Abstract
Background
Nilotinib is a tyrosine kinase inhibitor approved by the Ministry of Food and Drug Safety for frontline and 2nd line treatment of Philadelphia chromosome-positive chronic myeloid leukemia (Ph+ CML). This study aimed to confirm the safety and efficacy of nilotinib in routine clinical practice within South Korea.
Methods
An open-label, multicenter, single-arm, 12-week observational post-marketing surveillance (PMS) study was conducted on 669 Korean adult patients with Ph+ CML from December 24, 2010, to December 23, 2016. The patients received nilotinib treatment in routine clinical practice settings. Safety was evaluated by all types of adverse events (AEs) during the study period, and efficacy was evaluated by the complete hematological response (CHR) and cytogenetic response.
Results
During the study period, AEs occurred in 61.3% (410 patients, 973 events), adverse drug reactions (ADRs) in 40.5% (271/669 patients, 559 events), serious AEs in 4.5% (30 patients, 37 events), and serious ADRs in 0.7% (5 patients, 8 events). Furthermore, unexpected AEs occurred at a rate of 6.9% (46 patients, 55 events) and unexpected ADRs at 1.2% (8 patients, 8 events). As for the efficacy results, CHR was achieved in 89.5% (442/494 patients), and minor cytogenetic response or major cytogenetic response was achieved in 85.8% (139/162 patients).
Chronic myeloid leukemia (CML) is a clonal hematopoietic stem cell disorder caused by reciprocal translocation between the ABL1 locus of chromosome 9 and the BCR region of chromosome 22, which results in the BCR-ABL1 fusion gene, known as the Philadelphia chromosome (Ph) [1, 2].
Nilotinib is a novel aminopyrimidine available as an oral formulation that is an ATP-competitive inhibitor of the BCR-ABL protein tyrosine kinase activity, and which prevents the activation of BCR-ABL-dependent mitogenic and anti-apoptotic pathways, thus leading to death of the BCR-ABL phenotype. Multiple clinical trials have demonstrated the efficacy of nilotinib in various diseases — including CML. In a phase II open-label study in patients with imatinib-resistant or intolerant CML in the chronic phase (CP), nilotinib showed high efficacy and safety, with minimal cross-intolerance with imatinib [3]. In a multinational ENESTnd study comparing nilotinib with imatinib in newly diagnosed patients with CML-CP, nilotinib demonstrated significantly higher rates of complete cytogenetic response (CCyR) and major molecular response (MMR) [4]. As a result, nilotinib is indicated for the treatment of patients with newly diagnosed CML-CP, as well as for the treatment of patients with CML resistant or intolerant to previous treatment in the CP or accelerated phase (AP) [5].
In South Korea, nilotinib (Tasigna) treatment is administered at a dose of 300 mg twice a day (BID) for patients with newly diagnosed CML in the chronic phase (frontline CML-CP treatment), and 400 mg BID for patients with Ph+ CML resistant or intolerant to previous treatment including imatinib, in the chronic or accelerated phase (2nd-line CML treatment). These dosages and applications were approved on December 24, 2010, by the Ministry of Food and Drug Safety (MFDS) [6]. This study aims to evaluate the post-marketing surveillance (PMS) information of Tasigna in Korea under routine clinical practice, in addition to the safety and efficacy information approved by the MFDS — including (but not limited to) unknown adverse drug reactions (ADRs), especially serious adverse drug reactions (SADRs), incidences of adverse events (AEs), and efficacy.
An open-label, multicenter, single-arm, 12-week observational PMS study was conducted to evaluate the safety and efficacy of nilotinib in Korea. The study population included adult Ph+ CML patients in CP or AP according to "indications" of the current approved local product labeling [6]. Resistance or intolerance to previous treatments, including imatinib, was defined at the investigator’s discretion. Exclusion criteria for the PMS study were hypersensitivity to nilotinib, rare hereditary problems of galactose intolerance, severe lactase deficiency or glucose-galactose malabsorption, hypokalemia, hypomagnesemia, QT prolongation syndrome, and current participation in other interventional clinical trials. From December 24, 2010, to December 23, 2016, case report forms (CRFs) of 678 patients were collected from 29 physicians at 28 study sites. Among the patients treated with nilotinib at least once, the investigator registered the CRF of those who provided written informed consent. The investigator explained the nature, purpose, and anticipated duration of this study to the patients and obtained their completed consent forms. The study protocol was approved by the institutional review board of each participating institution and was conducted in accordance with the Declaration of Helsinki.
