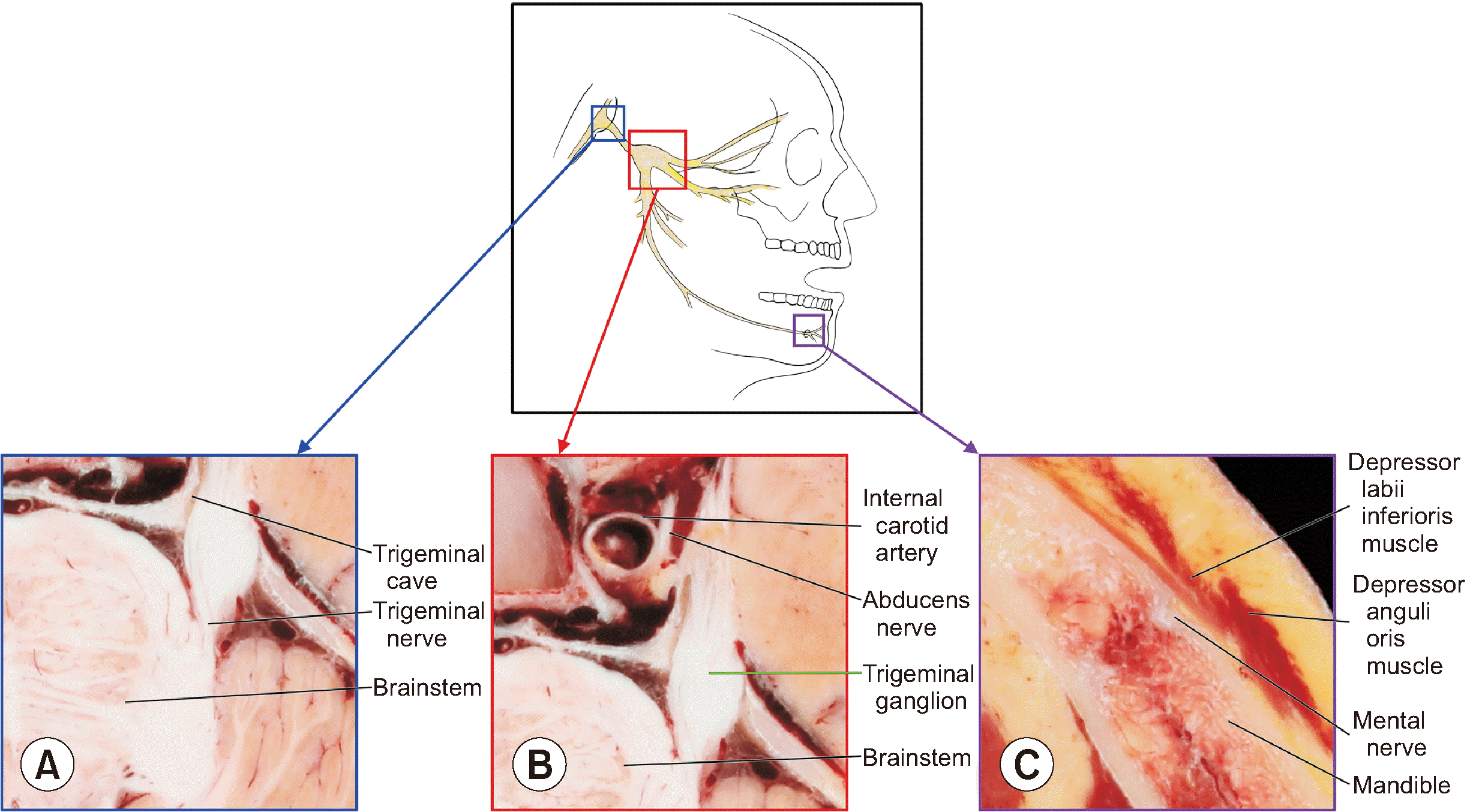 | Fig. 1Procedure to identify trigeminal ganglion in the horizontally sectioned images of a cadaver. To identify trigeminal ganglion, starting point (A) of the trigeminal nerve is observed in the sectioned images of brainstem. By tracking trigeminal nerve fibers, the ganglion (B) is observed. To confirm the location of the ganglion, ending point (C) of trigeminal nerve is observed. |
Table 1
| Main cranial nerve | Ganglion | Clinical features |
|---|---|---|
| III Oculomotor nerve | Ciliary ganglion | The ciliary ganglion can be injured during surgical intervention such as lateral orbitotomy, inferolateral endoscopic orbital approach, transmaxillary approach to the orbit [12]. |
| V Trigeminal nerve | Trigeminal ganglion | The trigeminal ganglion can be affected by herpes zoster virus infection [13]. |
| To treat trigeminal neuralgia, trigeminal ganglion can be selected for radiofrequency selective ablation [13]. | ||
| VII Facial nerve | Geniculate ganglion | The geniculate ganglion can be injured during surgical interventions such as the transmastoid approach, translabyrinthine approach, and middle cranial fossa approach due to abnormalities such as tumors [14]. |
