INTRODUCTION
MATERIALS AND METHODS
Patients
DMARDs prescription pattern and cancer treatment modality
Statistical analysis
RESULTS
Characteristics of enrolled patients and DMARDs prescription before cancer diagnosis
Table 1
|
Total (n=40) |
Surgery only (n=21) |
Chemotherapy (n=19)* |
|
|---|---|---|---|
| Age at cancer diagnosis (yr) | 67 (55, 73) | 70 (52, 77) | 66 (57, 71) |
| Female | 28 (70.0) | 16 (76.2) | 12 (63.2) |
| Disease duration (yr) | 4.5 (2.7, 4.5) | 3.4 (1.5, 11.4) | 4.2 (2,0, 10.0) |
| Seropositivity† | 36 (90.0) | 19 (90.5) | 17 (89.5) |
Table 2
| DMARDs prescription patterns |
Total (n=40) |
Surgery only (n=21) |
Chemotherapy (n=19)* |
|
|---|---|---|---|---|
| csDMARDs (n=31) | ||||
| Monotherapy (n=4) | MTX | 3 | 3 | 0 |
| SSZ | 1 | 1 | 0 | |
| Combination (n=27) | MTX (+) | 22 | 8 | 14 |
| MTX (–) | 5 | 2 | 3 | |
| Biologics (n=9) | MTX (+) | 5 | 4 | 1 |
| MTX (–) | 4 | 3 | 1 | |
DMARDs prescription patterns right after cancer diagnosis
Table 3
| Changes in DMARDs prescription patterns | Prescribed DMARDs | |
|---|---|---|
| Discontinuation (n=13)* | - | |
| Switching (n=14) | Monotherapy (n=8) | MTX (n=3), HCQ (n=4), SSZ (n=1) |
| Combination (n=6) | MTX+HCQ (n=1) | |
| SSZ+HCQ (n=4) | ||
| LEF+TC+HCQ (n=1) | ||
| Maintenance (n=13) | Monotherapy (n=4) | MTX (n=3), SSZ (n=1) |
| Combination (n=7) | MTX+SSZ+HCQ (n=1) | |
| MTX+LEF+HCQ (n=3) | ||
| MTX+BL+MR (n=1) | ||
| SSZ+HCQ (n=1) | ||
| LEF+BL+HCQ (n=1) | ||
| Biologics (n=2) | ETA (n=1)†, TCZ (n=1)‡ | |
DMARDs: disease modifying anti-rheumatic drugs, MTX: methotrexate, HCQ: hydroxychloroquine, SSZ: sulfasalazine, TC: tacrolimus, LEF: leflunomide, BL: bucillamine, MR: mizoribine, ETA: etanercept, TCZ: tocilizumab. *Four in the surgery only group (4/21, 19.0%), nine in the chemotherapy group (9/19, 47.4%) (p<0.05). †Patient with stage II colon cancer. ‡Patient with stage I bladder cancer.
Resumption of DMARDs in the discontinuation group
Table 4
DMARDs: disease modifying anti-rheumatic drugs, MTX: methotrexate, LEF: leflunomide, SSZ: sulfasalazine, HCQ: hydroxychloroquine, TC: tacrolimus, ETA: etanercept, ADA: adalimumab, IQR: interquartile range. *Time interval between discontinuation and resumption of DMARDs was median of 5.5 months (IQR 2.9, 18.3).
DMARDs prescription patterns at recent outpatient clinic visits
Table 5
| DMARDs prescription patterns | Prescribed DMARDs | ||
|---|---|---|---|
| No DMARD (n=4)† | |||
| csDMARDs (n=14) | |||
| Monotherapy (n=3) | MTX (n=3) | ||
| Combination (n=11) | MTX (+) | (n=8) | MTX+HCQ (n=1), MTX+HCQ+SSZ (n=1), MTX+TC (n=1), MTX+LEF (n=5) |
| MTX (–) | (n=3) | LEF+HCQ (n=1), SSZ+TC (n=1), SSZ+HCQ (n=1) | |
| Biologics (n=7) | MTX (+) | (n=3) | ADA (n=1), TCZ (n=1), ABA (n=1) |
| MTX (–) | (n=4) | ETA (n=2), TCZ (n=2) | |
DMARDs: disease modifying anti-rheumatic drugs, csDMARDs: conventional synthetic DMARDs, MTX: methotrexate, SSZ: sulfasalazine, HCQ: hydroxychloroquine, TC: tacrolimus, LEF: leflunomide, ADA: adalimumab, TCZ: tocilizumab, ABA: abatacept, ETA: etanercept, IQR: interquartile range. *At a median of 4.6 years (IQR 3.3, 6.7) after cancer diagnosis, five patients died (four out of 13 in the discontinuation group and one out of 27 in the switching or maintenance group) and 10 patients were lost (two out of 13 in the discontinuation group and eight out of 27 in the switching or maintenance group). †Three had cancer recurrence after chemotherapy and one achieved arthritis remission after cancer treatment.
