TO THE EDITOR: Plasma cell leukemia (PCL) is a rare disease that accounts for approximately 2% of all plasma cell myeloma (PCM) cases. It is defined as the presence of clonal plasma cells (PCs) in more than 20% of the total nucleated cells (TNCs) or when absolute TNC count is higher than 2×10-3/L in the peripheral blood. Primary PCL (pPCL) is the initial leukemic presentation of myeloma, which represents 60% of PCL cases [1]. Although patients with PCLs generally have a poor prognosis, early and aggressive chemotherapy and stem cell transplantation (SCT) may improve the prognosis [2]. Clonal PCs can appear in an atypical form, such as small-cell, cleaved, polymorphous, asynchronous, or blastic types, in addition to the Marshalko type, which is indistinguishable from a typical PC [3]. Therefore, the accurate identification of neoplastic PCs in the bone marrow and peripheral blood is important. Herein, we report a case of pPCL that was diagnosed by flow cytometry (FCM) due to morphological similarities between the clonal PCs and small-mature lymphocytes. This study was reviewed and approved by the institutional review board of Soonchunhyang University Hospital in Seoul, South Korea (IRB No. 2021-09-007).
In December 2019, a 65-year-old man visited the emergency room at Soonchunhyang University Seoul Hospital with acute chest pain. No specific abnormalities were observed in the lungs or on cardiovascular examination. The initial complete blood count (CBC) revealed pancytopenia as follows: Hb, 47 g/L (120–160 g/L); WBC, 2.8×109/L (4.0–10.0×109/L); and platelet count, 45×109/L (130–450×109/L). Peripheral blood smears demonstrated small-mature lymphoid cells, which accounted for up to 20% of the TNCs (Fig. 1A), and bone marrow aspirate smears revealed lymphoid cells comprising 88% of the TNCs (Fig. 1B) together with occasional multinucleated atypical lymphoid cells (Fig. 1C, D). Immunophenotype analysis by FCM (Navios EX flow cytometer and Kaluza analysis software, Beckman Coulter, Inc., Miami, FL, USA) of the bone marrow and peripheral blood specimens revealed abnormal PC populations accounting for 80% and 20% of TNCs, respectively. CD45-/CD19-/CD20+/CD38+/CD138+/CD56-/CD117+/cCD79a+/FMC7+ cells and cytoplasmic lambda light chain restriction (Fig. 2) was observed. Serum protein electrophoresis (PEP) and immunofixation electrophoresis (IFE) detected monoclonal gammopathy of the IgA and lambda types (M protein, 4.7 g/L). In the quantitative serum immunoglobulin test by an immunoturbidimetric assay (cobas c702 analyzer, Roche, Ibaraki, Japan; cobas ALB2 reagent, Mannheim, Germany), the IgA and free lambda light-chain were measured as 6.44 g/L (7.0–40.0 g/L) and 0.095 g/L (0.057–0.263 g/L), respectively. In the urine PEP and IFE, lambda-type Bence–Jones proteinuria was detected with an M-protein level of 0.12 g/L. FISH analysis (Fig. 1E) demonstrated IGH-CCND1 rearrangement and p53 (17p) deletion (dual-fusion probe, designed by Cytocell, Cambridge, UK and Metafer/Zeiss system, Metasystems, Altlussheim, Germany), and next-generation sequencing (NGS) identified suspected pathogenic mutations of TP53 (c.490A>G, p.Lys164Glu) (customized panel designed by Celemics, Inc., Seoul, Korea). On the basis of these results, the patient was diagnosed with pPCL. Allogenic hematopoietic SCT was performed after the patient achieved complete remission with chemotherapy (carfilzomib, lenalidomide, and dexamethasone). Nevertheless, the patient succumbed to death due to pneumonia 5 months after undergoing the SCT.
Small-cell-type PCs are small (mean size of 13 µm; compared with 21 µm for the Marschalko type) and have a round shape with only a narrow rim of a cytoplasm. Their nuclei have dense chromatin with rare nucleoli and mitoses resembling those of small lymphocytes [3]. Given their morphological similarities, the microscopic detection of small-cell-type PCL may be challenging to discriminate from small mature lymphocytes. While neoplastic PCs of PCM usually exhibit the CD19-/CD45variable/CD38+/CD56- or + immunophenotype, neoplastic PCs of PCL exhibit a more-frequent expression of CD20 and a less-frequent expression of CD56 [4, 5]. Several myeloma studies have shown that CD20 expression is associated with small-cell-type neoplastic PCs and t(11;14) in PCM [6, 7]. As CD56 is an adhesion molecule, PCs with no CD56 expression may reduce cell-to-cell interactions, easily escape from the bone marrow, invade extramedullary organs, including the peripheral blood, and proliferate abnormally beyond immune surveillance [4, 5].
To date, only 5 cases of small-cell-type PCL (Table 1) have been reported, while clonal PCs of the small-cell-type are found only in 3.4% of PCM [7-10]. The median age of the 6 cases including ours was 70.2 years with no demographic preponderance. The 6 cases of small-cell-type PCL demonstrated a variable expression of CD20 and loss of CD56. t(11;14) (IGH/CCND1 rearrangement), and overexpression of CCND1 or CCND2 was detected in 5 cases. Their secreting components were IgG or IgA with lambda or kappa light chains, including only one kappa light chain. The amount of M protein was not associated with the tumor burden. Two patients died within a year of starting chemotherapy.
In summary, we report a rare case of pPCL with t(11;14) presenting as the small-cell type. When diagnosing PCL of an atypical plasma cell type, it is mandatory to use FCM immunophenotyping for the bone marrow and peripheral blood specimens with morphology and IFE.
REFERENCES
1. Gonsalves WI, Rajkumar SV, Go RS, et al. 2014; Trends in survival of patients with primary plasma cell leukemia: a population-based analysis. Blood. 124:907–12. DOI: 10.1182/blood-2014-03-565051. PMID: 24957143. PMCID: PMC4126330.

