Abstract
Background
Children with bleeding disorders, such as hemophilia and von Willebrand disease (VWD), have an increased risk of acquiring transfusion-transmitted infections (TTI). Screening methods to exclude blood donations that are at risk of transmitting infection from donors to recipients are critical to preventing disease transmission. Nucleic acid testing (NAT) is the latest blood donor-screening method. This study aimed to determine the incidence of hepatitis C virus (HCV) infection in children with hemophilia and VWD at Dr. Cipto Mangunkusumo Hospital with a history of blood transfusion before and after implementation of a NAT screening method.
Methods
A cohort retrospective study was conducted on children aged 0‒18 years with bleeding disorders and a history of blood transfusion. In our center, all blood transfusions before 2015 were screened using non-NAT methods, while all blood transfusions were screened using NAT starting in 2015. Eligible patient characteristics were collected from medical records. From July to December 2019, blood samples were obtained from eligible patients for anti-HCV examination. HCV RNA examinations were performed on subjects with reactive anti-HCV results, and the relative risk was calculated.
Results
In total, 108 eligible participants were included in this study. We observed that 91 (94.3%) patients had history of receiving non-NAT blood transfusions, while 17 (15.7%) patients received NAT-screened blood transfusions. The proportion of anti-HCV reactivity in the non-NAT group and that in the NAT group were 3.3% (3/91) and 0% (0/17), respectively.
Children with hereditary bleeding disorders are most likely to receive blood component transfusions during their lives as part of treatment. The most common hereditary bleeding disorders are hemophilia and von Willebrand disease (VWD). Hemophilia is an X–linked recessive bleeding disorder that causes a deficiency in coagulation protein factor VIII (FVIII), leading to hemophilia A, or factor IX (FIX), leading to hemophilia B [1-3]. VWD is an autosomal recessive bleeding disorder due to defect of chromosome 12, which leads to deficiency of factor VWD (VWF) [4]. Immediate administration of replacement therapy is necessary to stop bleeding due to a deficiency in blood coagulation factors. The standard of care is to administer FVIII, FIX, or VWF concentrates as a replacement therapy [5]. However, in emerging countries, such as Indonesia, where the availability of clotting factor concentrates is limited, transfusion of cryoprecipitate or fresh-frozen plasma may be used. In cases of major blood loss, other blood products, such as red blood cells and platelets, for transfusions are also needed. Multiple blood transfusions have been linked to transfusion-transmitted infections (TTI), including hepatitis C virus (HCV) [6]. Approximately, 76% of chronic HCV infections progress to liver cirrhosis [7]. The American Association for the Study of Liver Diseases (AASLD) recommends screening high-risk populations, including patients with hemophilia and VWD [8]. Liver cirrhosis may worsen bleeding in hemophilia and VWD, which may lead to a poor quality of life, a high mortality rate, and an increased cost of therapy [9, 10].
Our center, Dr. Cipto Mangunkusumo Hospital (CMH), is a national referral hospital in Indonesia that provides comprehensive hemophilia care for children and adults with hemophilia and other bleeding disorders. The estimated number of people with hemophilia in Indonesia is 25,000, but data from the Indonesian Hemophilia Society 2020 reports the number of patients registered is 2,706. Blood transfusion services are available and coordinated by the hospital blood bank unit in cooperation with the Indonesian Red Cross, which supplies the blood products. In Indonesia, the first antibody screening method was the HCV rapid test in 1990, which has a window period of up to 3 months [11, 12]. Afterward, enzyme-linked immunosorbent assay (ELISA) and chemiluminescent immunoassay (ChLIA) were introduced in 1993 and 2004, respectively, with shorter window periods [13, 14]. Since 2015, nucleic acid testing (NAT) has been used as a blood donor screening method in our hemophilia center for CMH. NAT detects the genetic material of the virus with a window period of up to 3 days [15].
