INTRODUCTION
Atopic dermatitis (AD) is a chronic inflammatory skin disease characterized by pruritic eczematous skin lesions with repeated remission and relapse. It has become a major public health problem in modern industrialized countries owing to its high prevalence, intractability, and unknown etiologies [
1]. AD is implicated in multiple factors, such as bacterial infections, impaired skin barrier function, alteration of the immune system, and genetic background.
Staphylococcus aureus (
S.
aureus) has long been recognized as a key player in the pathogenesis of AD because it is observed to be colonizing the skin of patients with severe AD. Previous studies have shown that apoptotic cell death followed by colonization by
S.
aureus is common and plays a pivotal role in the development or aggravation of AD [
2]. Many virulence factors in
S.
aureus induce apoptosis in various cell types, including keratinocytes and immune cells [
3]. Apoptotic cell death of the host immune cells may accelerate infection by pathogenic staphylococci [
4]. Under physiological conditions, apoptosis and subsequent phagocytic clearance are immunologically silent. However, dysregulated apoptosis can stimulate abnormal immune responses such as cytokine production and T lymphocyte differentiation [
5,
6]. Given that the phagocytic activities of immune cells were significantly impaired in the AD patients along with apoptotic cell death and/or improper clearance of apoptotic cells in the skin, skin colonization by
S.
aureus, may be implicated in the pathogenesis of inflammatory skin diseases.
Adipose-derived stem cells (ASCs), the mesenchymal stem cells (MSCs) derived from adipose tissues, have been widely used not only for tissue regeneration and tissue engineering [
7] but also in the treatment of various human diseases, such as myocardial infarction [
8], liver disease [
9], ischemic brain injury [
10], and muscular dystrophy [
11]. Moreover, ASCs have recently been considered as an attractive alternative therapy for a variety of immune-related human diseases because of their anti-inflammatory and immune-regulatory properties [
12,
13]. Administration of ASCs-culture medium or ASCs themselves has been reported to considerably improve AD and psoriasis [
14,
15]. The therapeutic effects of ASCs on inflammatory or allergic skin diseases are likely to involve the differentiation of keratinocytes [
10], attenuation of Th2 inflammation [
12], and regulation of B lymphocytes [
15]. However, it is not yet clear whether ASCs affect apoptosis and phagocytic activities of host immune cells.
In the present study, we investigated whether cutaneous infection by S. aureus affects both apoptosis and phagocytic activity of peripheral blood mononuclear cells (PBMC) and whether ASCs could reverse AD-related pathologies caused by S. aureus. Our data indicate that ASCs treatment not only prevents apoptotic cell death of PBMCs but also enhances the phagocytic activities of PBMCs in AD conditions. In addition, our results show that ASCs treatment increases antimicrobial peptides (AMPs) in the skin; that can control S. aureus pathogenesis.
Go to :

METHODS
Animal model
All experiments were approved by the Korea University of Medicine Animal Research Policies Committee (Korea-2017-0145). Newborn rat pups were injected with capsaicin (50 mg/kg, s.c.; Sigma-Aldrich, St. Louis, MO, USA) within 48 h of birth to induce AD [
16]. All animals were raised in a room maintained under a 12 h light/dark cycle (light on at 07:00 h) at 22°C –25°C, with free access to food and water.
Evaluation of cutaneous lesions
Cutaneous lesions were carefully inspected and evaluated by scoring [
17]. In brief, a lesion of 25 mm
2 was adopted as the unit size for the extent of skin lesions. The dermatitis score was calculated by summing up the score of all the lesions. The lesions were assessed according to their severity, as shown in
Table 1.