| Pterygopalatine ganglion | To perform a pterygopalatine ganglion block, the intranasal approach, transoral approach, or infrazygomatic arch approach can be selected [15]. | |
| Submandibular ganglion | To treat sialorrhea, the submandibular ganglion can be selected for injection of botulinum toxin A or surgical removal [16]. | |
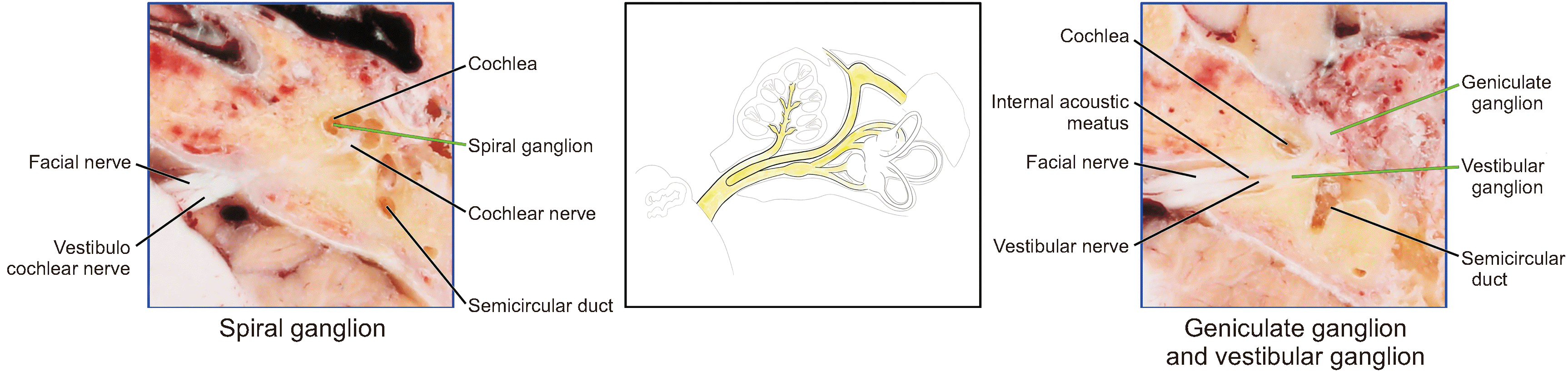
| VIII Vestibulocochlear nerve | Spiral ganglion | To treat sensoneural hearing loss, studies to identify novel treatment to prevent and reverse the damage of spiral ganglion neurons including gene therapy and stem cell therapy [17]. |
| Vestibular ganglion | To treat vestibular schwannomas of the middle cranial fossa, the translabyrinthine, and retrosigmoid approaches can be selected [18]. | |
| IX Glossopharyngeal nerve | Superior ganglion (IX) | To treat glossopharyngeal neuralgia, percutaneous radiofrequency coagulation of nerves inside the jugular foramen can be selected [19]. |