Currently, there are no data in Indonesia regarding the incidence of HCV infection in children with hemophilia and VWD before and after the implementation of NAT blood donor screening. This study aimed to determine the incidence of HCV infection in children with hemophilia and VWD before and after implementation of NAT as a blood donor screening method.
A cohort retrospective study of HCV infection in patients with hemophilia and patients with VWD receiving blood component transfusion was carried out in the Pediatric Hematology-Oncology Division, Dr. Cipto Mangunkusumo Hospital (CMH), Faculty of Medicine Universitas Indonesia, Jakarta. This study was conducted between July and December 2019. The inclusion criteria were children aged 0–18 years with hemophilia and VWD who had a history of blood transfusions before the start of the study, including those with red blood cells, platelets, cryoprecipitate, and fresh frozen plasma. Data collected from the medical records included sex, age, diagnosis, severity of hemophilia, inhibitor status, history of major surgery, major bleeding episodes, and history of clotting factor concentrates received as replacement therapy. Data collected on blood transfusion history included the time of receiving transfusion (date/month/ year), type of blood component product used, age of the first transfusion, and method of blood donor screening. Informed consent was obtained from the parents of the eligible patients. Patients with a history of sharing needles and a mother with a history of HCV infection were excluded from the study. Participants who were unwilling to participate in the study were also excluded.
Blood specimens were collected from all eligible subjects, and anti-HCV examination was performed using a chemiluminescent microparticle immunoassay Architect Anti-HCV (Abbot Laboratories, Wiesbaden, Germany). The sample/cut-off (S/CO) value used to determine the reactivity was ≥1.0. HCV RNA examination using the Xpert HCV Viral Load Assay GeneXpert (Cepheid, AB, CE) was performed only on subjects with reactive anti-HCV results. In this study, we did not assess the presence of other viral or bloodborne pathogens. All blood collection and tests were performed at the clinical pathology laboratory of CMH throughout the study period from July 2019 to December 2019. The relative risk (RR) of using NAT for blood donor screening related to the incidence of HCV infection was calculated.
The protocol for this study was reviewed and approved by the health research ethics commission of the Faculty of Medicine, University of Indonesia, as well as by the Head of Research Centre CMH with ethics approval numbers 537/UN2. F1/ETIK/2019 and LB.02/2.2/0686/2019.
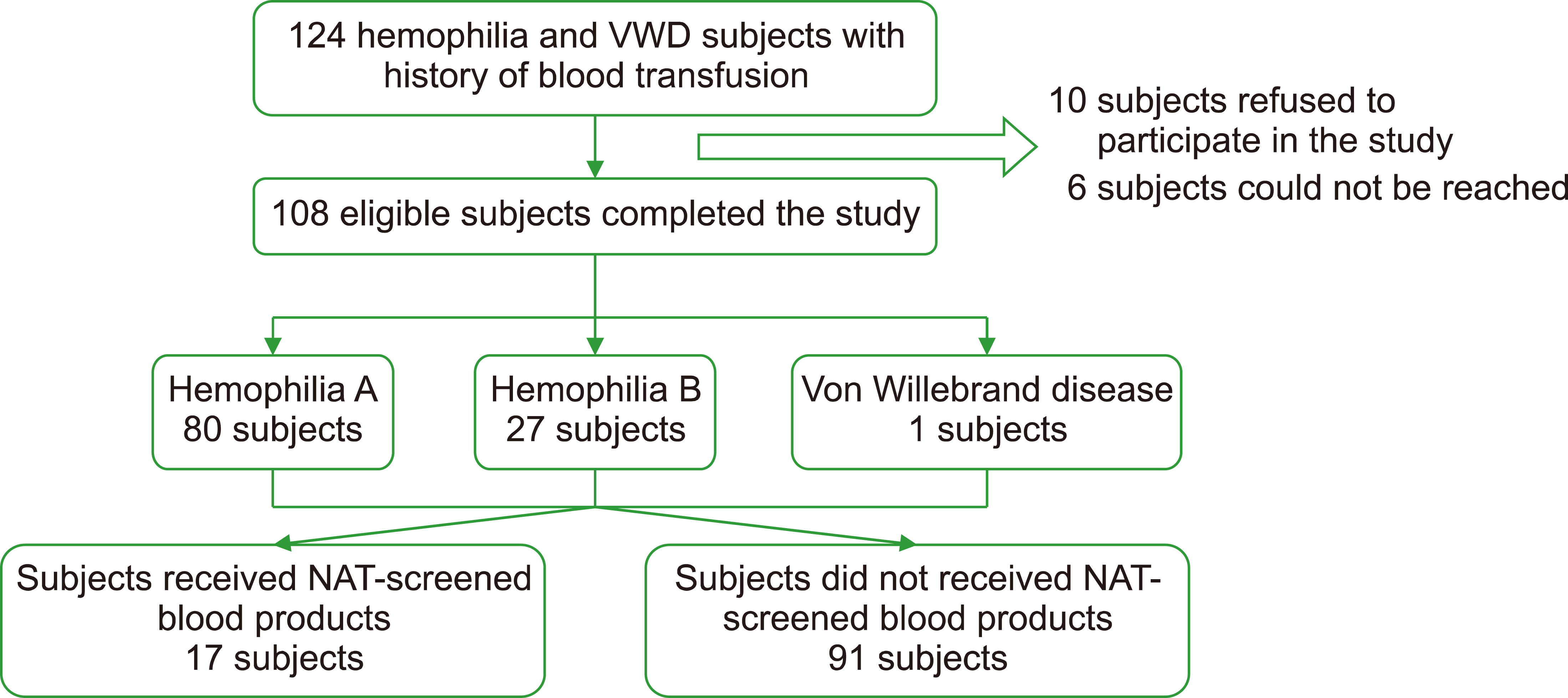
We identified 124 children with hemophilia and VWD who were eligible for inclusion in the study (Fig. 1). Ten patients refused to participate in the study, and six patients could not be reached or had moved far away from the CMH. A total of 108 subjects completed the study. The characteristics of the participants are presented in Table 1.
Throughout the study period, all eligible patients were exposed to plasma-derived clotting factor concentrates available at our center as replacement therapy. The plasma-derived concentrates underwent two steps of viral inactivation using solvent/detergent and heat treatment or solvent/detergent and terminal dry heat treatment.
Of the 108 eligible subjects, 80 (74%) had hemophilia A, 27 (25%) had hemophilia B, and 1 (1%) had VWD (Table 1). The median age of all the subjects was 10.91 (IQR, 8) years. The number of subjects who received NAT-screened blood products were 17 (16%) and non-NAT blood products were 91 (84%).