Table 1
Severity index for skin lesions
|
Region |
Score |
Skin condition |
|
Face |
0 |
Normal |
|
1 |
Wispy fur |
|
2 |
Alopecia and flare |
|
3 |
Bleeding or ulcerative lesion |
|
Ears |
0 |
Normal |
|
1 |
Flare |
|
2 |
Bleeding |
|
3 |
Loss of part of the ear |
|
Back |
0 |
Normal |
|
1 |
Wispy fur |
|
2 |
Alopecia and flare |
|
3 |
Bleeding or ulcerative lesion |

Cells and flow cytometric assay
Subcutaneous adipose tissue was obtained from 10-week aged naïve male rats. ASCs were isolated and expanded as previously described [
18]. The tissues were washed several times with PBS and minced on ice, followed by incubation with an equal volume of 0.075% collagenase I at 37°C for 1 h. After centrifugation, pellet was suspended in 5% DMEM and passed through a 70 μm cell strainer. ASCs were cultured in 5% DMEM (Welgene, Gyeongsan, Korea) containing 100 μg/ml of streptomycin and 100 U/ml of penicillin, supplemented with 5% CO
2. ASCs at passage number 3 or 4 were used for the present study. ASCs (1 × 10
6 cells/10 μl Hartman solution) were injected into AD rats
via the retro-orbital sinus once a week for a month [
19].
PBMCs were isolated from whole blood by density gradient centrifugation using HISTOPAQUE-1077 (density: 1.077 g/ml; Sigma). After centrifugation, a white cloudy layer was obtained. Cells were incubated with adequate antibodies such as anti-CD4 (BD Bioscience, San Jose, CA, USA), anti-IFN-γ (eBioscience, San Diego, CA, USA), anti-IL-4 (eBioscience), anti-CD163 (GHI/61), or isotype controls (BioLegend, San Diego, CA, USA).
Transwell assays were performed using fresh PBMCs obtained from AD and naïve animals. PBMCs (1 × 106 cells) and cultured ASCs (1 × 104 cells) were plated in the bottom and top chambers (0.4 μm; SPL, Pocheon, Korea) of a transwell, respectively, and then incubated for 24 h at 37°C in 5% CO2.
All cells were counted using a BD FACSCalibur (BD Biosciences). Data were analyzed using FCS express software (ver. 5, De Nove Software, Pasadena, CA, USA).
Phagocytosis assay
As previously described [
20,
21], phagocytic activity was evaluated by flow cytometry using a pHrodo-conjugated zymosan A bioparticles Phagocytosis kit (Invitrogen, Life Technologies, Carlsbad, CA, USA), according to the manufacturer’s instructions. PBMCs (1 × 10
6 cells) were incubated with zymosan A bioparticles (0.5 mg/ml) at 37°C in the incubator for 1–2 h and then washed twice with PBS. Data are represented as the fraction of cells bearing fluorescent zymosan A bioparticles to the total monocyte gate.
Evaluation of apoptosis
The apoptosis of PBMCs was assayed using annexin V/7-AAD staining [
22]. PBMCs were incubated successively with annexin V-FITC and 7-AAD in the dark for 15 and 5 min, respectively. Annexin V
+/7-AAD
+ cells were counted by flow cytometry. Data are presented as percentages of total PBMCs.
Serological and histological analysis
Serum IgE levels were measured using enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (CUSABIO, Wuhan, China). Rat skin obtained one week after the last ASC treatment, were fixed with 4% formalin and embedded in paraffin. Sections (5 μm in thickness) were prepared and subjected to hematoxylin and eosin (H&E), Gram, and immunofluorescent staining with anti-S. aureus (Abcam, Cambridge, UK).
Quantification of cutaneous S. aureus
Cutaneous
S.
aureus was cultured and evaluated, as previously described [
17]. Skin samples (2 mm in diameter) were obtained
via punching biopsy. The samples (each 30 mg) were homogenized in pure water (Welgene) and serially diluted by a factor of 10. The diluted sample (100 μl) was inoculated on HiCropme Aureus Agar Base (Sigma-Aldrich) plate and then incubated at 37°C for 24 h. The number of colonies were counted in duplicate and expressed as colony-forming units (CFU) per mg of skin sample. The logarithmic scale of the number of colonies was used for the statistical analysis.