Safety was monitored throughout the study period. An AE was defined as the presence of any undesirable sign, symptom, or medical condition that occurred after treatment and does not necessarily have a causal relationship to nilotinib. All AEs were identified, regardless of the reporting route, including spontaneous reports by patients and clinicians’ reports obtained through the patient’s physical examination, laboratory test, and other interactions. All AEs were categorized based on the MedDRA version 19.1 classification standards to the system organ class (SOC) and preferred term (PT). All AEs, except for those with no causal relationship to nilotinib and which were recorded as “unlikely”, were categorized as ADRs. A serious adverse event (SAE) was defined as any undesirable sign, symptom, or medical condition that: 1) was fatal or life-threatening; 2) required inpatient hospitalization or prolongation of existing hospitalization; 3) resulted in persistent or significant disability/incapacity; 4) constituted a congenital anomaly or a birth defect; 5) was medically significant, in that it may jeopardize the patient and may require medical or surgical intervention; and 6) the transmission of infectious agents via nilotinib.
For efficacy, the complete hematological response (CHR) and cytogenetic response (CyR) rates were evaluated. Hematologic response was evaluated in reference to complete blood count (CBC) conducted at baseline and at week 12 (or at the discontinuation visit) as CHR, absence of CHR, treatment failure, and unknown. CHR was defined as follows: WBC <10×109/L, basophils <5%, no myelocytes, promyelocytes, myeloblasts in differential, platelet count <450×109/L, and nonpalpable spleen. CyR was evaluated with an optional bone marrow study at baseline and at week 12 (or at the discontinuation visit). CyR was categorized as follows: complete (CCyR): no Ph+ metaphases, partial (PCyR): 1–35% Ph+ metaphases, minor (mCyR): 36–65% Ph+ metaphases, minimal (minCyR): 66–95% Ph+ metaphases, and none (noCyR): >95% Ph+ metaphases.
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA). Continuous variables were presented as descriptive statistics, and categorical variables were presented as frequencies and percentages.
Safety analyses: For the incidence of AEs, the number of events and percentages were categorized and calculated based on the severity, outcomes, causal relationship to nilotinib, action taken in connection to nilotinib, and treatment. The incidence of AEs by type, number of events, and percentages were categorized and calculated based on severity, outcomes, causal relationship to nilotinib, action taken to nilotinib, and treatment. The number of subjects and cases was calculated for each of the categories of unexpected SAEs (USAEs) and unexpected SADRs (USADRs); SAEs and SADRs; unexpected AEs (UAEs) unexpected ADRs (UADRs); and AEs and ADRs. The incidence and 95% confidence intervals (CI) of incidences were estimated. The number of subjects with AEs and the number of incidences were calculated according to demographic characteristics such as age and sex. The AE incidences according to demographic characteristics and the corresponding 95% CI were estimated and analyzed using the chi-square test or Fisher’s exact test.
Efficacy analyses: The ratio of the overall assessment of CBC was estimated. CHR and 95% CI were estimated. The CHR rates and 95% CI according to demographic characteristics such as sex and age, were also calculated. The chi-square test or Fisher’s exact test was used for the analysis.