2. Mina R, Joseph NS, Kaufman JL, et al. 2019; Survival outcomes of patients with primary plasma cell leukemia (pPCL) treated with novel agents. Cancer. 125:416–23. DOI: 10.1002/cncr.31718. PMID: 30332496.

3. Bartl R, Frisch B, Fateh-Moghadam A, Kettner G, Jaeger K, Sommerfeld W. 1987; Histologic classification and staging of multiple myeloma. A retrospective and prospective study of 674 cases. Am J Clin Pathol. 87:342–55. DOI: 10.1093/ajcp/87.3.342. PMID: 3825999.

4. Raja KR, Kovarova L, Hajek R. 2010; Review of phenotypic markers used in flow cytometric analysis of MGUS and MM, and applicability of flow cytometry in other plasma cell disorders. Br J Haematol. 149:334–51. DOI: 10.1111/j.1365-2141.2010.08121.x. PMID: 20201947.

5. Pellat-Deceunynck C, Barillé S, Jego G, et al. 1998; The absence of CD56 (NCAM) on malignant plasma cells is a hallmark of plasma cell leukemia and of a special subset of multiple myeloma. Leukemia. 12:1977–82. DOI: 10.1038/sj.leu.2401211. PMID: 9844928.

6. Robillard N, Avet-Loiseau H, Garand R, et al. 2003; CD20 is associated with a small mature plasma cell morphology and t(11;14) in multiple myeloma. Blood. 102:1070–1. DOI: 10.1182/blood-2002-11-3333. PMID: 12702507.

7. Heerema-McKenney A, Waldron J, Hughes S, et al. 2010; Clinical, immunophenotypic, and genetic characterization of small lymphocyte-like plasma cell myeloma: a potential mimic of mature B-cell lymphoma. Am J Clin Pathol. 133:265–70. DOI: 10.1309/AJCPUS3PRRT5ZXVS. PMID: 20093236. PMCID: PMC4433023.

8. Gounari E, Kaiafa G, Koletsa T, et al. 2018; CD5+ B lymphoproliferative disorder with subsequent development of plasma cell leukaemia: diagnostic and aetiologic reasoning. Cytometry B Clin Cytom. 94:688–94. DOI: 10.1002/cyto.b.21596. PMID: 29024518.

