Abstract
Objective
Methods
Results
Notes
FUNDING
This research was supported by a faculty research grant of Yonsei University College of Medicine (6-2019-0184) and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (HI14C1324).
AUTHOR CONTRIBUTIONS
J.Y.P., S.S.A., and S.W.L. designed the study. J.Y.P. and S.W.L. drafted the manuscript. J.Y.P., S.S.A., J.J.S., and S.W.L. contributed to the acquisition and analysis of data. J.J.S. and Y.B.P. validated and reviewed the drafted manuscript. All authors approved the final manuscript.
REFERENCES
Fig. 1
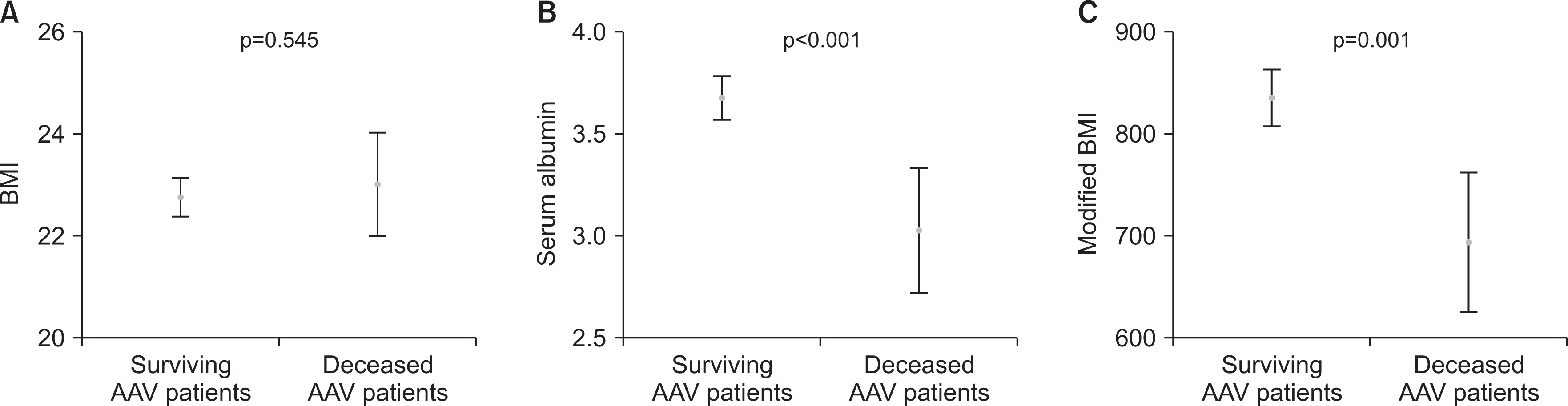
Fig. 2
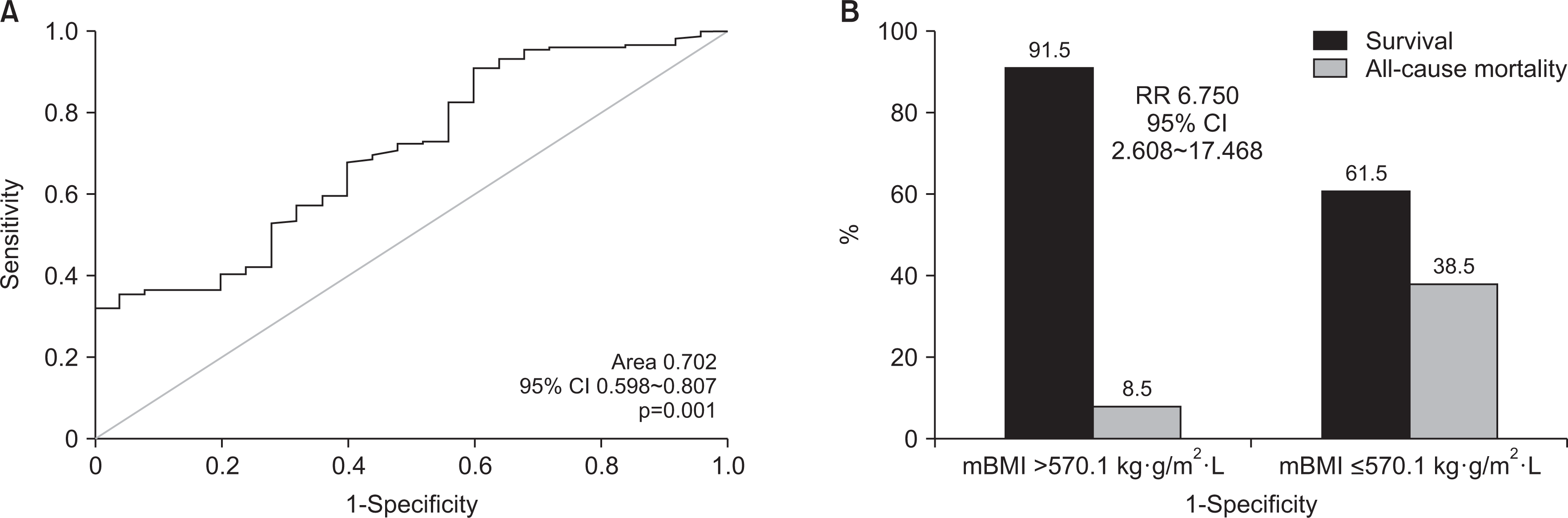
Fig. 3
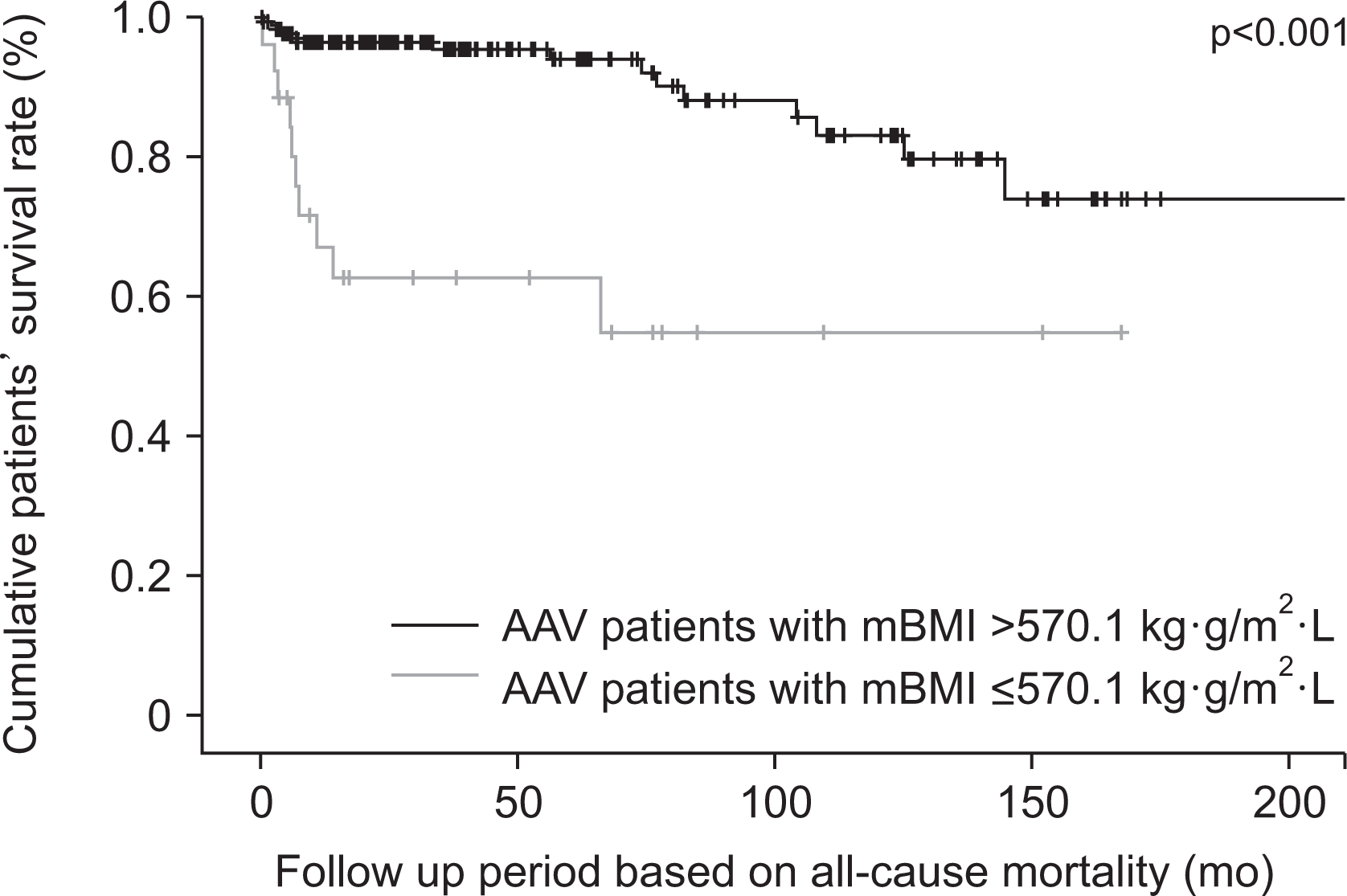
Table 1
| Variables | Values |
|---|---|
| At the time of diagnosis | |
| Demographic data | |
| Age (yr) | 59.0 (18.5)* |
| Male sex | 72 (35.5) |
| AAV subtypes | |
| MPA | 112 (55.2) |
| GPA | 54 (26.6) |
| EGPA | 37 (18.2) |
| ANCA positivity | |
| MPO-ANCA (or P-ANCA) positivity | 136 (67.0) |
| PR3-ANCA (or C-ANCA) positivity | 34 (16.7) |
| Both ANCA positivity | 8 (3.9) |
| ANCA negativity | 41 (20.2) |