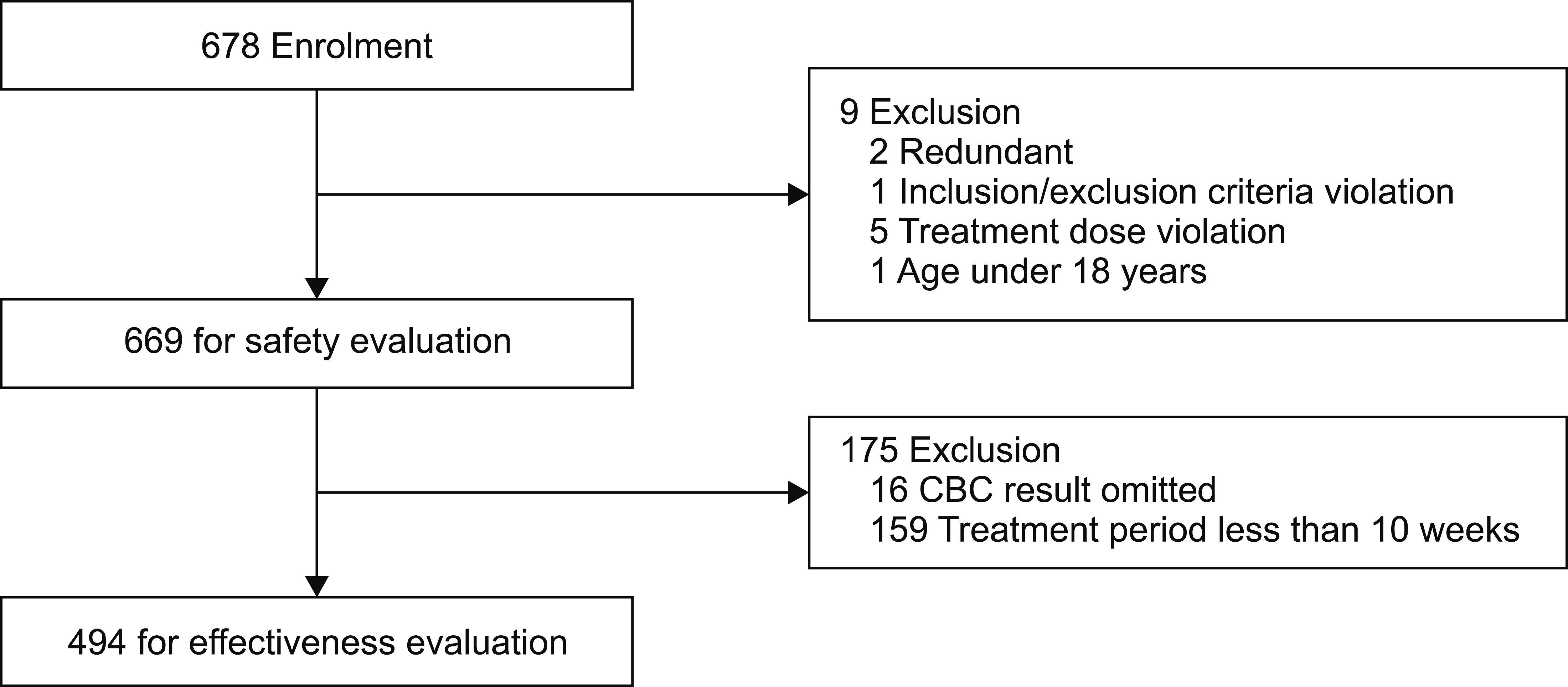
During the study period, the CRFs were retrieved from 678 patients. Of the 678 patients, 2 redundant patients, 2 patients who violated the inclusion/exclusion criteria and 5 patients who violated the dosing administration were excluded; 669 patients were included in the safety evaluation population. Of the 669 patients, 16 patients whose baseline CBC results were omitted and 159 patients who were administered nilotinib for less than 10 weeks were excluded;494 patients were included in the efficacy evaluation (Fig. 1).
Among the 669 patients included in the safety population, 59.5% (398 patients) were male and 40.5% (271 patients) were female. The median age of the patients was 56 years (range, 19–89 yr). In terms of age distribution, the majority were 60 years or older (33.5%, 224 patients), followed by 50–59 years (24.1%, 161 patients), under 40 years (22.7%, 152 patients), and 40–49 years (19.7%, 132 patients). There were no pediatric patients, as this study excluded any patients aged <18 years (Table 1). The mean disease duration was 1.6±5.4 months ranging from 0 to 65 months. The proportion of newly diagnosed CML-CP patients was 49.0% (328 patients), and CML-CP or AP patients with resistance or intolerance to prior therapies, including imatinib, was 51.0% (341 patients). The number of patients with CML-CP with resistance was 149 (22.3%) and CML-CP with intolerance was 168 (25.1%); the number of patients with CML-AP with resistance was 11 (1.6%) and CML-AP with intolerance was 13 (1.9%, Table 1).