Among subjects who received non-NAT blood products, three out of 91 (3.3%) showed anti-HCV reactivity (Table 2), whereas no subjects with reactive anti HCV were found among subjects who received NAT-screened blood trans-fusions. All three subjects underwent further testing for HCV RNA, all of which were negative. The duration to confirmatory HCV RNA test was approximately 2–3 weeks after testing positive using the anti-HCV screening test. The RR of contracted HCV infection with a history of NAT exposure was 1.034, with a confidence interval of 95% CI: 0.996–1.074. The characteristics of subjects with anti HCV reactivity are shown in Table 3. All three subjects claimed that they had never previously experienced any signs or symptoms of HCV infection.
Patients with hemophilia and other bleeding disorders are at high risk of transfusion-associated hepatitis C, mainly when clotting factor concentrates are not available. To date, there are no published data on the prevalence of transfusion-transmissible HCV infections among blood donors in Indonesia. However, based on data from Jakarta Blood Center that supplied CMH, the blood donor’s screening using enzyme immunoassay (EIA) method, approximately 0.54% donors were reactive to anti-HCV from 2005 to 2021 (data was not published).
In 2005, Oswari et al. [16] reported that 39 children with hemophilia in CMH were diagnosed with HCV infection (RNA PCR-positive) due to transfusion. A total of 24 out of 39 children had chronic infection, while 15 out of 39 (39%) had spontaneous clearance [16]. CMH Internal Medicine Department reported there were 38% of adults with hemophilia acquired HCV infection in 2012. Of these, 3% of them developed into liver cirrhosis. Liver cirrhosis is the end-stage of chronic liver disease, which may be caused by hepatitis C infection. In liver cirrhosis, the synthesis of coagulation factors, such as FVIII and IX, is decreased due to the destruction of liver cells [17]. This condition may worsen the level of coagulation factors that are genetically low in patients with hemophilia. Hypersplenism in liver cirrhosis may lead to a low number of platelets. Furthermore, esophageal varices may be found in patients with liver cirrhosis. Patients with hemophilia who suffer from esophageal varices rupture experience massive bleeding. This condition may require massive transfusion, which includes not only coagulation factors but also packed red cells and platelets. The early initiation of therapy may prevent the progression of HCV infection to liver cirrhosis and its complications [18, 19].
Our study showed a decreased number of children with chronic HCV infection compared with a previous study in 2005. Our study supported the evidence of a better outcome of the ChLIA screening method over the rapid test and ELISA in transfusion-related HCV infection. More importantly, this study found 0% of subjects with HCV infection who received NAT screening blood transfusion. Studies have shown that NAT is superior to rapid tests, ELISA, and ChLIA because of its ability to detect nucleic acid particles of hepatitis C RNA [12-15]. In contrast, rapid tests, ELISA, and ChLiA detect antibodies against hepatitis C infection [12-14]. The formation of antibodies needs time after the infection of hepatitis C virus. Therefore, antibody-based screening methods have a longer window than NAT. Rapid tests and ELISA using ChLiA have window periods of up to 6 months, 4 months, and 21 days, respectively. A longer window may increase the number of undetected hepatitis C infections in blood products. Above all, NAT has the shortest window period, which can detect nucleic acid particles of hepatitis C virus for up to 3 days [15].
We found that three patients were anti-HCV reactive among those who received non-NAT blood transfusions; however, HCV RNA was negative. The possibility of false-positives by anti-HCV tests using EIA method based on the literature was considerably high, with estimation of 15% to 62% [20]. Moreover, a study related to the diagnostic performance of our HCV screening test immunoassays showed a 96.7% diagnostic sensitivity and a 52.1% positive predictive value, which may result in false positive results in three subjects of this study [21]. Furthermore, HCV infection may occur spontaneously. In 2006, Zhang et al. [22] reported several