| AAV-specific indices | |
| BVAS | 12.0 (10.5)* |
| FFS | 1.0 (1.0)* |
| Clinical manifestations at diagnosis | |
| Generalized symptoms | 88 (43.3) |
| Skin | 44 (21.7) |
| Mucous membrane and eyes | 11 (5.4) |
| Ear nose throat | 92 (45.3) |
| Lungs | 120 (59.1) |
| Heart | 43 (21.2) |
| Gastrointestine | 9 (4.4) |
| Kidneys | 127 (62.6) |
| Central or peripheral nervous systems | 61 (30.0) |
| Comorbidities at diagnosis | |
| Diabetes mellitus | 52 (25.6) |
| Hypertension | 78 (38.4) |
| Chronic kidney disease (stages 3~5) | 58 (28.6) |
| Hyperlipidaemia | 35 (17.2) |
| Interstitial lung disease | 56 (27.6) |
| Acute-phase reactants | |
| ESR (mm/hr) | 54.0 (68.0)* |
| CRP (mg/L) | 11.7 (60.5)* |
| BMI (kg/m2) | 22.8 (3.6)* |
| Serum albumin (g/dL) | 3.7 (1.1)* |
| mBMI (kg · g/m2 · L) | 813.2 (270.7)* |
| During the follow-up period | |
| Mortality during follow-up | |
| All-cause mortality | 25 (12.3) |
| Follow-up duration based on all-cause mortality (mo) | 36.6 (66.1)* |
| Medications administered during follow-up | |
| Glucocorticoids | 189 (93.1) |