AE incidence rate: During the study period, the incidence of AEs collected through CRF was 61.3% (95% CI, 57.5–65.0; 410 patients, 973 events) and that of ADRs was 40.5% (95% CI, 36.8–44.3; 271 patients, 559 events, Table 2). Of the AEs that occurred during study period, 4.5% (95% CI, 3.1–6.3; 30 patients, 37 events) were SAEs, and 0.7% (95% CI, 0.2-1.7; 5 patients, 8 events, Table 3) were serious adverse drug reactions (SADRs). UAEs and UADRs occurred in 6.9% (46 patients, 55 events) and 1.2% of patients, respectively. The reported UADRs include eyelid function disorder, dysmenorrhea, myopathy, epididymitis, orbital cellulitis, scleroderma, hepatitis, and cerebral artery occlusion. Among these, cerebral artery occlusion was reported as a USADR, and where nilotinib was discontinued permanently.
AE by line of therapy: The analysis of AE incidence by different subgroups of patients was conducted in this PMS study. The analysis of AE incidence by diagnosis indicated the rate of incidence of AEs in new CML-CP patients at 72.0% (236/328 patients) and in CP or AP patients who failed prior TKIs at a rate of 51.0% (174/341 patients), where the difference between the two groups was statistically significant (P<0.001). In second-line treatment, the incidence of AEs in CML-CP with resistance and intolerance was 47.0% (11/341 patients) and 52.4% (88/168 patients), respectively, whereas the incidence of AEs for CML-AP with resistance or intolerance was 72.7% (8/11 patients) and 61.5% (8/13 patients), respectively (Fig. 2).
AE by severity: To evaluate severity, AEs were analyzed using CTCAE version 4.0. The incidence of AEs according to severity was as follows: grade 1, 66.7% (649/973 events), grade 2 at 21.8% (212/973 events), grade 3 at 10.7% (104/973 events), grade 4 at 0.6% (6/973 events), and grade 5 at 0.2% (2/973 events, Fig. 3A). The investigators reported that no action was taken in 81.8% (796/973 events) of AEs, temporary discontinuation of nilotinib in 11.1% (108/973 events), dose reduction in 4.1% (40/973 events), permanent discontinuation in 2.8% (27/973 events), and 0.2% (2/973 events) were reported as not applicable (Fig. 3B).
AE by patient demographic: An analysis of the AE incidence by sex was conducted, and the incidence of AEs in males was 58.0% (231/398 patients) and in female was found to be 66.1% (179/271 patients). The difference in AE incidence according to sex was statistically significant (P=0.037, Fig. 3C). Pregnancy tests were performed on 32 of the 271 female patients, and the results were negative. An analysis of the AE incidence by age showed ‘Over 60 years’ at 67.0% (150/224 patients), ‘40–49 years’ at 65.9% (87/132 patients), ‘50–59 years’ at 60.9% (98/161 patients), and ‘Under 40 years’ at 49.3% (75/152 patients). The difference in AE incidence according to age was statistically significant (P=0.004, Fig. 3D).
Of the 494 patients included in the efficacy evaluation, 89.5% (442/494) achieved CHR, 9.9% (49/494) were evaluated as having no CHR, and 0.6% (3/494) were evaluated as experiencing treatment failure. Patients underwent an optional bone marrow study at week 12 of nilotinib treatment or early termination. A total of 162 patients underwent optional bone marrow study for cytogenetic response, which resulted in 85.8% achievement of mCyR or MCyR (139/162 patients). Thirteen patients (8%) achieved minCyR, 7 patients (4.3%) experienced treatment failure, and 3 patients (1.9%) had unknown outcomes.
In this study, 61.3% of AEs, 40.5% of ADRs, 4.5% of SAEs, and 0.7% of SADRs occurred. Unexpected AEs and UADRs occurred at a rate of 6.9% and 1.2%, respectively. However, drug discontinuation was not observed because the rate of permanent discontinuation of nilotinib was 2.8% when the overall AE incidence was 61.3%. As previously reported, the most common non-hematological ADRs (>10%) of nilotinib are rash, pruritus, and fatigue [4, 5, 7]. However, in this PMS study, the incidence of these non-hematological ADRs — with the exception of rash, which was reported in 11.1% of patients — was less than 10%. In addition, the incidence of hematological ADRs in this study was 4.7%, 2.8%, and 0.7% for thrombocytopenia, neutropenia, and anemia, respectively, and have already been reported in the Tasigna label as thrombocytopenia (17%), neutropenia (15%), and anemia (7%) [6].
