Abstract
Gastric malignant peripheral nerve sheath tumors (MPNSTs) are extremely rare spindle cell sarcomas that arise within the peripheral nerves of the gastrointestinal tract. MPNST can present as a mass that may or may not be accompanied by obstruction or bleeding. Type 1 neurofibromatosis (NF) is an autosomal dominant genetic disorder with an incidence of 1 in 2,500-3,000. Plexiform neurofibromas in Type 1 NF can undergo a malignant transformation to MPNSTs. Approximately half of the incidence of MPNST is associated with the NF-1 gene. MPNST behaves aggressively, and radical excisional surgery is important for treatment. Recurrence and metastasis are significant, even after a radical excision. Despite multidisciplinary treatment, the five-year survival rate is only 30-50%. This paper reports the case of a 47-year-old man with Type 1 NF who presented with hemorrhage of a gastric subepithelial lesion. He underwent surgery under the suspicion of a gastrointestinal stromal tumor, but it was diagnosed as MPNST after confirming the histopathological appearance and immunohistochemical profiles. In addition, the large mass invaded the spleen and diaphragm. Radical surgery was performed, and additional chemotherapy was administered. This paper reports the experience of a patient with NF 1 with advanced MPNST discovered due to a subepithelial lesion.
A malignant peripheral nerve sheath tumor (MPNST) was previously called malignant schwannoma or neurofibrosarcoma.1 Malignant schwannoma, neurofibrosarcoma, and malignant nerve sheath tumor were all categorized as MPNST by the World Health Organization in 1978.2 Gastric MPNST is malignant sarcoma arising within Schwann cells, fibroblasts, and perineural cells of the gastrointestinal tract. Atypical neurofibromas, spindle cell melanomas, and leiomyosarcomas need to be considered in a differential diagnosis. Sarcomas are rare cancers, and MPNST is a rare type of sarcoma. MPNSTs commonly grow in the trunk, head, neck, and paravertebral region.3 On the other hand, MPNST of the gastrointestinal tract and gastric MPNST are rare. MPNST is more common in patients with genetic Type 1 neurofibromatosis (NF). The annual incidence of MPNST is 1.58 per 1,000 patients with Type 1 NF,4 higher than 1.46 person per million people in the general population.5
Approximately 5.9-6.4% of patients with Type 1 NF develop MPNST,4 and despite multidisciplinary treatment, it shows an aggressive disease course, resulting in a poor prognosis. The five-year survival rate is only 30-50%.6 This paper presents the case of a 47-year-old man diagnosed with MPNST after the surgical resection of a 5 cm-sized subepithelial lesion in the stomach.
A 47-year-old man was referred to the hospital for a further evaluation of anemia diagnosed from the complete blood cell count test performed because of aggravated general weakness. He had been in a state of alert mentality and had multiple café au lait spots over his entire body. His medical history revealed that he was previously diagnosed with Type 1 NF, but he did not visit the hospital regularly. The patient had a family history; his mother had Type 1 NF. He had never undergone a routine endoscopy and had no history of alcohol consumption or smoking.
His vital signs were as follows: blood pressure, 100/70 mmHg; pulse rate, 72 times/min; body temperature, 36.3℃; and no abdominal pain or tenderness. He stated that melena was present when he visited the hospital, and had melena for 1 month.
The complete blood cell count test revealed a hemoglobin level of 5.5 g/dL, white blood cell count of 19,030/μL (73.4% neutrophils), and platelet count of 901,000/μL. The biochemical test revealed the serum levels of aspartate aminotransferase, alanine aminotransferase, blood urea, and creatinine to be 4 U/L, 7 U/L, 19.1 mg/dL, and 0.5 mg/dL, respectively.
