INTRODUCTION
METHODS
Animals and artery preparation
Oxytocin-induced vasorelaxation
Oxytocin-induced vasoconstriction
Human umbilical vein endothelial cells (HUVECs) culture
Western blot
Statistical analysis
RESULTS
Oxytocin increased the protein expression of eNOS phosphorylation through the activation of PI3K/Akt pathway in HUVECs
 | Fig. 1Effects of oxytocin on the protein expressions of phospho-endothelial nitric oxide synthesis (peNOS, Ser 1177) and eNOS in HUVECs.Oxytocin time-dependently increased peNOS (A) but not total eNOS expression (B), oxytocin dose-dependently increased peNOS (C) but not total eNOS expression (D). Data is expressed as mean ± SE. n = 6. HUVECs, human umbilical vein endothelial cells; OT, oxytocin. *p < 0.05 vs. control group.
|
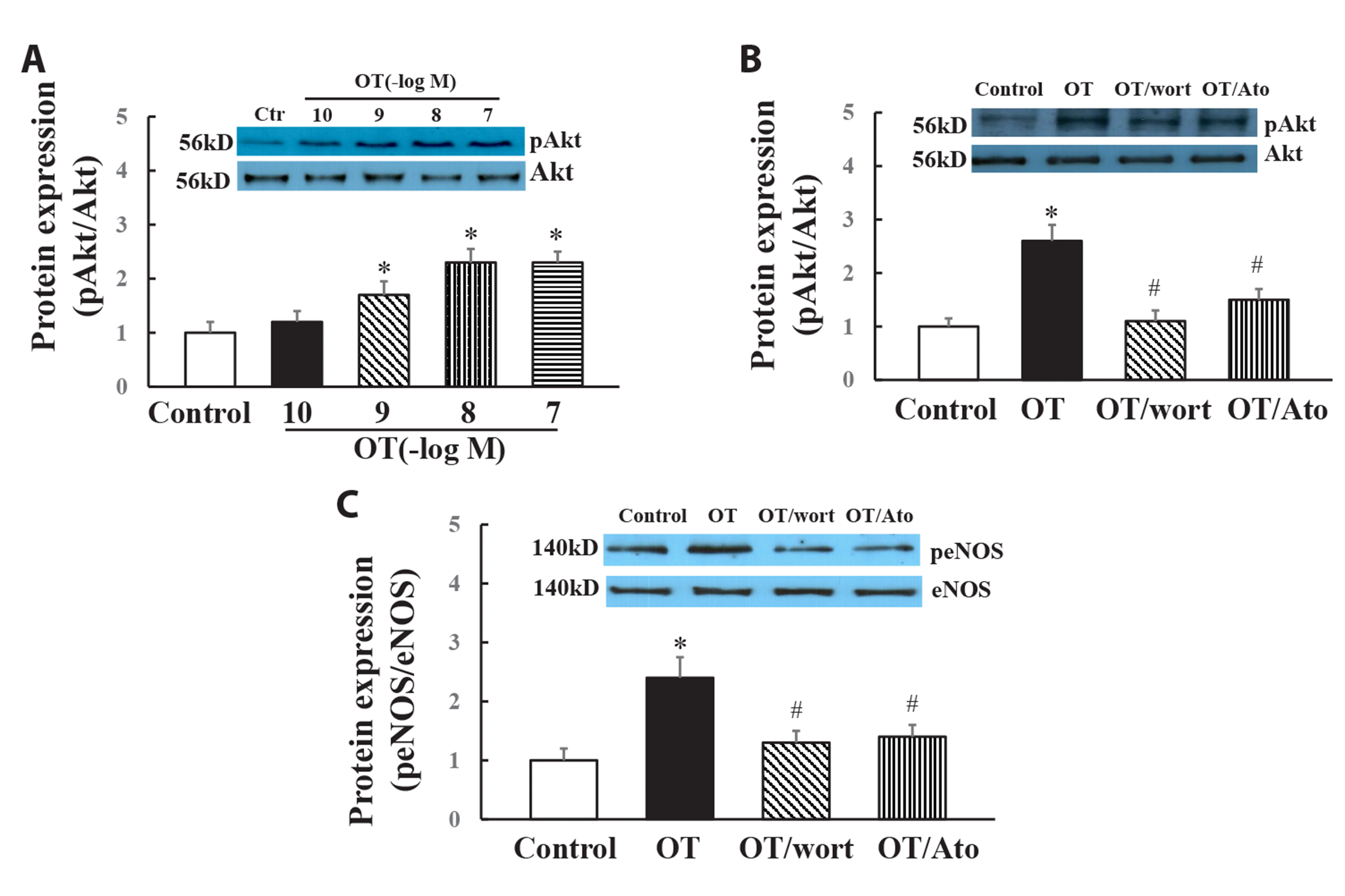 | Fig. 2Effects of PI3K inhibitor wortmannin or oxytocin/vasopressin 1a receptor antagonist atosiban on pAkt (tyr473) or peNOS (ser1177) in the cultured HUVECs.Oxytocin dose-dependently increased Akt phosphorylation (A); Either wortmannin or atosiban significantly attenuated oxytocin-induced Akt (B) or eNOS phosphorylation (C). PI3K, phosphatidylinositol 3-kinase; HUVECs, human umbilical vein endothelial cells; OT, oxytocin; Wort, wortmannin; Ato, atosiban. n = 6, *p < 0.05 vs. control group, #p < 0.05 vs. oxytocin group.