In the case of SADRs, thrombocytopenia, neutropenia, and anemia were reported at an incidence rate greater than 1.0% in a previous study of nilotinib for CML-CP [4], and thrombocytopenia, neutropenia, febrile neutropenia, pneumonia, leukopenia, intracranial hemorrhage, elevated lipase, and pyrexia were reported at an incidence rate greater than 1.0% in a 24 month follow up study of nilotinib for CML-AP [7]. In this study, thrombocytopenia and neutropenia were reported at an incidence rate of only 0.1% (1/669 patients). The overall incidence of AEs reported in the PMS study was low. However, the overall types and trends of the identified AEs were consistent with those of previous studies of nilotinib.
The incidence of AE was statistically different according to sex and age group (Fig. 3C, D). However, owing to the observational nature of the study, the influence of other factors should be taken into consideration, and further studies may be required to draw meaningful conclusions on the clinical significance of these results.
In terms of efficacy, 89.5% of the patients achieved CHR by 12 weeks, and among 162 patients whose bone marrow biopsy results at 12 weeks were available, 85.8% achieved at least mCyR. Among those who did not undergo bone marrow biopsies, 89 patients achieved CCyR within 12 weeks of nilotinib administration, and some patients were evaluated for molecular response by PCR. Combining 89 patients who had already achieved CCyR at 3 months, 90.8% of the patients achieved optimal cytogenetic response according to the 2009 ELN recommendations. The 2009 ELN recommendation, which was applied when this study was planned, recommends the criteria for the optimal response at 3 months as CHR and at least mCyR [8]. The GIMEMA study evaluating nilotinib in frontline CML-CP (published in 2009) reported that the CHR rate was 100% at 3 months and the optimal cytogenetic response rate including mCyR and MCyR was 91.8% at 3 months (N=73) [9]. This result was similar to the optimal response rate of 90.8% in this study. The European LeukemiaNet published revised recommendations for the management of CML in 2013; therefore, during the study period, real-world clinical practice has changed, particularly in treatment response-monitoring methods and milestones.
This study has several limitations. First, 12 weeks of treatment follow-up was not sufficient to observe the long-term safety and efficacy of nilotinib. In a recent phase 4 clinical study, in which 114 Korean Ph+ CML-CP patients were treated with nilotinib for 24 months, the incidence of AEs was similar to that in this study; however, the incidence rate was higher [10]. Furthermore, considering that nilotinib requires long-term use because of the overall survival of patients treated with 300 mg BID and 400 mg BID for 5 years was 93.7% and 96.2%, respectively [11], the observation of AE incidence in long-term use should be considered. Second, the molecular response was not evaluated in this study. The ELN guidelines for CML in 2013 and the Korean guidelines in 2015 recommend the evaluation of molecular responses along with hematological and cytogenetic responses for clinical evaluation in patients with CML [12, 13]; in addition, the recently published (2020) ELN guidelines for CML recommend only monitoring molecular response [14]. Since the planned clinical guidelines published by ELN in 2009 defined the optimal response at 3 months as CHR and at least mCyR (Ph+ ≤65%), we planned to evaluate mCyR at least in the initial protocol, which is different from the current guidelines. Third, since this study was dependent on medical records, the collected data had limitations. As this study was performed in a non-interventional manner under routine practice, controlling factors that can influence safety and efficacy was difficult. The inevitable influence of the non-interventional nature of this study and the restrictive conditions of local medical practice on the outcomes of this study must be recognized. Nevertheless, given that there were no data to confirm the safety and efficacy of nilotinib in a large number of Korean patients under routine clinical practice, the results of this study may provide some information on AEs and discontinuation of treatment, especially in the early period of nilotinib administration.