Table 6
Cancer recurrence in patients with rheumatoid arthritis
Table 7
F: female, M: male, CBD: common bile duct, CML: chronic myelogenous leukemia, DMARDs: disease modifying antirheumatic drugs, MTX: methotrexate, LEF: leflunomide, TCZ: tocilizumab, ADA: adalimumab, HCQ: hydroxychloroquine, SSZ: sulfasalazine, S: surgical therapy, C: chemotherapy, FU: follow-up, RA: rheumatoid arthritis.
DISCUSSION
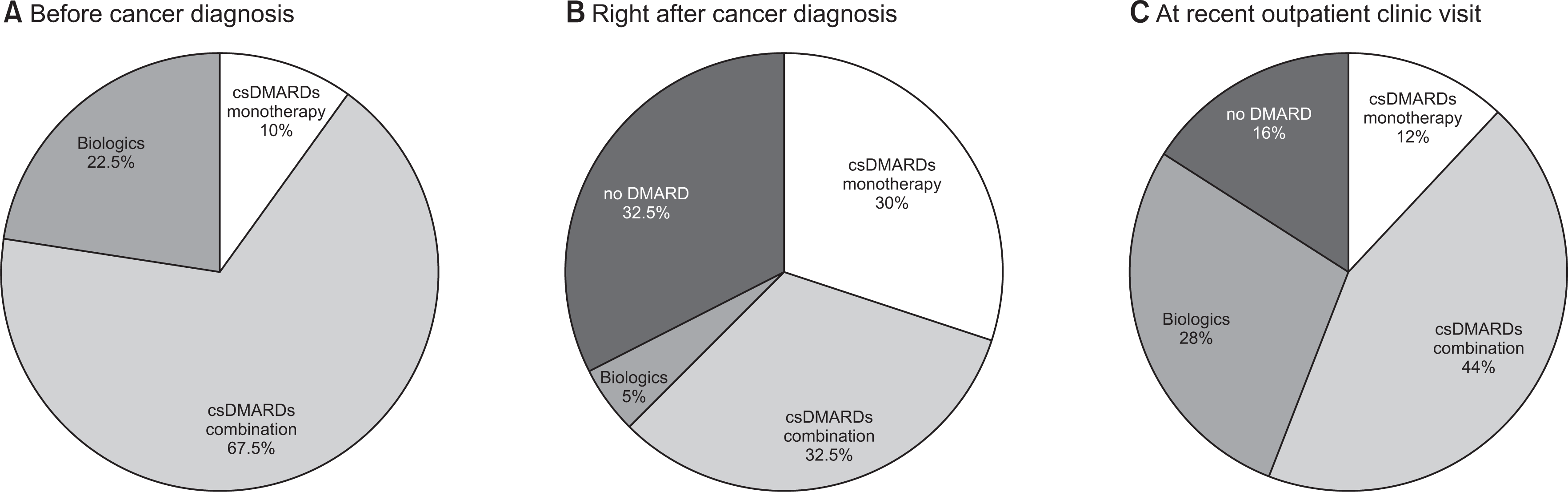 | Fig. 1DMARDs prescription patterns before and after cancer diagnosis in RA patients. (A) Before cancer diagnosis (n=40); 27 patients were treated with csDMARDs combination (67.5%), 4 with csDMARDs monotherapy (10%), and 9 with biologics (22.5%). (B) Right after cancer diagnosis (n=40); 13 patients discontinued DMARDs (32.5%) and 13 patients were treated with csDMARDs combination (32.5%), 12 with csDMARDs monotherapy (30%), and 2 with biologics (5%). (C) At recent outpatient clinic visit (median 4.6 years [IQR 3.3, 6.7] after cancer diagnosis) (n=25): 4 patients were prescribed no DMARD (16%), 11 csDMARDs combination (44%), 3 csDMARDs monotherapy (12%), and 7 biologics (28%). RA: rheumatoid arthritis, csDMARDs: conventional synthetic DMARDs, IQR: interquartile range. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download