|
Oxytocin induced eNOS phosphorylation in rat aorta ex vivo
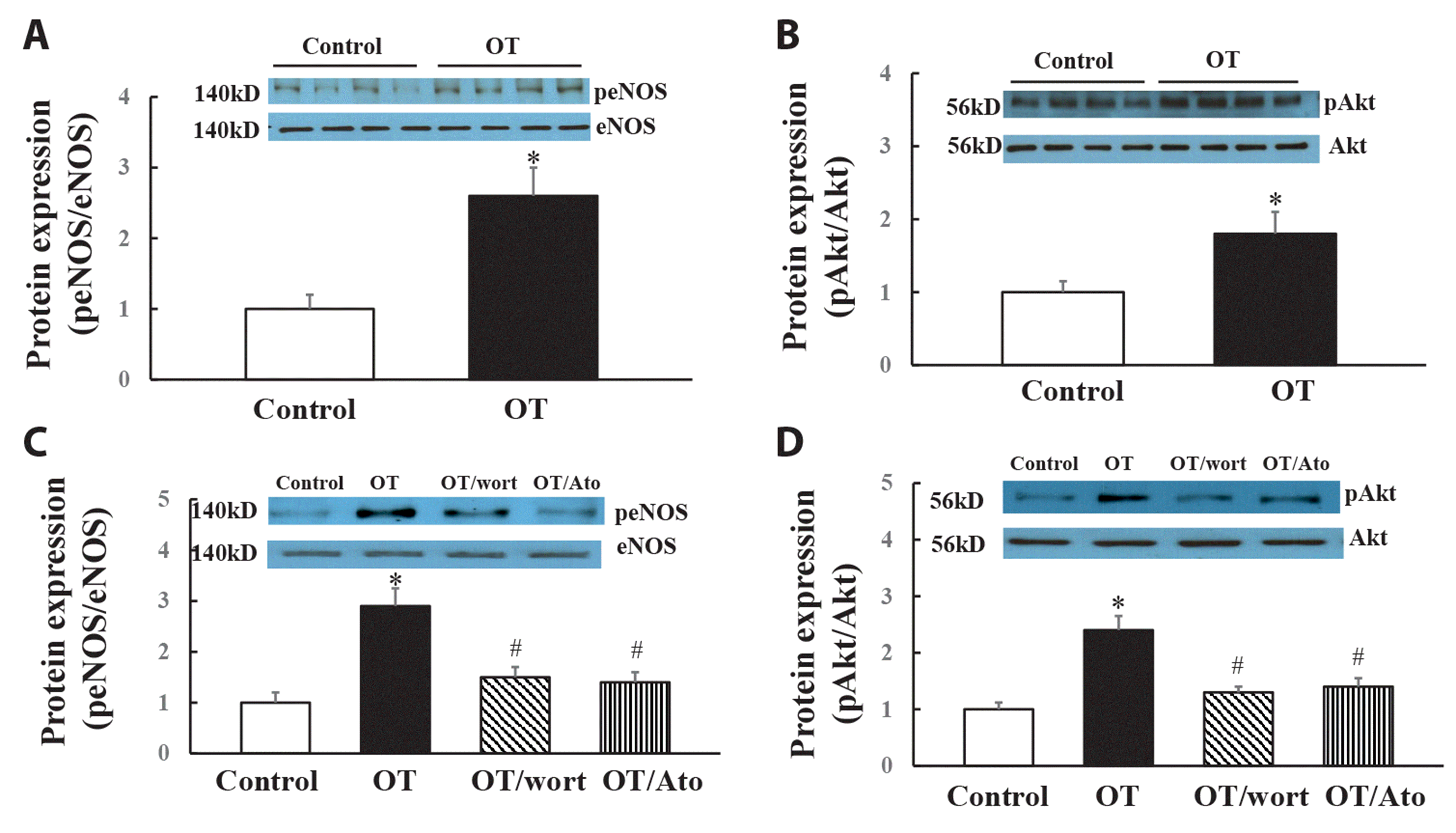 | Fig. 3Effects of oxytocin on pAkt (Tyr 473) and peNOS (Ser 1177) expressions in the rat aorta ex vivo.Incubation of oxytocin (10 nM) for 5 min ex vivo significantly increased pAkt (Tyr 473, A) and peNOS (Ser 1177, B) expression. Either wortmannin or atosiban prevented oxytocin-indued pAkt (C) and peNOS (D) expression. OT, oxytocin; Wort, wortmannin; Ato, atosiban. n = 8, *p < 0.05 vs. control group; #p < 0.05 vs. oxytocin group.