In conclusion, the results of the PMS study performed on nilotinib treatment were consistent with previously reported studies in terms of safety and early CyR achievement. Treatment with nilotinib in patients receiving frontline and second-line CML treatments was well tolerated and effective.
REFERENCES
1. Faderl S, Talpaz M, Estrov Z, O'Brien S, Kurzrock R, Kantarjian HM. 1999; The biology of chronic myeloid leukemia. N Engl J Med. 341:164–72. DOI: 10.1056/NEJM199907153410306. PMID: 10403855.

2. Sawyers CL. 1999; Chronic myeloid leukemia. N Engl J Med. 340:1330–40. DOI: 10.1056/NEJM199904293401706. PMID: 10219069.

3. Jabbour E, Hochhaus A, le Coutre P, et al. 2008; Minimal cross- intolerance between nilotinib and imatinib in patients with imatinib-intolerant chronic myelogenous leukemia (CML) in chronic phase (CP) or accelerated phase (AP). J Clin Oncol (ASCO Annual Meeting Abstracts). 26(Suppl):7063. DOI: 10.1200/jco.2008.26.15_suppl.7063.

4. Saglio G, Kim DW, Issaragrisil S, et al. 2010; Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 362:2251–9. DOI: 10.1056/NEJMoa0912614. PMID: 20525993.

5. Giles FJ, le Coutre PD, Pinilla-Ibarz J, et al. 2013; Nilotinib in imatinib-resistant or imatinib-intolerant patients with chronic myeloid leukemia in chronic phase: 48-month follow-up results of a phase II study. Leukemia. 27:107–12. DOI: 10.1038/leu.2012.181. PMID: 22763385.

6. Novartis. 2010. Highlights of prescribing information: Tasigna (nilotinib) [package insert]. Novartis Pharma Stein;Stein, Switzerland: DOI: 10.1038/leu.2012.181.
7. le Coutre PD, Giles FJ, Hochhaus A, et al. 2012; Nilotinib in patients with Ph+ chronic myeloid leukemia in accelerated phase following imatinib resistance or intolerance: 24-month follow-up results. Leukemia. 26:1189–94. DOI: 10.1038/leu.2011.323. PMID: 22076466.

8. Baccarani M, Cortes J, Pane F, et al. 2009; Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 27:6041–51. DOI: 10.1200/JCO.2009.25.0779. PMID: 19884523. PMCID: PMC4979100.

9. Rosti G, Palandri F, Castagnetti F, et al. 2009; Nilotinib for the frontline treatment of Ph(+) chronic myeloid leukemia. Blood. 114:4933–8. DOI: 10.1182/blood-2009-07-232595. PMID: 19822896.

10. Shin J, Koh Y, Yoon SH, et al. 2018; A phase 4 study of nilotinib in Korean patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTKorea. Cancer Med. 7:1814–23. DOI: 10.1002/cam4.1450. PMID: 29577674. PMCID: PMC5943463.

11. Hochhaus A, Saglio G, Hughes TP, et al. 2016; Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 30:1044–54. DOI: 10.1038/leu.2016.5. PMID: 26837842. PMCID: PMC4858585.

12. Baccarani M, Deininger MW, Rosti G, et al. 2013; European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 122:872–84. DOI: 10.1182/blood-2013-05-501569. PMID: 23803709. PMCID: PMC4915804.
13. Kim DY, Lee JO, Kim KH, et al. 2015; Korean guidelines for treating chronic myelogenous leukemia - The Korean Society of Hematology Chronic Myelogenous Leukemia Working Party. Korean J Med. 88:406–19. DOI: 10.3904/kjm.2015.88.4.406.

14. Hochhaus A, Baccarani M, Silver RT, et al. 2020; European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 34:966–84. DOI: 10.1038/s41375-020-0776-2. PMID: 32127639. PMCID: PMC7214240.

Fig. 2
Distribution of AE incidence by diagnosis.Abbreviations: AE, adverse events; AP, accelerated phase; CP, chronic phase.