characteristics of the spontaneous clearance of HCV infection in patients with hemophilia. This study revealed that the likelihood of spontaneous HCV clearance increased in patients younger than 16 years of age. Another study in 2006 [23] also reported that spontaneous clearance was more likely to occur in younger patients, with a higher proportion of spontaneous clearance reported in the pediatric cohort than in adults. Spontaneous clearance was also associated with the female sex, viral load, viral coinfections, alcohol use, and genetic factors [22, 23]. Three subjects in our study had several characteristics of spontaneous clearance, including younger age and a non-compromised immune system. However, other associated factors were not evaluated in this study.
The probability of HCV infection may increase with the severity of hemophilia. Comorbidities also play an important role in causing more bleeding episodes. For example, patient A with severe hemophilia A in our study was also diagnosed with epilepsy and consumed valproic acid regularly, which has the side effect of thrombocytopenia. Hemophilia combined with thrombocytopenia may lead to frequent and massive bleeding episodes. The number of blood transfusions increases when massive bleeding episodes occur more frequently. In this case, the patient might have required transfusions of packed red cells, cryoprecipitate, and platelets. Consequently, the high frequency of transfusions required might lead to a higher chance of HCV infection.
This study has several limitations. First, the number of subjects who received NAT-screened blood transfusions was small; hence, further multicenter studies in Indonesia should be considered to identify more patients with hemophilia and other bleeding disorders with HCV infection. The NAT-screened blood products were only available since 2015 in our center, and during the last 5 years, the use of factor concentrates for children with hemophilia A and B has increased, causing a small number of subjects to receive NAT-screened blood transfusion. Second, the precise age of HCV infection in the subjects was estimated using the first age at transfusion. Thus, the factors associated with spontaneous clearance in this study may have been imprecise.
This is the first study in Indonesia to evaluate the number of HCV infections in children with hemophilia who underwent NAT compared to that in those who underwent non-NAT blood transfusion. The three subjects were observed to have reactive anti-HCV, and this is a finding in our study should be recognized as important. Due to its limited budget, NAT is not applied as a routine blood donor screening method in the majority of provinces in our country. We strongly suggest that NAT be considered in all regular blood donor screening methods for children with hereditary bleeding disorders, such as hemophilia and VWD. HCV infection could lead to liver cirrhosis, which causes failure to produce other coagulation factors and may pose major life-threatening bleeding and catastrophic burden on quality of life and treatment cost.
ACKNOWLEDGMENTS
All authors contributed equally to this study. We thank Dr. Aria Kekalih, MTI, for providing assistance with the statistical analysis. We also thank Dr. Saptuti Chunaeni, M.Biomed, from the Indonesian Red Cross Society. We thank Dr. Raisa Cecilia Sarita, BMedSc (Hons.) for assistance with the manuscript construction.
REFERENCES
1. Gatot D, Moeslichan MZ. Permono HB, Sutaryo , Ugrasena IDG, Windiastuti E, Abdulsalam M, editors. 2010. Gangguan pembekuan darah yang diturunkan: hemofilia. Buku ajar hematologi onkologi anak. Badan Penerbit Ikatan Dokter Anak Indonesia;Jakarta, Indonesia: p. 174–6.
2. Scott JP, Montgomery RR. Kliegman RM, Behrman RE, Jenson HB, Stanton BF, editors. 2007. Hereditary clotting factor. Nelson textbook of pediatrics. 18th ed. WB Saunders Company;Philadelphia, PA: p. 2066–74. DOI: 10.1016/b978-1-4377-0755-7.00470-x.
3. Srivastava A, Santagostino E, Dougall A, et al. 2020; WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 26(Suppl 6):1–158. DOI: 10.1111/hae.14046. PMID: 32744769.