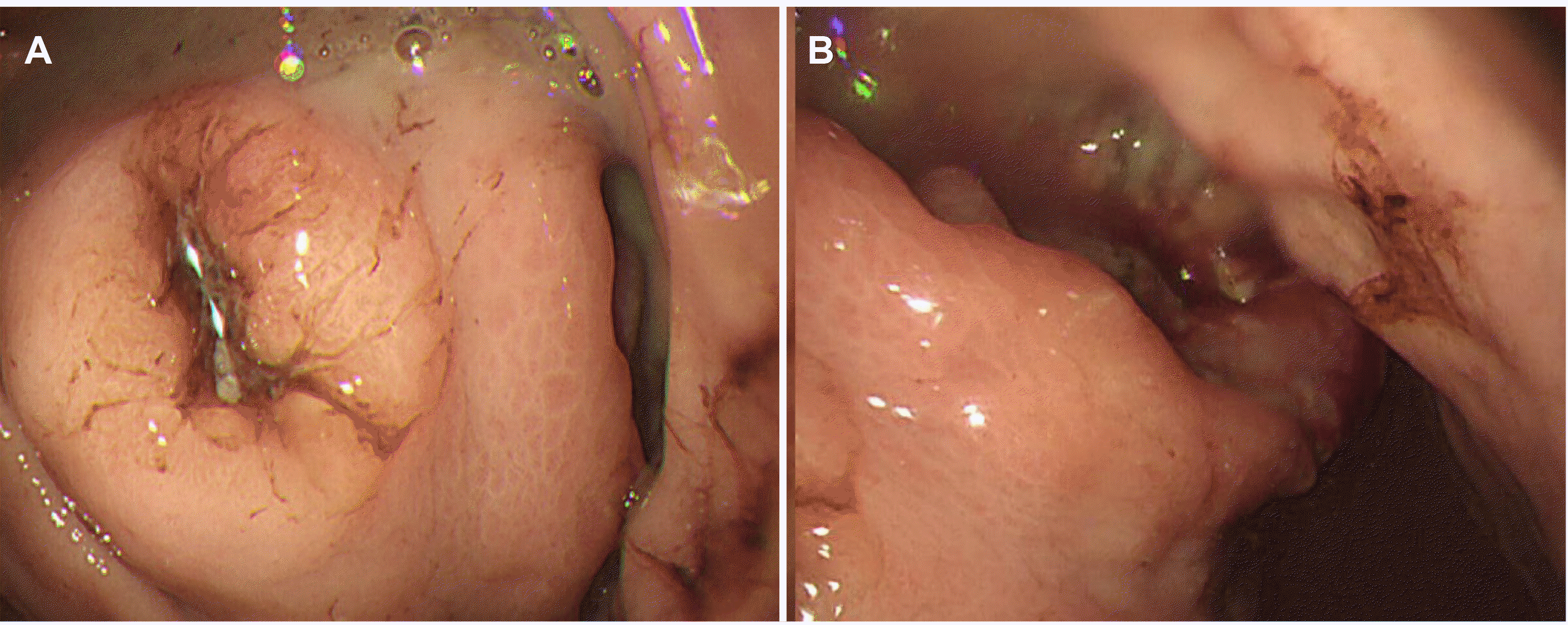
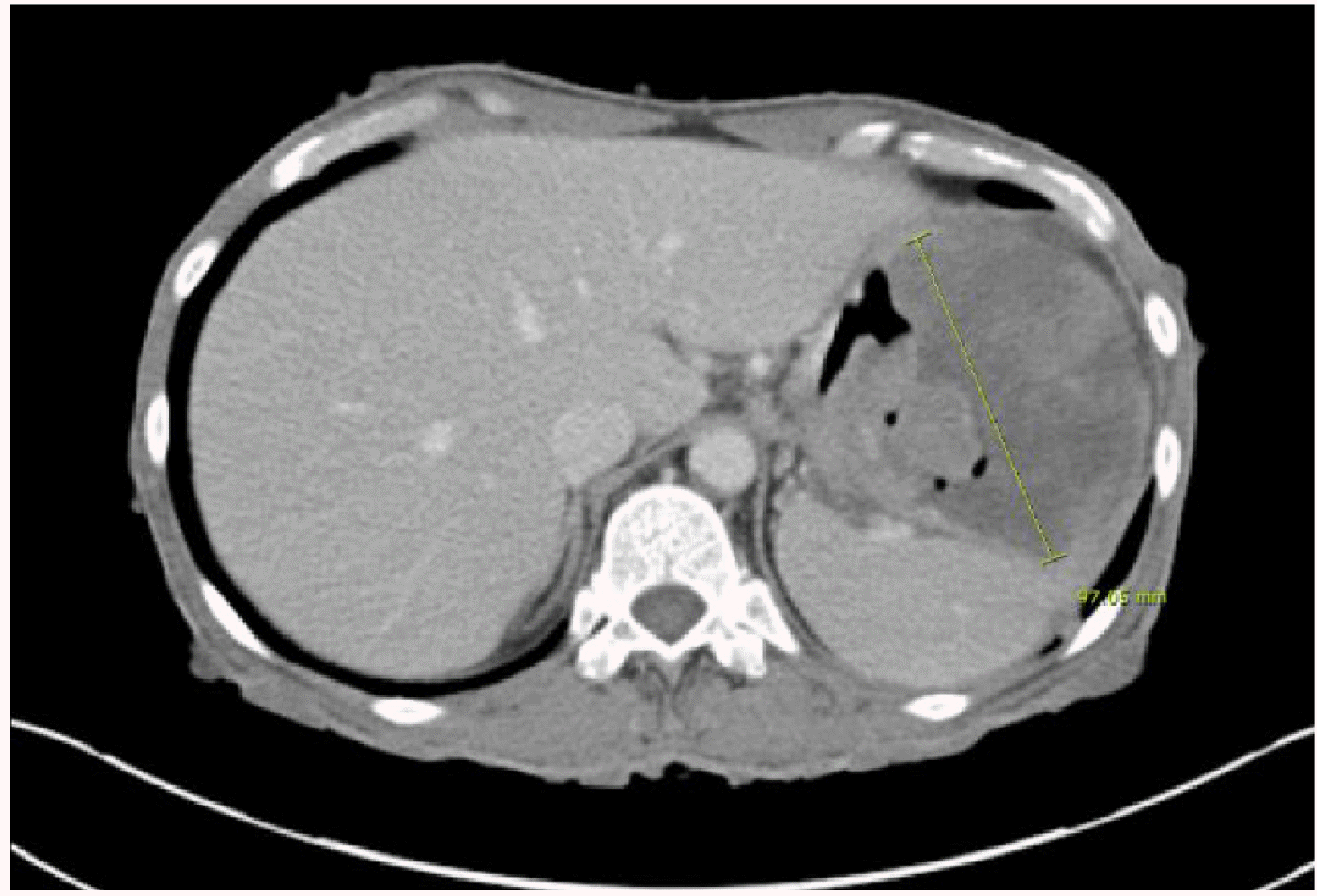
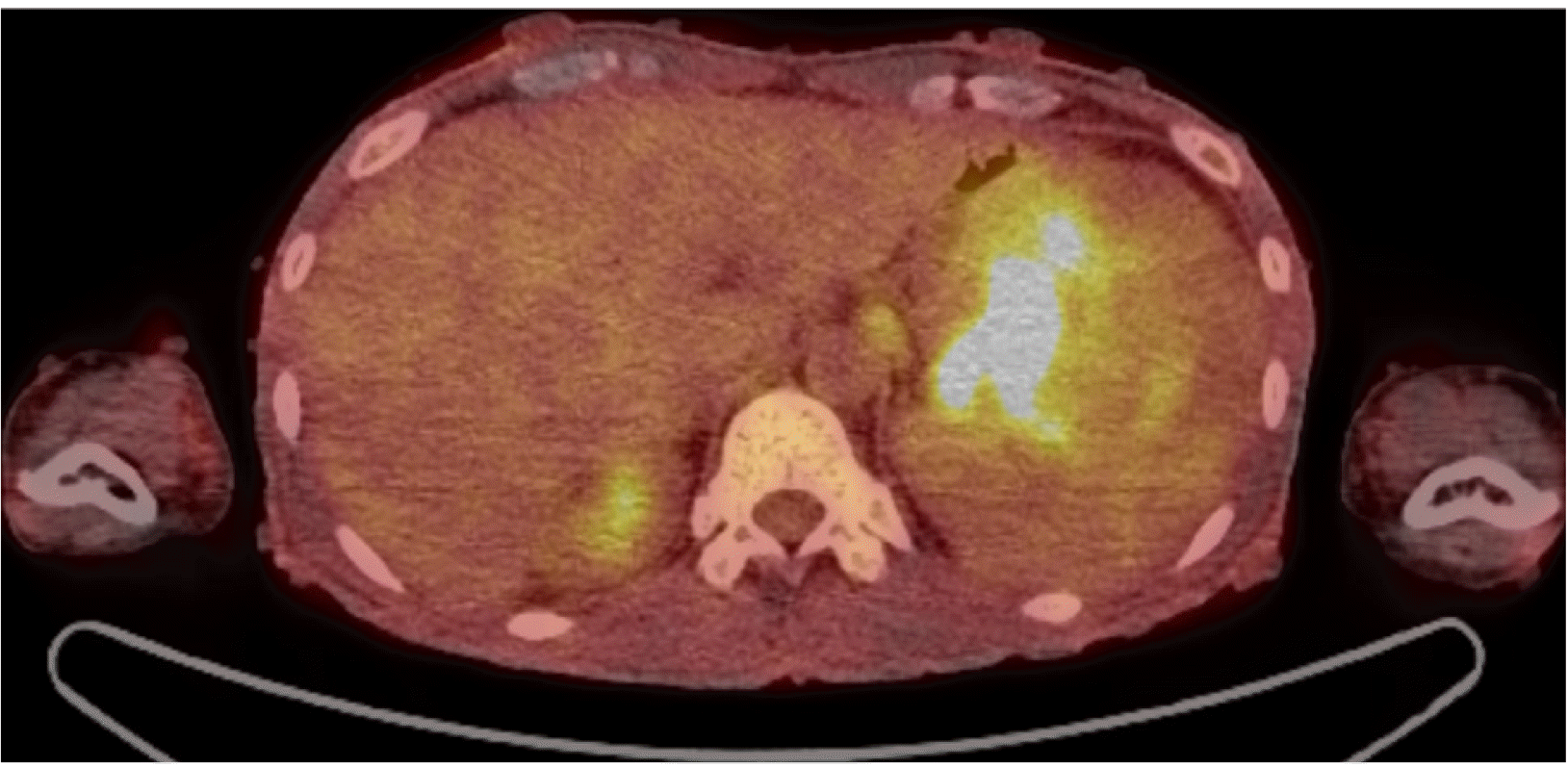
Esophagogastroduodenoscopy (EGD) was performed to evaluate the anemia. On EGD, subepithelial lesions measuring 4.2 cm (Fig. 1A) and 5 cm (Fig. 1B) were identified in the fundus and the great curvature side of the upper body, respectively, with central ulcers. A large amount of hematin was embedded in the stomach. The ulcer had an ulcerofungating shape, and no active bleeding was observed. Therefore, a biopsy was performed. CT revealed an exophytic 9.7×8.1 cm mass and another 5.1 cm similar mass adjacent to the gastric body/fundus (Fig. 2). In addition, PET-CT revealed enlarged lymph nodes (LNs) with fluorodeoxyglucose uptake in the left gastric and subclavicular areas, suggesting metastatic lymphadenopathy (Fig. 3). In chest CT and abdomen-pelvic CT, however, enlarged LN correlating with FDG uptake was not observed. The initial biopsy result of gastroscopy revealed an atypical spindle cell tumor that appeared to be intermediate between sarcoma (not otherwise specified) and KIT-negative gastrointestinal stromal tumors (GISTs). A definite method for a pathologic diagnosis in this young patient was needed, so surgical treatment was planned, and the patient was discharged.
On the other hand, he returned to the emergency room with melena and hematemesis shortly after. Therefore, the patient underwent gastric surgery under the suspicion of GIST. Large subepithelial lesions of 5 cm and 4.2 cm in size were observed in the operation field, and a radical total gastrectomy with a Roux en Y esophagojejunostomy was performed. Intra-operation invasion of the spleen and diaphragm were confirmed, and a splenectomy, pulmonary left lower wedge resection, and diaphragm resection were performed.