| Cyclophosphamide | 101 (49.8) |
| Rituximab | 33 (16.3) |
| Azathioprine | 113 (55.7) |
| Mycophenolate mofetil | 24 (11.8) |
| Tacrolimus | 10 (4.9) |
| Methotrexate | 21 (10.3) |
Values are expressed as a median (interquartile range)* or number (%). AAV: ANCA-associated vasculitis, ANCA: antineutrophil cytoplasmic antibody, BMI: body mass index, MPA: microscopic polyangiitis, GPA: granulomatosis with polyangiitis, EGPA: eosinophilic GPA, MPO: myeloperoxidase, P: perinuclear, PR3: proteinase 3, C: cytoplasmic, BVAS: Birmingham vasculitis activity score, FFS: five-factor score, CRP: C-reactive protein, mBMI: modified body mass index.
Table 2
AAV: ANCA-associated vasculitis, ANCA: antineutrophil cytoplasmic antibody, HR: hazard ratio, CI: confidence interval, MPO: myeloperoxidase, P: perinuclear, PR3: proteinase 3, C: cytoplasmic, BVAS: Birmingham vasculitis activity score, FFS: five factor score, ESR: erythrocyte sedimentation rate, CRP: C-reactive protein: BMI: body mass index, mBMI: modified body mass index.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download