Quantitative real-time PCR
PBMCs and skin samples were collected from 8-week old AD and ASC-treated rats. Total RNA was isolated according to the manufacturer’s instructions using TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA). The purified total RNA was reverse-transcribed to cDNA using the Reverse Transcription System (Promega, Madison, WI, USA). For quantitative analysis, we used the following pairs of primers: GAPDH (F: ACTTTGGCATCGTGGAAGGG, R: ACATTGGGGGTAGGAACACG), FasL (F: AACTCCGTGAGTTCACCAACC, R: CCTCATTGATCACAAGGCCG), Cathelicidin (F: CCTGGATTCTGAGCCCCAAG, R: TGTATACCAGGCGCATCACA), and β-defensin (F: GGGTGCTGGCATTCTCACAA, R: TCCTGCAACAGTTGGGCTTAT). The relative expression of each gene was calculated using the 2ΔΔCT method and each group was normalized to naïve values.
Statistics
All data are presented as mean ± SEM. Student’s t-test and One-way ANOVA were used wherever appropriate for statistical analysis. Statistical significance was set at p < 0.05. All statistical analyses were performed using the Sigma Stat (ver. 3.5; Systat Software Inc., San Jose, CA, USA).
Go to :

DISCUSSION
S.
aureus has long been recognized as an important player in the pathogenesis of AD; therefore, decolonization of