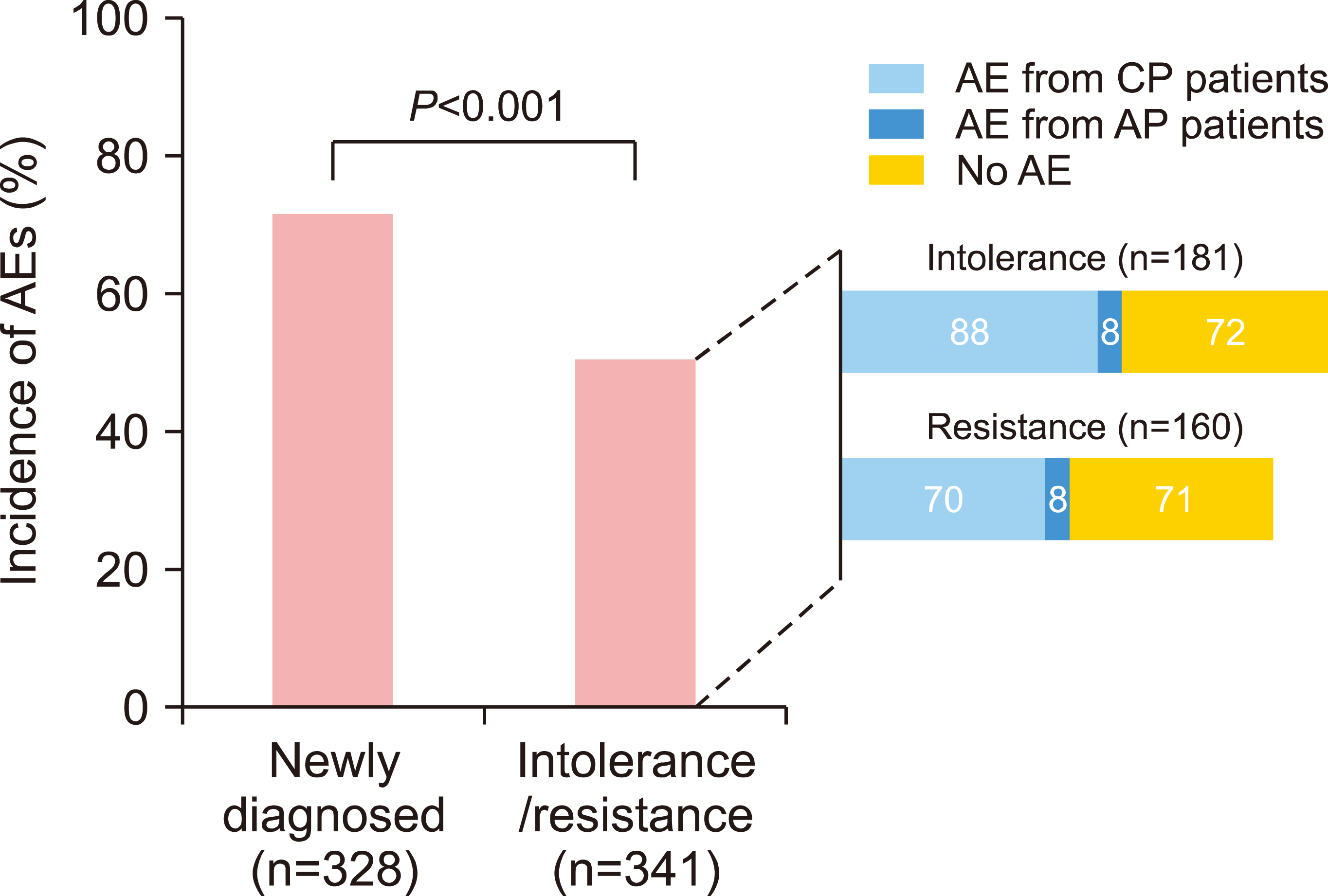
Fig. 3
The incidence of adverse events (AEs) by categories. (A) AEs have been classified and evaluated along five severity categories: mild, moderate, severe, life threatening consequences, and death. (B) Action taken due to AEs has been evaluated along six categories as: none, permanent discontinuation, temporary discontinuation, dose reduction, dose escalation, and not applicable. (C) The incidence of AEs by sex. (D) The incidence of AEs by age.
Table 1
Demographics of patients.
Table 2
Adverse events and adverse drug reactions.
Table 3
Serious adverse events and serious adverse drug reactions.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download