9. Loureiro AD, Gonçalves MV, Ikoma MR, et al. 2017; Plasma cell leukemia with t(11;14)(q13;q32) simulating lymphoplasmacytic lymphoma - a diagnostic challenge solved by flow cytometry. Rev Bras Hematol Hemoter. 39:66–9. DOI: 10.1016/j.bjhh.2016.10.001. PMID: 28270351. PMCID: PMC5339392.

10. Teriaky A, Hsia CC. 2011; Plasma cell leukemia mimicking chronic lymphocytic leukemia. Blood. 117:2991. DOI: 10.1182/blood-2010-02-269977. PMID: 21528511.

Fig. 1
Microscopic findings and FISH analysis of the small-cell type of plasma cell leukemia. Peripheral blood and bone marrow aspirate smears: small-cell type neoplastic plasma cells and atypical multinu-clear plasma cells (A), peripheral blood, Wright–Giemsa stain, ×400; (B), bone marrow, Wright–Giemsa stain, ×400; (C, D) bone marrow, Wright–Giemsa stain, ×1,000. (E) FISH analysis showing an IGH/ CCND1 rearrangement and p53 (17p) deletion (white arrow).
Abbreviation: FISH, fluorescence in situ hybridization.
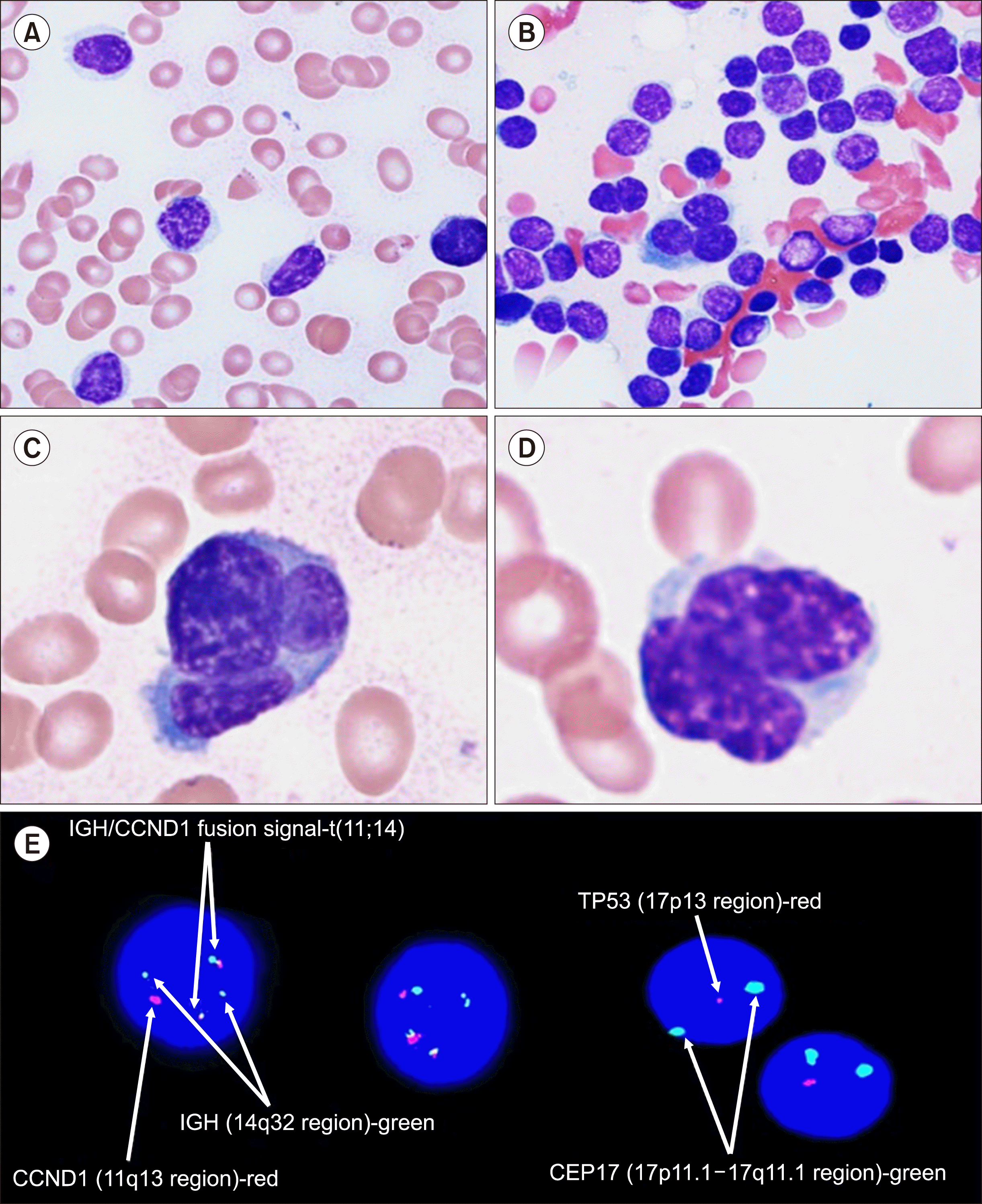
Fig. 2
Flow-cytometric immunophenotyping of the small-cell type of plasma cell leukemia. Clonal plasma cells in the bone marrow exhibited a CD19-/CD20+/CD38+/CD56-/CD138+ phenotype and cytoplasmic lambda light-chain restriction gated by CD45- and low side scatter.
Table 1
Basic clinical characteristics of six patients with small-cell-type plasma cell leukemia.
| Present patient | Heerema-McKenney et al. [7] | Heerema-McKenney et al. [7] | Gounari et al. [8] | Loureiro et al. [9] | Teriaky et al. [10] | |
|---|---|---|---|---|---|---|
| Sex/age (yr) | M/65 | F/59 | M/74 | M/61 | F/77 | F/85 |