S.
aureus is considered essential in AD treatment [
23]. Our results revealed that systemic administration of ASCs not only prevented apoptosis of PBMCs, but also enhanced the phagocytic activity of PBMCs in AD rats. In addition, we demonstrated that ASCs were competent enough to increase AMPs in the skin of AD rats. These effects of ASCs are likely to be partly responsible for the decolonization of
S.
aureus.
Accumulating evidence has demonstrated that cutaneous colonization and/or infection by
S.
aureus induces apoptotic cell death in patients with AD, which is pivotal for the development or aggravation of AD [
3,
24]. Dysregulated apoptosis is known to upset immune homeostasis by abnormal differentiation of immune cells and cytokine production [
25,
26], which makes the environment susceptible to the development of inflammatory skin diseases. Many virulence factors in
S.
aureus induce apoptotic cell death through various apoptotic processes [
27]. Among these processes, the Fas/FasL signaling pathway is likely to be involved in
S.
aureus toxin-induced apoptosis of PBMCs [
3,
24]. FasL (CD95L or CD178), a type-II transmembrane glycoprotein belonging to the tumor necrosis factor (TNF) family, plays a key role in apoptotic cell death in target cells expressing Fas on the surface. A previous study reported that staphylococcal enterotoxin B increased Fas-mediated apoptosis of PBMCs obtained from patients with AD [
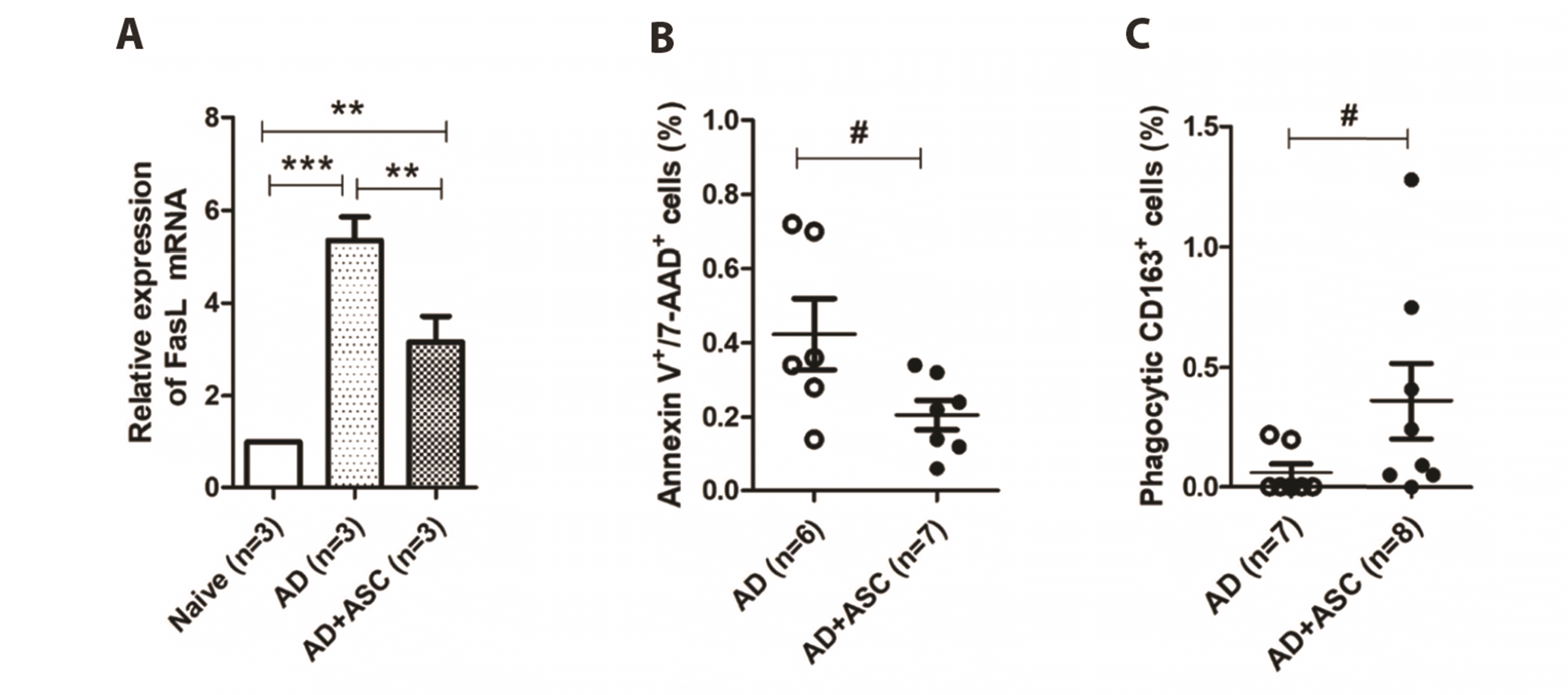
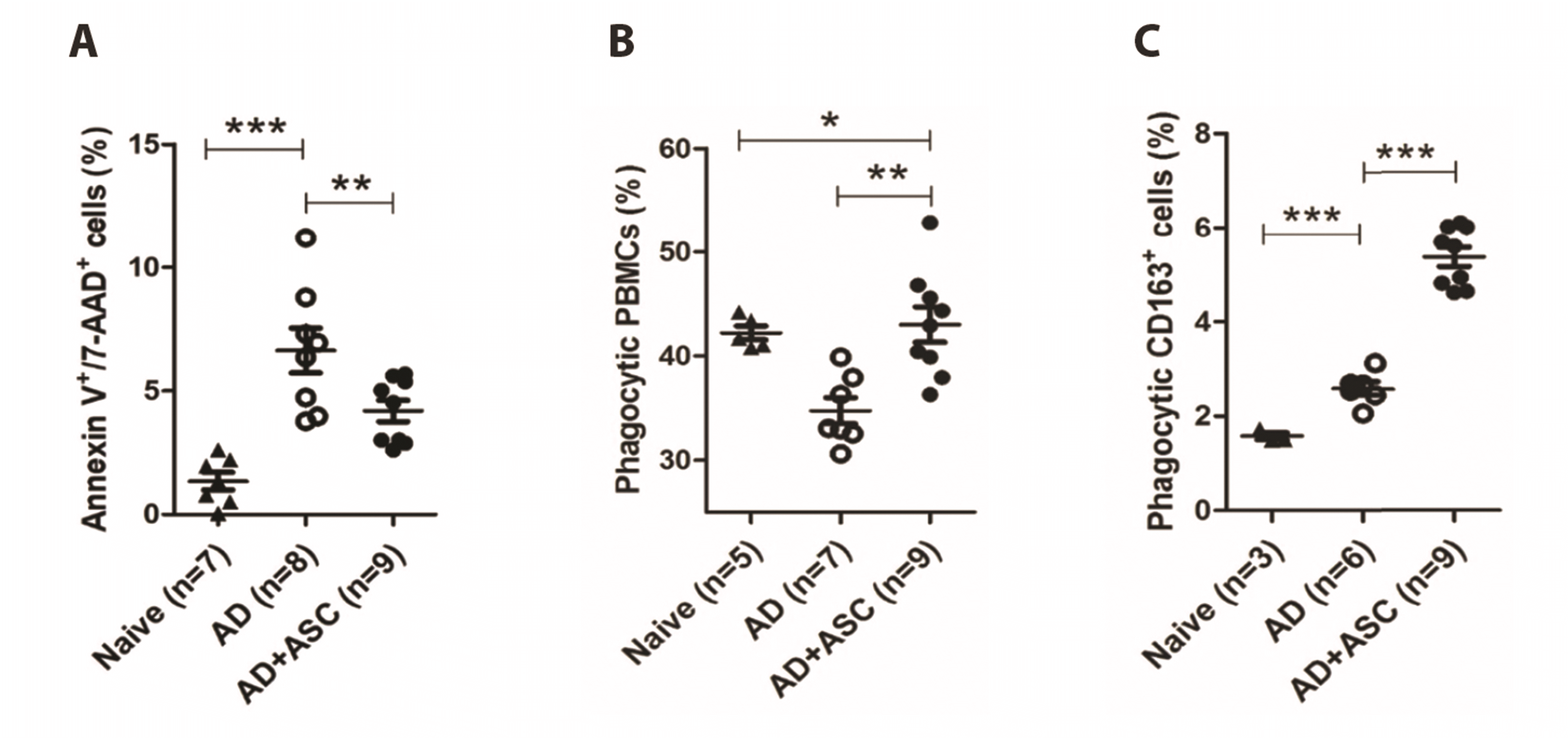
24]. The authors also reported that FasL expression was significantly increased in peripheral monocytes of patients. Consistently, in the present study, we also observed increase in FasL mRNA expression and apoptotic cell death in PBMCs of AD rats (
Fig. 3A). However, in PBMCs co-cultured with ASCs, FasL mRNA expression and apoptotic cells were significantly reduced, although the underlying mechanisms are not suggested herein. It has been also reported that Fas/FasL signals induce keratinocyte apoptosis [
28] and skin inflammation that is independent of caspase activity [
29], aggravating eczematous lesions in AD patients. It is noteworthy that pharmacological intervention in cell apoptosis, including interference of Fas/FasL interaction and some cytokines related to cell apoptosis, has therapeutic effects in murine models of
S.
aureus-induced sepsis [
30].