4. Lillicrap D, James P. 2009; Von Willebrand disease: an introduction for the primary care physician. Québec, Canada:. World Federation of Hemophilia,. 1–7.
5. Farrugia A, Evers T, Falcou PF, Burnouf T, Amorim L, Thomas S. 2009; Plasma fractionation issues. Biologicals. 37:88–93. DOI: 10.1016/j.biologicals.2009.01.005. PMID: 19289290.

6. Dean CL, Wade J, Roback JD. 2018; Transfusion-transmitted infections: an update on product screening, diagnostic techniques, and the path ahead. J Clin Microbiol. 56:e00352–18. DOI: 10.1128/JCM.00352-18. PMID: 29669792. PMCID: PMC6018323.

7. Toshikuni N, Arisawa T, Tsutsumi M. 2014; Hepatitis C-related liver cirrhosis - strategies for the prevention of hepatic decompensation, hepatocarcinogenesis, and mortality. World J Gastroenterol. 20:2876–87. DOI: 10.3748/wjg.v20.i11.2876. PMID: 24659879. PMCID: PMC3961980.

8. Gupta E, Bajpai M, Choudhary A. 2014; Hepatitis C virus: screening, diagnosis, and interpretation of laboratory assays. Asian J Transfus Sci. 8:19–25. DOI: 10.4103/0973-6247.126683. PMID: 24678168. PMCID: PMC3943138.

9. Muciño-Bermejo J, Carrillo-Esper R, Uribe M, Méndez-Sánchez N. 2013; Coagulation abnormalities in the cirrhotic patient. Ann Hepatol. 12:713–24. DOI: 10.1016/S1665-2681(19)31312-2. PMID: 24018489.

10. Kessler CM. 2006; Update on liver disease in hemophilia patients. Semin Hematol. 43(1 Suppl 1):S13–7. DOI: 10.1053/j.seminhematol.2005.11.021. PMID: 16427378.

11. Unit Transfusi Darah Pusat. 2018. Sejarah unit transfusi darah palang merah Indonesia. Unit Transfusi Darah Pusat;Jakarta, Indonesia: at http://home.utdp-pmi.or.id/page/detail/sejarah-utd-pmi. Accessed December 10, 2021.
12. Fagan EA. 1991; Testing for hepatitis C virus. BMJ. 303:535–6. DOI: 10.1136/bmj.303.6802.535. PMID: 1655135. PMCID: PMC1670862.

13. Kotwal GJ, Baroudy BM, Kuramoto IK, et al. 1992; Detection of acute hepatitis C virus infection by ELISA using a synthetic peptide comprising a structural epitope. Proc Natl Acad Sci U S A. 89:4486–9. DOI: 10.1073/pnas.89.10.4486. PMID: 1374903. PMCID: PMC49107.

14. Dufour DR, Talastas M, Fernandez MD, Harris B. 2003; Chemiluminescence assay improves specificity of hepatitis C antibody detection. Clin Chem. 49:940–4. DOI: 10.1373/49.6.940. PMID: 12765991.

15. Roth WK. 2019; History and future of nucleic acid amplification technology blood donor testing. Transfus Med Hemother. 46:67–75. DOI: 10.1159/000496749. PMID: 31191192. PMCID: PMC6514489.

16. Oswari H, Damardjati F, Gatot D, Munasir Z, Bisanto J. 2007; Clinical and laboratory profiles of hepatitis C in hemophiliac children. Paediatr Indones. 47:229–3. DOI: 10.14238/pi47.5.2007.229-33.

17. Senzolo M, Burra P, Cholongitas E, Burroughs AK. 2006; New insights into the coagulopathy of liver disease and liver transplantation. World J Gastroenterol. 12:7725–36. DOI: 10.3748/wjg.v12.i48.7725. PMID: 17203512. PMCID: PMC4087534.