| Inferior ganglion (IX) | To treat glossopharyngeal neuralgia, percutaneous radiofrequency coagulation of the inferior ganglion and nerves inside the jugular foramen can be selected [19]. | |
| Otic ganglion | Frey’s syndrome can occur by aberrant regeneration of cut parasympathetic fibers between the otic ganglion and subcutaneous vessels [20]. | |
| To treat trigeminal autonomic cephalalgias, the otic ganglion can be selected for injection of botulinum toxin A [21,22]. | ||
| X Vagus nerve | Superior ganglion (X) | Vagal paraganglioma can originate from the superior ganglion of the vagus nerve [23]. |
| Inferior ganglion (X) | Vagal paraganglioma mostly originates from the inferior ganglion of the vagus nerve [23]. |
RESULTS
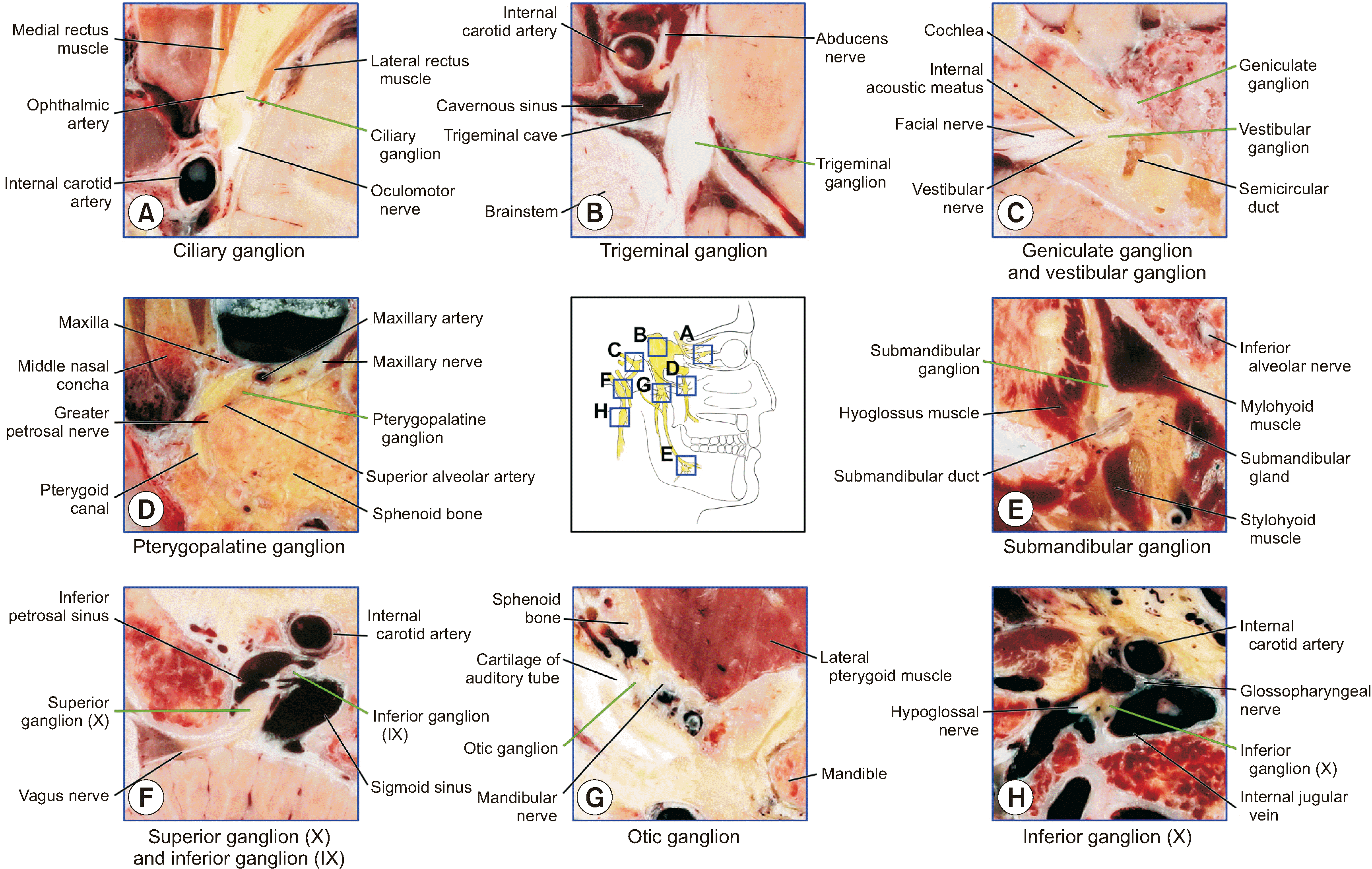 | Fig. 2Ten cranial nerve ganglia and adjacent structures identified in the horizontally sectioned images of a cadaver. Ten cranial nerve ganglia (A, ciliary ganglion; B, trigeminal ganglion; C, geniculate ganglion and vestibular ganglion; D, pterygopalatine ganglion; E, submandibular ganglion; F, superior ganglion of vagus nerve; G, otic