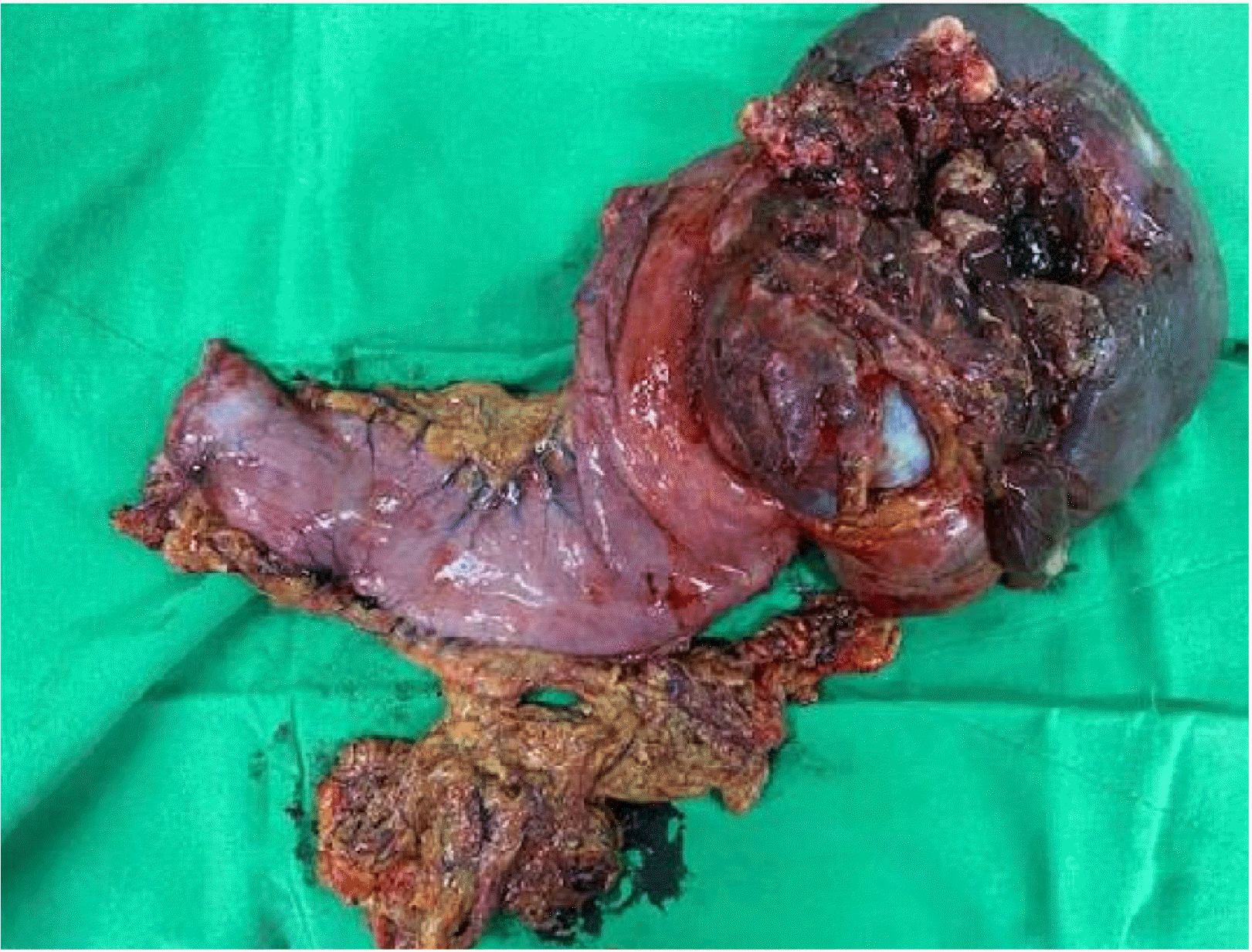
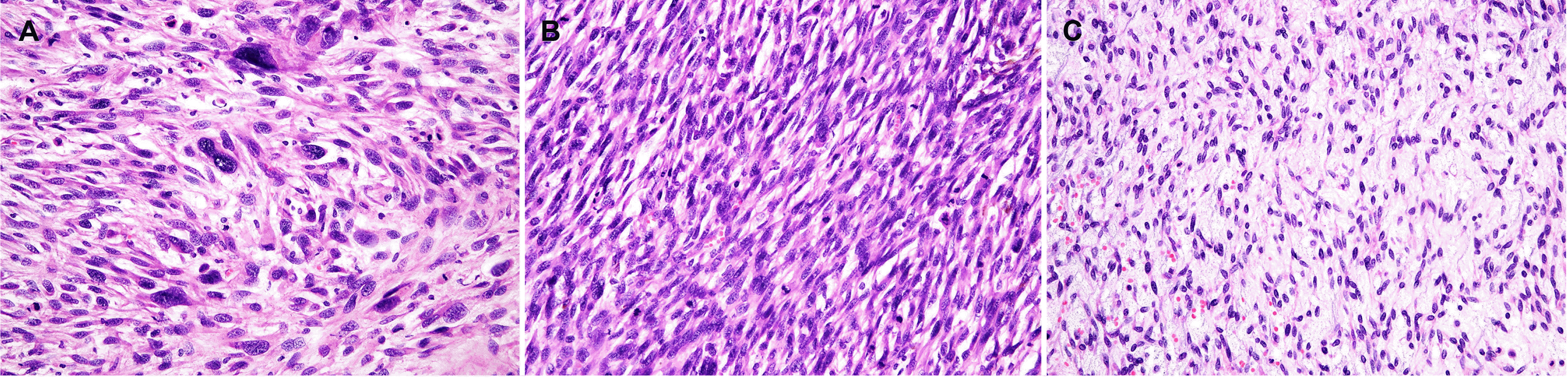
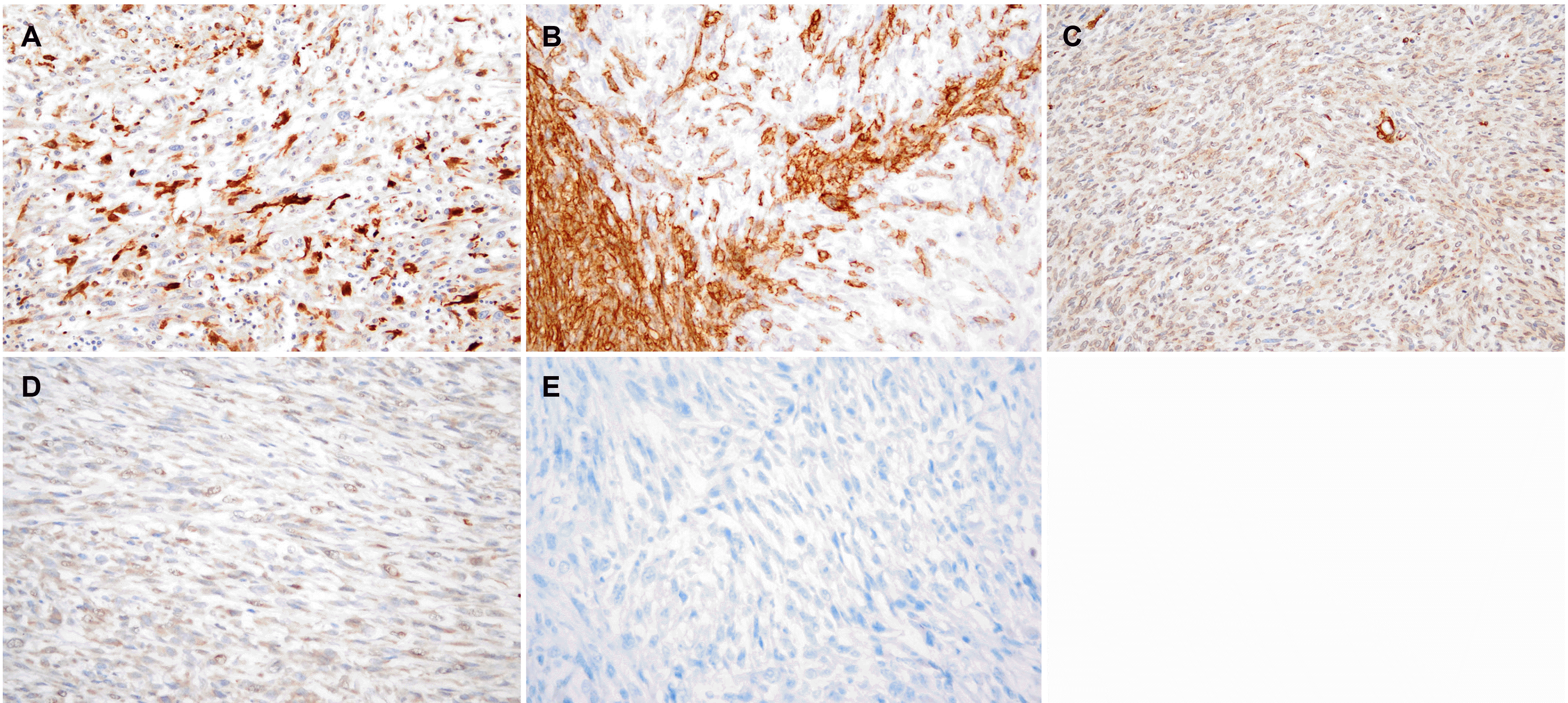
Histologically, the gastrectomy specimen revealed a large fungating mass (Fig. 4), measuring 13.0×11.0×9.0 cm obtained from the fundus, and another mass, measuring 6.0×6.0×2.0 cm, obtained from the great curvature side of upper-body. The tumor was composed of fascicles of spindle cells with many large pleomorphic cells (Fig. 5A) and alternating hypercellular (Fig. 5B) and hypocellular (Fig. 5C) areas. The spindle cells were hyperchromatic with wavy nuclei and eosinophilic cytoplasm. Immunohistochemically, the tumor showed focal faint positivity (<10%) for S100 (Fig. 6A), CD34 (Fig. 6B), and smooth muscle actin (Fig. 6C). The tumor demonstrated focal faint positivity for C-kit (Fig. 6D) and complete negativity for DOG-1 (Fig. 6E). These findings were compatible with a diagnosis of MPNST, Federation Nationale des Centres de Lutte le Cancer (FNCLCC) grade 3 (tumor differentiation 2, 28 mitotic counts per 10 HPF, and 30% of tumor necrosis). The tumor was resected entirely without metastasis in 33 regional lymph nodes. Three cycles of doxorubicin-based combination chemotherapy (CYVADIC) were administered for lymphovascular invasion, but it recurred as peritoneal seeding. The 2nd line of PD-L1 inhibitor combined with CTLA-4 inhibitor immunotherapy was administered for two cycles, but the patient died due to a systemic infection.