| Initial CBC |
WBC: 2.8×109/L Hb: 47 g/L Platelet: 45×109/L |
NR | NR |
WBC: 12.3×109/L Hb: 98 g/L Platelet: 89×109/L |
WBC: 8.6×109/L Hb: 101 g/L Platelet: 140×109/L |
WBC: 28.2×109/L Hb: 92 g/L Platelet: 238×109/L |
| Plasma cells (BM/PB), % | >80.0 | >80.0 | >80.0 | 54.0 | 90.0 | 75.0 |
| 20.0 | 40.0 | 52.0 | 22.7 | 43.5 | 74.0 | |
| Immunophenotyping results | CD45-/CD19-/CD20+/CD38+/CD138+/CD56-/CD117+/cCD79a+/ FMC7+ and lambda restriction | CD45-/CD20-/CD38+/CD138+/CD56-/CD117- | CD45-/CD38+/CD138+ | CD45-/CD19-/CD20dim+/CD38+/CD138+/CD56-/CD200+ and kappa restriction | CD45-/CD19-/ CD38+/CD138dim+/CD56-/CD117-/CD28-/CD81+ and kappa restriction | CD45-/CD19partia+l/CD20dim+/CD38+/CD138+ and kappa restriction |
| PEP/IFE | IgA-lambda (5 g/La)) | IgG-lambda (4g/La)) | IgG-lambda (30g/La)) | IgA-kappa | IgG-kappa (44g/La)) | kappa |
| Cytogenetic study | 42,XY,-1,-9,-10, ?t(11;14)(q13;q32),-14,-16,?add(17)(p11.2)der(19) t(1;19)(p13;p13.3),-20,-21, +der(?)t(?;21)(?;q11.2), +2mar[6]/46,XY [14] | 46,XX[19]/45,XX,−20[11] | 46,XY, t(11;14)(q12∼13.1;q32), del(13)(q14q22),?del(17)(p12)[cp2]/46,XY[18] | Hyperdiploid clone with trisomy 2, 3, 12, and 18 | NR | NR |
| Molecular study | IGH/CCND1 rearrangement, 13q14 deletion, p53 (17p) deletionb), TP53 (c.490A>G, p.Lys164Glu)c) | Overexpression of MS4A1/CD20 and CCND2d) | Overexpression of CCND1d) | Overexpression CCND1e) | IGH/CCND1 rearrangementb) | NR |
| Disease course | Died after 5 months after allo-PBSCT | NR | NR | CR after CTx | Died 3 months after diagnosis | CR after CTx |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download