INTRODUCTION
Primary biliary cholangitis (PBC), previously known as primary biliary cirrhosis, is an autoimmune cholestatic liver disease that is characterized by a positive antimitochondrial antibodies (AMA) test and progressive destruction of small intrahepatic bile ducts.
1 PBC is often associated with other extrahepatic autoimmune diseases. According to a recent large-scale study, 28.3% of patients with PBC had extrahepatic autoimmune diseases. Ankylosing spondylitis (AS) is a chronic systemic inflammatory disease affecting the joints of the spine and sacroiliac joints. Among these, only 0.2% of PBC patients had concurrent AS.
2 The association of PBC with AS is very low, and only two case reports
3,4 and three cases in a recent large-scale study
2 have been found. On the other hand, to the best of the authors’ knowledge, there are no reports on PBC with AS in Korea. This paper reports a rare case of a man with AS who developed PBC.
Go to :

CASE REPORT
A 43-year-old man visited the Pusan National University Yangsan Hospital because of a liver function test (LFT) abnormality. He was diagnosed with AS 12 years earlier. His pain and disease activity showed improvement, and he was treated with a nonsteroidal anti-inflammatory drug. He received indomethacin at a dose of 25 mg three times for 5 months. He had stopped drinking alcohol for 12 years and did not take any toxic substances or herbal medications. The physical examination was unremarkable. The laboratory test showed the following: a white blood cell count of 7,610/mm
3, hemoglobin level of 15.9 g/dL, platelet count of 401,000/mm
3, AST of 148 U/L, ALT of 239 U/L, ALP of 655 U/L, GGT 351 U/L, albumin of 3.8 g/dL, PT of 12.0 sec, total bilirubin level of 1.5 mg/dL without evidence of a hepatitis virus infection (negative for HBsAg and anti-HCV). The serological test for ANA was positive (>1:2,560), whereas the anti-LKM, anti- smooth muscle antibody, and AMA were negative. The serum levels of IgG and IgM were 1,683 mg/dL and 507 mg/dL, respectively. Abdominal CT was performed for a differential diagnosis of other possible causes of LFT abnormality. The abdominal CT findings showed biliary hamartoma and enlarged lymph nodes along the common hepatic artery and portocaval area, suggesting reactive hyperplasia but no intra- or extrahepatic duct dilatation. Although the clinical findings, including an elevation of cholestatic liver enzymes and IgM level, implied PBC, the elevated ALT and IgG levels and negative AMA indicated autoimmune hepatitis or overlap syndrome. Therefore, a liver biopsy was undergone, and histology showed focal lymphoplasma cell aggregates and bile duct damage compatible with PBC (
Fig. 1). These histological and clinical findings were consistent with PBC. Accordingly, the patient was diagnosed with PBC and treated with high-dose ursodeoxycholic acid (UDCA). After 8 months of UDCA at 900 mg a day, the laboratory tests improved with an AST of 49 U/L, ALT of 71 U/L, ALP of 307 U/L, and GGT of 179 U/L.
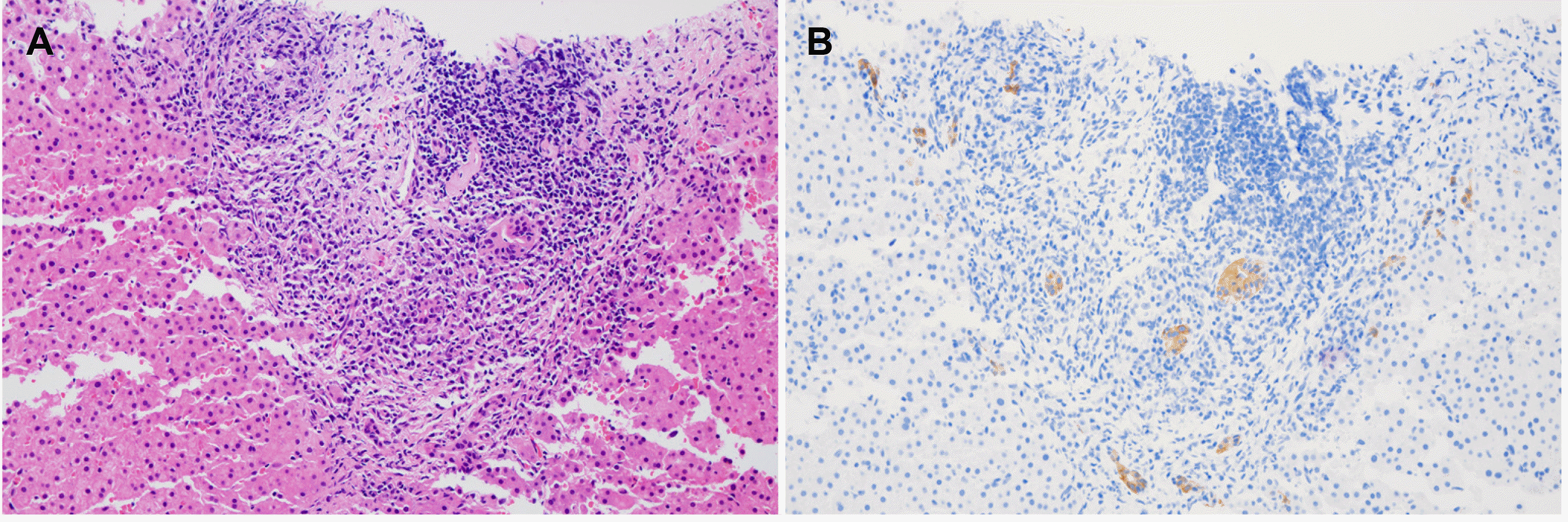 | Fig. 1Histology findings of liver biopsies consistent with primary biliary cholangitis. (A) A portal area shows focal lymphoplasma cell aggregates and bile duct damage (hematoxylin and eosin stain, ×200). (B) The immunohistochemistry shows an irregular shape, nuclear stratification, and vacuolar degeneration of the interlobular bile ducts (K7 stain, ×200). 
|
Go to :