In this case, MPNST exhibited as a gastric subepithelial lesion in a patient with Type 1 NF. Type 1 NF is one of the most common autosomal dominant genetic disorders with an incidence of 1 in 2,500-3,000.7 It is a genetic disorder that exhibits systemic manifestations, causing abnormalities of tumor suppressor genes due to a mutation in the NF-1 gene, hence increasing the rate of malignancy.8 Gastrointestinal tract lesions are uncommon in Type 1 NF. On the other hand, neurofibromas, carcinoid tumors, and GISTs, which may appear as subepithelial lesions, occur more commonly in Type 1 NF than in the general population. MPNST is characterized by focal positive staining for the immunochemical staining of S100.9 Neurofibroma has strong positivity in S100 and fingerprint- like positivity in CD34.10 Carcinoid tumors are positive for keratin, EMA, NSE, synaptophysin, CD57/Leu7, and chromogranin A.11 GIST is positive for DOG1, CD34, CD117.12
MPNST is more common in patients with genetic Type 1 NF.13 Approximately half of MPNST are associated with the NF-1 gene.13 MPNST has a high risk of local recurrence and distant metastasis.14
MPNST is uncommon in the gastrointestinal tract.13 MPNST may present in the gastrointestinal tract as bleeding or obstruction, but it is often clinically silent compared to other painful tumors. MPNST occurs aggressively, and early detection is the most effective against its progression. On the other hand, the early detection of malignant changes within preexisting large tumors is difficult. In the present case, the intraoperative findings revealed an invasion of the spleen and diaphragm, but a complete tumor resection was possible. In 2011, Watanabe et al.15 reported a case of laparoscopy- assisted partial gastrectomy for malignant schwannoma in the gastric body without metastasis. The patient had no evidence of recurrence for 2 years after the resection.15 In 2012, Takemura et al.16 reported a case of a distal gastrectomy with a regional lymph node dissection for a malignant schwannoma in the lesser curvature of the gastric body with multiple liver metastases. The patient died 5 months after surgery without undergoing any additional treatment.16 In the present case, liver metastasis and peritoneal seeding occurred despite the tumor being completely resected. The patient eventually died of a systemic infection despite chemotherapy and 2nd line immunotherapy, suggesting a poor prognosis. The present case is the first case report of gastric MPNST in Korea diagnosed with surgical treatment in a Type 1 NF patient.
Based on this case report, the following conclusions can be drawn: gastric MPNST has a very aggressive disease course and can appear in the form of subepithelial lesions on endoscopy. MPNST must also be considered in a differential diagnosis for gastric subepithelial lesions in patients with a background of Type 1 NF. Tissue confirmation may be considered for the gastric subepithelial lesions detected in patients with Type 1 NF. Surgery should also be considered as a complete resection is the only option for cure.
REFERENCES
1. Czarnecka AM, Sobczuk P, Zdzienicki M, Spałek M, Rutkowski P. 2018; Malignant peripheral nerve sheath tumour (MPNST). Oncol Clin Pract. 14:364–376. DOI: 10.5603/OCP.2018.0050. PMID: 19835664.

2. Kwon Y, Lee SE, Hwang DW, Lim CS, Jang JY, Kim SW. 2008; Malignant peripheral nerve sheath tumor of the pancreas: a case report. Korean J Hepatobiliary Pancreat Surg. 12:307–311. DOI: 10.1016/j.ijscr.2019.02.011,. PMID: 30785006. PMCID: PMC6383168.