|
Oxytocin induced endothelial NO dependent vasorelaxation
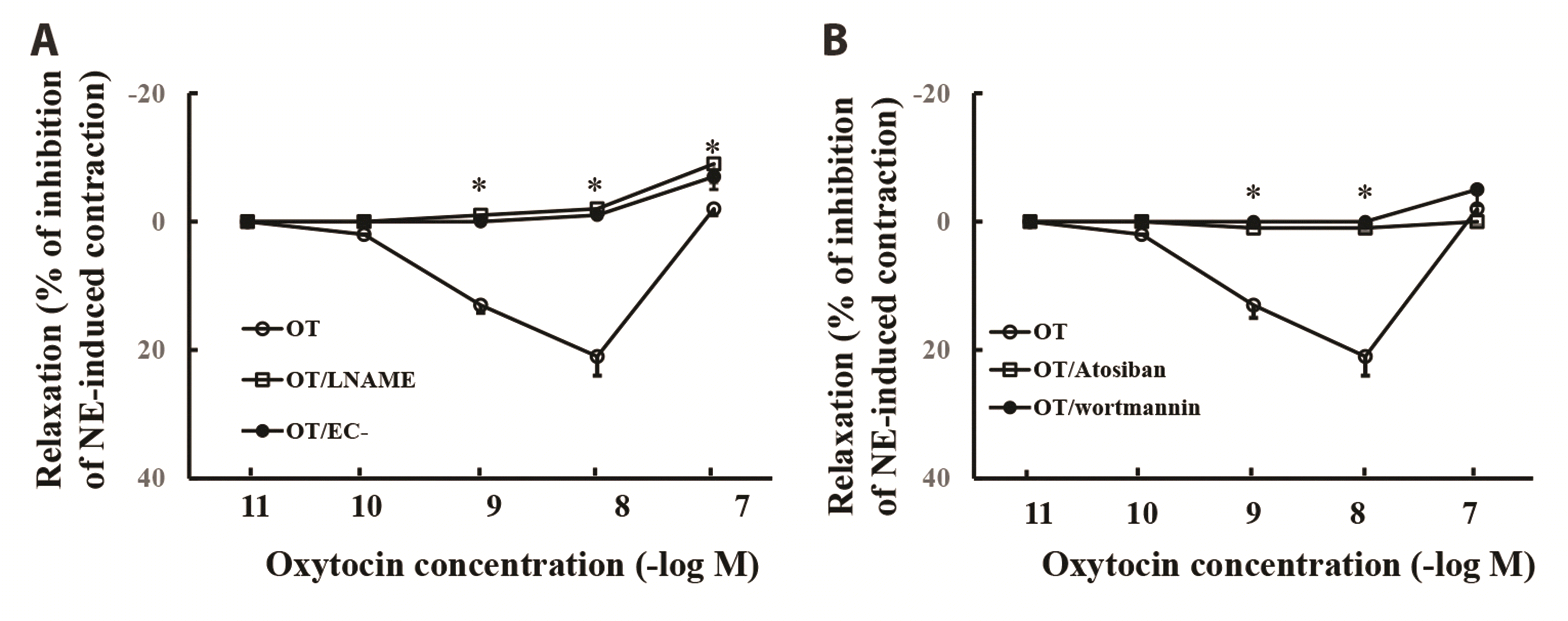 | Fig. 4Effect of oxytocin (OT) on the vascular reactivity in the rat’s aortic rings pre-constricted by norepinephrine.(A) L-NAME (an inhibitor of nitric oxide synthase) or endothelium removal (EC–) abolished oxytocin-induced vasorelaxation and slightly enhanced oxytocin-induced vasoconstriction; (B) Atosiban or wortmannin abolished oxytocin-induced vasorelaxation. n = 8. *p < 0.05 vs. oxytocin group.
|
Oxytocin induced vasoconstriction in the unstimulated aortic rings
 | Fig. 5Oxytocin induced vasoconstriction in the unstimulated rat’s aortic rings.L-NAME (an inhibitor of nitric oxide synthase) (A) or endothelium removal (B) slightly enhanced oxytocin-induced vasoconstriction; (C) SQ29548 (an inhibitor of prostaglandin H2/thromboxane A2) did not affect oxytocin-induced vasoconstriction; (D) Atosiban completely abolished oxytocin-induced vasoconstriction; (E) PD98059 (an inhibitor of ERK1/2 activation) partially inhibited oxytocin-induced vasoconstriction; (F) Effect of oxytocin on phospho-ERK1/2 expression. OT, oxytocin; Ato, atosiban. n = 8. *p < 0.05 vs. control group, #p < 0.05 vs. oxytocin group.
|




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download