ganglion; H, inferior ganglion of vagus nerve) and adjacent structures are identified and labeled in the sectioned images (IX, glossopharyngeal nerve; X, vagus nerve). |
1. Oculomotor nerve and ciliary ganglion
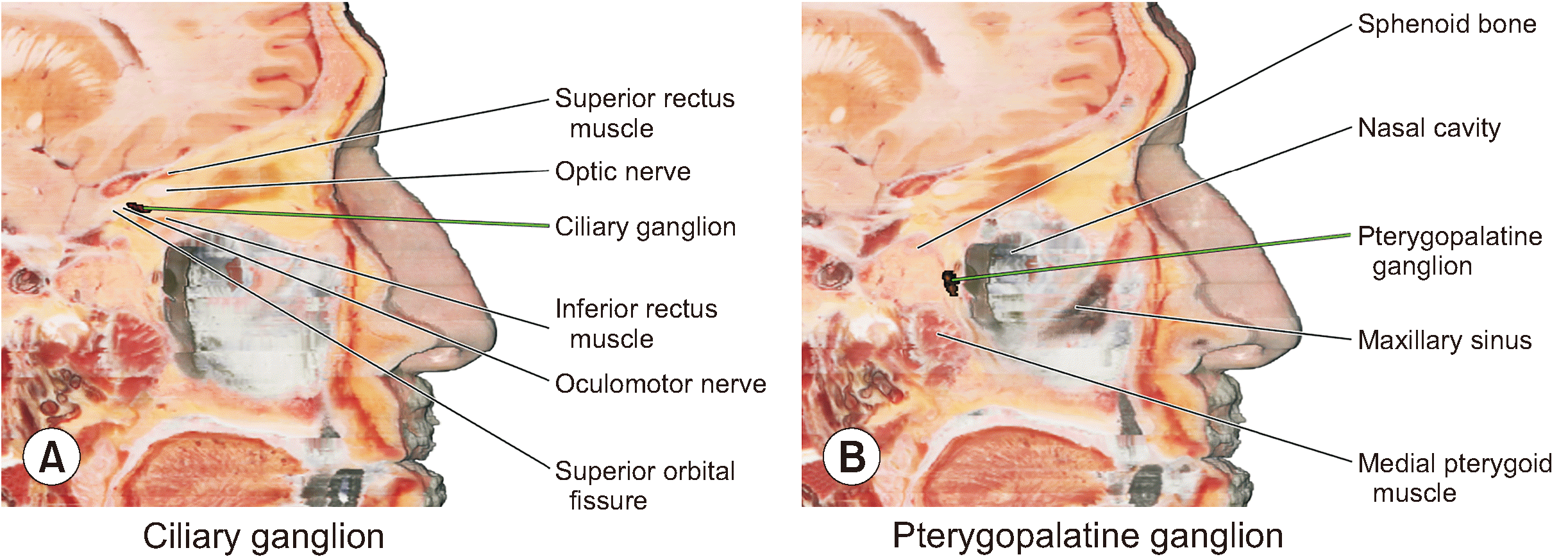 | Fig. 5Cranial nerve ganglia identified in the sagittal section of the volume model. The spatial relationships of the ciliary ganglion (A) and pterygopalatine ganglion (B) can be understood with adjacent structures in the sagittal section of the volume model. |
Table 2
|
Main cranial nerve |
Ganglion | Anatomical features | ||||||
|---|---|---|---|---|---|---|---|---|
| Location |
Lengtha (mm) |
Widtha (mm) |
Heighta (mm) |
Shape | Color | |||
| III Oculomotor nerve | Ciliary ganglion | In the orbital apex, medial to lateral rectus muscle, infralateral to optic nerve | 3.62 | 1.80 | 1.44 | Oval, flattened | Reddish | |
| V Trigeminal nerve | Trigeminal ganglion | Near the posterolateral part of the cavernous sinus, lateral to the internal carotid artery and abducens nerve | 7.05b | 6.79b | 3.29b | Semilunar | White | |