Another important finding of the present study is that the systemic administration of ASCs enhances the phagocytic activity of PBMCs and M2 macrophages. The removal of apoptotic cells by phagocytes is important for maintaining tissue homeostasis, and the process is immunologically silent under physiological conditions [
6]. Thus, it is not hard to imagine that defects in apoptotic cell clearance are closely linked to the development of many diseases, such as chronic inflammation and autoimmune diseases [
6]. Conversely, the enhancement of phagocytosis is an attractive therapeutic method for these diseases. In the present study, using a phagocytic assay, we showed that the uptake of fluorescent zymosan bioparticles reduced significantly in the PBMCs of AD rats as compared to naïve healthy donors (
Fig. 4B), indicating a reduction in phagocytic activity of these cells in AD conditions. Additionally, ASCs treatment drastically enhanced the phagocytic activity of both PBMCs and CD163
+ cells. Consistent with our results, a recent clinical study also reported defects in the phagocytic activity of mononuclear cells obtained from patients with AD [
31]. The authors of the clinical study suggested that excessive activation of the complement system is a possible cause. It is unclear how ASCs enhance the phagocytic activity of PBMCs and M2 macrophages. Several lines of evidence indicate that upregulation of anti-inflammatory cytokines, such as IL-10, following ASC treatment are related to the enhancement of phagocytic activity [
32-
34]. It has also been suggested that upregulation of CD206, an important scavenger receptor of M2 macrophages, enhances phagocytic activity in systemic lupus erythematosus [
5]. However, it should be noted that unlike in autoimmune diseases such as systemic lupus erythematosus; MSCs prevent differentiation of CD163
+ M2 macrophages in AD conditions characterized by Th2- and M2 macrophage-dominant inflammation [
35]. In the present study, the systemic administration of ASCs reduced the total number of CD163
+ cells in AD rats. However, many more CD163
+ cells that had taken fluorescent zymosan bioparticles were counted in AD rats treated with ASCs, indicating functional enhancement of M2 macrophages. Han
et al. [
36] reported that M2 macrophages functionally activated by pharmacological intervention have therapeutic effects on AD in mice. Thus, ASCs treatment may be useful for restoring or enhancing the phagocytic activity of phagocytes.
AMPs, also known as host defense peptides, are key components of the innate immune system that provide protection against invading pathogens [
37]. In the human skin, nonpathogenic commensal bacteria and keratinocytes produce AMPs that inhibit colonization by pathogenic microbes. However, AMPs, especially cathelicidin (LL-37) and β-defensin, which have antimicrobial activities against
S.
aureus, are diminished in the human AD skin. Overexpression of Th2 cytokines, a hallmark of AD, has been known to be involved in the downregulation of AMPs [
38,
39]. Here, we showed that mRNA levels of cathelicidin and β-defensin were significantly increased in the skin of AD rats following systemic administration of ASCs. Cutaneous increases in AMPs might be correlated with skin decolonization of
S.
aureus and, therefore, improvement of skin conditions in AD rats. The upregulation of AMPs seems to be associated with the role of ASCs in the differentiation of Th1 and Th2 cells, both of which are identified by their representative cytokines, INF-γ and IL-4, respectively. In the present study, we showed that ASCs treatment led to an increase in the IFN-γ
+/IL-4
+ cell ratio, suggesting the alleviation of Th2 responses. Consistently, ASCs treatment reduced serum levels of IgE and the total number of CD163
+ cells, both of which are upregulated by Th2 cytokines. A previous study demonstrated that topical application of ASCs-cultured medium promotes the production of AMPs in mouse skin [
40]. ASCs are known to produce antimicrobial factors [
41]. In conclusion, ASCs administration can help in decolonizing
S.
aureus from AD skin by upregulating AMPs.
S.
aureus infection is a major challenge in the management of AD because of its diverse roles in the development or aggravation of the disease [
2-
4]. Many virulence factors of
S.
aureus, such as cytolytic toxins, exfoliative toxins, superantigens, destructive enzymes and protein A, are involved in skin inflammation and further immune dysregulation, causing a defective skin barrier [
42]. In addition, cutaneous colonization of
S.
aureus reduces the diversity of the normal microflora of the skin that maintains immune homeostasis and prevents the growth of pathogens, making more susceptible environment for outgrowth of
S.
aureus [
7,
8,
42]. Thus, decolonization may be essential for improving the clinical symptoms of AD.
In the present study, we showed that ASCs not only prevent apoptotic cell death of the PBMC but also enhance the phagocytic activities of PBMC with inhibiting Th2- and M2 macrophage-dominant inflammation in AD condition, contributing to decolonization of S. aureus in the skin. Antibiotics could be chosen for decolonizing S. aureus. However, it’s effectiveness is doubtful due to the occurrence of antibiotic resistance and bactericidal effects on beneficial normal flora, such as S. epidermidis and S. hominis. Therefore, the use of MSCs could be an alternative therapy for the decolonization of S. aureus.
Go to :






 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download