18. Dustin LB, Bartolini B, Capobianchi MR, Pistello M. 2016; Hepatitis C virus: life cycle in cells, infection and host response, and analysis of molecular markers influencing the outcome of infection and response to therapy. Clin Microbiol Infect. 22:826–32. DOI: 10.1016/j.cmi.2016.08.025. PMID: 27592089. PMCID: PMC5627509.

19. Morozov VA, Lagaye S. 2018; Hepatitis C virus: morphogenesis, infection and therapy. World J Hepatol. 10:186–212. DOI: 10.4254/wjh.v10.i2.186. PMID: 29527256. PMCID: PMC5838439.

20. Ballani N, Gupta E. 2015; Low signal-to-cutoff ratio (S/Co) in the diagnosis of hepatitis C: a diagnostic dilemma? Indian J Gastroenterol. 34:413–4. DOI: 10.1007/s12664-015-0593-0. PMID: 26498021.

21. Choi MS, Lee K, Hong YJ, Song EY, Kim DS, Song J. 2018; The role of the signal-to-cutoff ratio in automated anti-HCV chemiluminescent -immunoassays by referring to the nucleic acid amplification test and the recombinant immunoblot assay. Ann Lab Med. 38:466–72. DOI: 10.3343/alm.2018.38.5.466. PMID: 29797818. PMCID: PMC5973922.

22. Zhang M, Rosenberg PS, Brown DL, et al. 2006; Correlates of spontaneous clearance of hepatitis C virus among people with hemophilia. Blood. 107:892–7. DOI: 10.1182/blood-2005-07-2781. PMID: 16204310. PMCID: PMC1895891.

23. Yeung LT, To T, King SM, Roberts EA. 2007; Spontaneous clearance of childhood hepatitis C virus infection. J Viral Hepat. 14:797–805. DOI: 10.1111/j.1365-2893.2007.00873.x. PMID: 17927616.

Table 1
Summary of the characteristics of the subjects.
|
Group 1 (NAT), N=17 (16%) |
Group 2 (Non-NAT), N=91 (84%) |
|
|---|---|---|
| Gender | ||
| Male | 17 (100%) | 88 (97%) |
| Female | 0 (0%) | 3 (3%) |
| Age | ||
| <2 years old | 0 (0%) | 0 (0%) |
| 2–15 years old | 14 (82%) | 72 (79%) |
| ≥16 years old | 3 (18%) | 19 (21%) |
| Diagnosis | ||
| Hemophilia A | 12 (71%) | 68 (75%) |
| Hemophilia B | 5 (29%) | 22 (24%) |
| Von Willebrand | 0 (0%) | 1 (1%) |
| Severity | ||
| Severe hemophilia | 11 (65%) | 54 (59%) |
| Moderate hemophilia | 6 (35%) | 30 (33%) |
| Mild hemophilia | 0 (0%) | 6 (7%) |
| VWD type 1 | 0 (0%) | 1 (1%) |
| Inhibitor FVIII | ||
| Low titer | 0 (0%) | 1 (1%) |
| High titer | 0 (0%) | 3 (3.3%) |
| Negative | 16 (94%) | 85 (93.4%) |
| History of inhibitor | 1 (6%) | 2 (2.2%) |
| Number of bagsa) during transfusion | ||
| 1–4 bags | 9 (53%) | 42 (46%) |
| 5–10 bags | 6 (35%) | 36 (40%) |
| >10 bags (volume of each bag ±50–180 mL) | 2 (12%) | 13 (14%) |
| Age of first transfusion | ||
| <2 years old | 6 (35%) | 34 (37%) |
| 2–15 years old | 11 (65%) | 57 (63%) |
| ≥16 years old | 0 (0%) | 0 (0%) |
| Major surgery | ||
| Yes | 0 (0%) | 18 (20%) |
| No | 17 (100%) | 73 (80%) |
Table 2
Association between using NAT and the anti-HCV result.
| Anti-HCV | Total | P | RR (95% CI) | ||
|---|---|---|---|---|---|
| Reactive, % | Non-reactive, % | ||||
| NAT | 0 (0) | 17 (100) | 17 | 0.448 | 1.034 (0.996–1.074) |
| Non-NAT | 3 (3.3) | 88 (96.7) | 91 | ||
Table 3
Summary of the characteristics of thesubjects with reactive anti-HCV.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download