| VII Facial nerve | Geniculate ganglion | Lateral to the cochlea, anterior to the semicircular duct, near the fundus of the internal acoustic meatus | 3.00 | 1.35 | 1.74 | Sharp bend | White | |
| Pterygopalatine ganglion | Posterosuperior to the maxillary sinus in the pterygopalatine fossa | 2.41 | 2.40 | 3.46 | Conical | Reddish gray | ||
| Submandibular ganglion | Medial to the superior part of the submandibular gland | 2.66 | 1.74 | 3.63 | Swollen | White | ||
| VIII Vestibulocochlear nerve | Spiral ganglion | Near the lamina of the modiolus of the cochlea | NMc | NMc | NMc | Spot | Bright white | |
| Vestibular ganglion | Near the fundus of the internal acoustic meatus | 1.17 | 3.14 | 1.00 | Ampullated | Dark brown | ||
| IX Glossopharyngeal nerve | Superior ganglion | In the superior part of the jugular foramen, posterior to the inferior petrosal sinus | 1.29 | 2.55 | 1.63 | Expanded | Grayish orange | |
| Inferior ganglion | In the middle part of the jugular foramen, anterolateral to the superior ganglion (X), between the inferior petrosal sinus and sigmoid sinus | 1.74 | 3.03 | 2.62 | Expanded | Grayish orange | ||