3. Dare AJ, Gupta AA, Thipphavong S, Miettinen M, Gladdy RA. 2020; Abdominal neoplastic manifestations of neurofibromatosis type 1. Neurooncol Adv. 2(Suppl 1):i124–i133. DOI: 10.1093/noajnl/vdaa032. PMID: 32642738. PMCID: PMC7317050.

4. McCaughan JA, Holloway SM, Davidson R, Lam WW. 2007; Further evidence of the increased risk for malignant peripheral nerve sheath tumour from a Scottish cohort of patients with neurofibromatosis type 1. J Med Genet. 44:463–466. DOI: 10.1136/jmg.2006.048140. PMID: 17327286. PMCID: PMC2598004.

5. Bates JE, Peterson CR, Dhakal S, Giampoli EJ, Constine LS. 2014; Malignant peripheral nerve sheath tumors (MPNST): a SEER analysis of incidence across the age spectrum and therapeutic interventions in the pediatric population. Pediatr Blood Cancer. 61:1955–1960. DOI: 10.1002/pbc.25149. PMID: 25130403.

6. Shanmugam S, Govindasamy G, Hussain SA, Prasanna Srinivasa Rao H. 2017; Gastric malignant peripheral nerve sheath tumor - a rare mesenchymal tumor in the era of C-Kit. Journal Of Medical Science And Clinical. 5:24387–24391. DOI: 10.18535/jmscr/v5i7.13.

7. Leroy K, Dumas V, Martin-Garcia N, et al. 2001; Malignant peripheral nerve sheath tumors associated with neurofibromatosis type 1: a clinicopathologic and molecular study of 17 patients. Arch Dermatol. 137:908–913. PMID: 11453810.
8. Patil S, Chamberlain RS. 2012; Neoplasms associated with germline and somatic NF1 gene mutations. Oncologist. 17:101–116. DOI: 10.1634/theoncologist.2010-0181. PMID: 22240541. PMCID: PMC3267808.
9. Malignant peripheral nerve sheath tumor (MPNST). [Internet]. Bingham Farms (MI): PathologyOutlines.com, Inc.;2021. Dec. 8. cited 2022 Mar 17. Available from: https://www.pathologyoutlines.com/topic/softtissuempnst.html.
10. Neurofibroma-general. [Internet]. Bingham Farms (MI): PathologyOutlines.com, Inc.;2022. Jan. 4. cited 2022 Mar 17. Available from: http://www.pathologyoutlines.com/topic/softtissueneurofibroma.html.
11. Carcinoid tumor. [Internet]. Bingham Farms (MI): PathologyOutlines.com, Inc.;2020. Dec. 22. cited 2022 Mar 17. Available from: https://www.pathologyoutlines.com/topic/mediastinumcarcinoid.html. PMID: 11453810.
12. GIST. [Internet]. Bingham Farms (MI): PathologyOutlines.com, Inc.;2022. Feb. 24. cited 2022 Mar 17. Available from: https://www.pathologyoutlines.com/topic/stomachGIST.html.
13. Garrouche N, Ben Abdallah A, Arifa N, et al. 2018; Spectrum of gastrointestinal lesions of neurofibromatosis type 1: a pictorial review. Insights Imaging. 9:661–671. DOI: 10.1007/s13244-018-0648-8. PMID: 30187267. PMCID: PMC6206377.

14. Doorn PF, Molenaar WM, Buter J, Hoekstra HJ. 1995; Malignant peripheral nerve sheath tumors in patients with and without neurofibromatosis. Eur J Surg Oncol. 21:78–82. DOI: 10.1016/S0748-7983(05)80073-3. PMID: 7851559.

15. Watanabe A, Ojima H, Suzuki S, et al. 2011; An individual with gastric schwannoma with pathologically malignant potential surviving two years after laparoscopy-assisted partial gastrectomy. Case Rep Gastroenterol. 5:502–507. DOI: 10.1159/000331561. PMID: 21960956. PMCID: PMC3180670.

16. Takemura M, Yoshida K, Takii M, Sakurai K, Kanazawa A. 2012; Gastric malignant schwannoma presenting with upper gastrointestinal bleeding: a case report. J Med Case Rep. 6:37. DOI: 10.1186/1752-1947-6-37. PMID: 22277785. PMCID: PMC3292804.

Fig. 1
(A) Esophagogastroduodenoscopy (EGD) shows a 4.2 cm sized central ulcerated subepithelial lesion in the fundus. (B) EGD shows a 5.0 cm sized central ulcerated subepithelial lesion in the upper-body.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download