DISCUSSION
PBC is an autoimmune liver disease characterized by positive AMA and destruction of the small intrahepatic bile ducts, resulting in cholestasis.
1 This leads to fibrosis, which can progress to cirrhosis and end-stage liver disease and its associated complications. The disease was first discovered in 1851 and called primary biliary cirrhosis in 1949, but the terminology was changed from primary biliary cirrhosis to primary biliary cholangitis in 2014 because cirrhosis only appears when the disease has progressed.
5 PBC predominantly affects middle- aged women, and the prevalence rate varies from 40 to 400 per million persons.
6 The diagnosis of PBC can be established if there is no extrahepatic biliary obstruction, no comorbidity affecting the liver, and at least two of the following conditions are met: 1) elevated ALP and GGT; 2) Presence of AMA or other PBC specific autoantibodies (sp100 or gp210), if AMA is negative; 3) Histologic evidence of PBC (nonsuppurative destructive cholangitis and destruction of the interlobular bile ducts).
7 Although a liver biopsy is not necessary for diagnosing PBC, it is still necessary when PBC-specific antibodies are negative or when coexistent autoimmune hepatitis or nonalcoholic steatohepatitis is suspected. A liver biopsy can be useful to stage the disease for fibrosis and ductopenia and evaluate the treatment effect and prognosis. The clinical symptoms varied from asymptomatic to complications of liver cirrhosis. PBC may be asymptomatic, or they may present with symptoms, such as fatigue and pruritus. If left untreated, the signs and symptoms of end-stage liver disease can develop. Treatment with UDCA or obeticholic acid can slow disease progression to end-stage liver disease in PBC.
1
AS is a chronic inflammatory disease of the spine and sacroiliac joints causing new bone formation that eventually leads to ankyloses.
8,9 Although the cause of AS is unknown, they are believed to involve a combination of genetic and environmental factors. Approximately 80-90% of patients with AS have the HLA-B27 genotype. A diagnosis is typically based on the clinical and radiologic tests. The underlying mechanism is believed to be autoimmune or autoinflammatory.
10 Epidemiological studies have found higher incidences of extra-articular manifestations to be a consequence of uncontrolled systemic inflammation.
11 The prevalence and clinical significance of extra-articular manifestations vary widely. The most common extra-articular manifestations are eye, gastrointestinal tract, lung, heart, skin, bone, and kidney involvement.
12
PBC is classified as an autoimmune disorder because its pathogenesis has an immunological basis. PBC often coexists with various autoimmune diseases, including autoimmune thyroid disease, Sjogren's syndrome, systemic sclerosis, and rheumatoid arthritis.
2 These coexistences can be explained by the mosaic of autoimmunity. This suggests that different combinations of genetic, immunological, hormonal, and environmental factors result in a diversity of expression of autoimmune diseases. With the alteration of the immune system, the presence of one autoimmune condition may lead to the development of another autoimmune disease.
13 Although the pathological mechanisms of PBC in patients with AS remain unclear, both environmental factors and genetic variants increase the disease susceptibility. Genetic factors are strongly implicated in the pathogenesis of autoimmune disease. The association between the HLA-DRB1, -DQA1, and -DQB1 loci and PBC was reported. Other non-HLA genes are also suggested to contribute to PBC development. Many non-HLA loci have been found, including IL12A, IL12RB2, STAT4, CTLA4, TNF-a, SPIB, and STAT4.
14 In addition, many genes involved in the pathogenesis of PBC have also been linked to other autoimmune diseases. As a non-HLA risk locus, ETS1 has been associated with PBC and AS, suggesting the role of a common genetic predisposition in the pathogenesis of coexistence.
15 In addition to the possibility that PBC and AS share a genetic variant, molecular mimicry, one of the most common mechanisms through which infectious or chemical agents may induce autoimmunity, may be involved in the development of both diseases in response to common infectious triggers.
16 The intestinal microbiota that can cause PBC include intestinal microbiota, such as
Escherichia coli and
Lactobacillus.
17,18 Intestinal microbiota has also been associated with AS.
19
Drug‐induced liver injury has been reported in association with anti-TNF-α agents, which can be used to treat AS, and is usually hepatocellular-type hepatitis.
20,21 Although cholestatic liver injury can also occur,
22 the patient has not been treated with anti-TNF-α agents. In the present case, the patient showed an elevation in ALT as well as ALP and GGT. The overlap syndrome of autoimmune hepatitis and PBC was also suspected. On the other hand, the patient was finally diagnosed with PBC based on the laboratory results and histological findings.
In conclusion, this paper describes a rare case of a patient who had AS and developed PBC. The association of PBC with AS is very low. To the best of the authors’ knowledge, this is the first case report of such coexistence in Korea. When cholestatic hepatitis occurs in patients with an autoimmune disease like AS, physicians should always consider the possibility of the coexistence of PBC. Further studies will be needed to identify the relationship between these two diseases.
Go to :






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download