| Otic ganglion | Inferior to the foramen ovale, medial to the mandibular nerve, lateral to the cartilage of the auditory tube, anterior to the medial pterygoid muscle | 2.08 | 1.82 | 1.84 | Oval, small | White | ||
| X Vagus nerve | Superior ganglion | In the middle part of the jugular foramen, posterior to the inferior ganglion (IX) | 5.61 | 3.07 | 3.32 | Expanded | Bright yellow | |
| Inferior ganglion | Inferior to the jugular foramen, lateral to the hypoglossal nerve | 2.81 | 3.55 | 6.64 | Expanded | Dark brown | ||
2. Trigeminal nerve and trigeminal ganglion
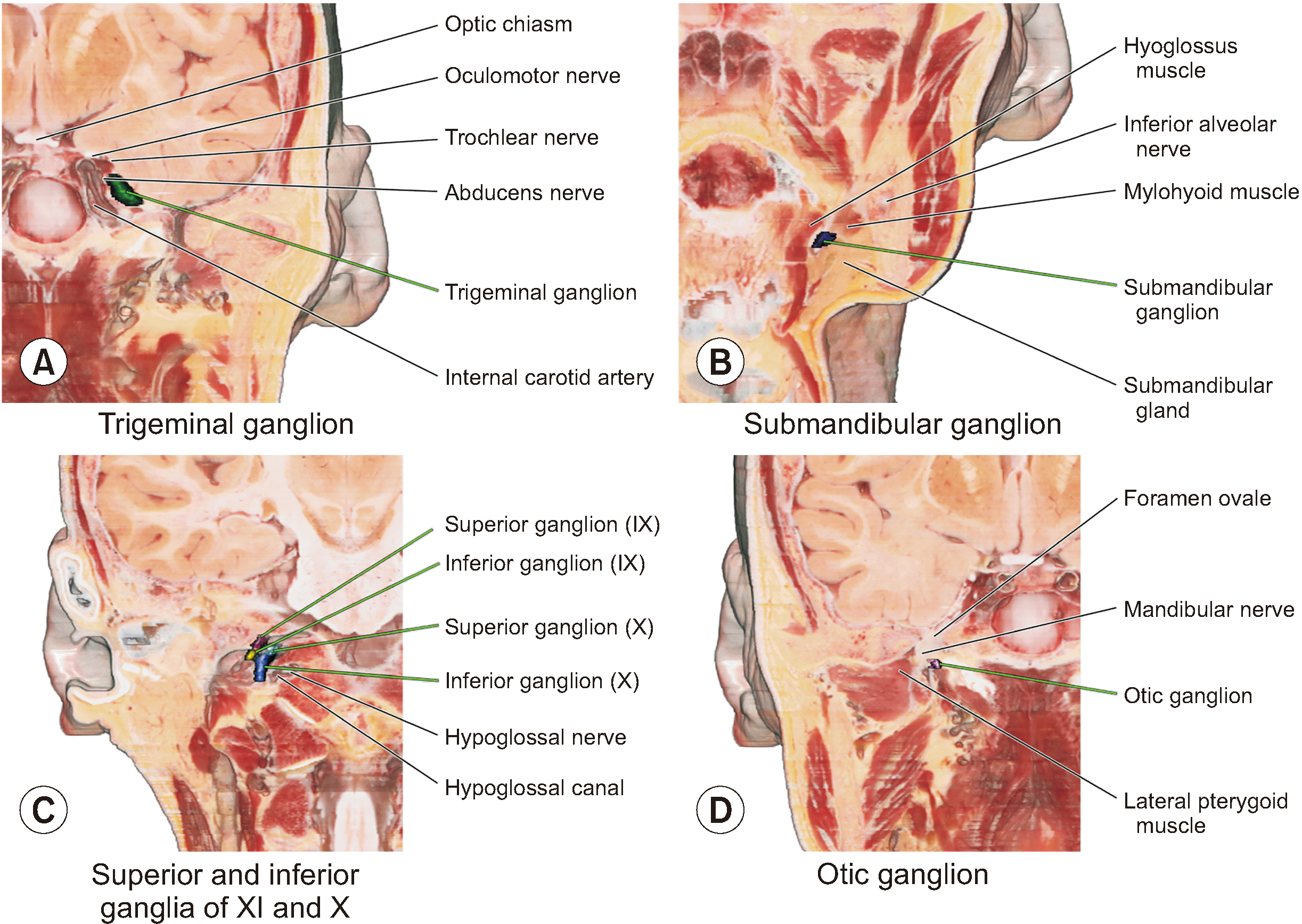 | Fig. 6Cranial nerve ganglia identified in the coronal section of the volume model (IX, glossopharyngeal nerve; X, vagus nerve). The spatial relationships of the cranial nerve ganglia (A, trigeminal ganglion; B, submandibular ganglion; C, superior and inferior ganglia of glossopharyngeal and vagus nerve; D, otic ganglion) can be understood with adjacent structures in the coronal section of the volume model. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download