CASE REPORT
A 52-year-old woman presented with abdominal distension and weight loss that had persisted for several months. She complained of an inability to tolerate oral intake. She had never undergone esophagogastroduodenoscopy before. Her vital signs were stable, and the physical examination revealed marked abdominal distension without remarkable tenderness. The laboratory investigations were within normal limits except for the elevated carcinoembryonic antigen (CEA) level (856.9 ng/mL, reference value: 0-5 ng/mL). Endoscopy revealed pyloric stenosis caused by an infiltrating mass at the pylorus (
Fig. 1A, B). A biopsy of the mass confirmed the diagnosis of adenocarcinoma. Abdominal CT revealed multiple hepatic metastases in both liver lobes. A 20 mm×80 mm SEMS (Niti-S ™ D pyloric stent, TaeWoong Medical, Gimpo, Korea) was inserted to relieve gastric stasis (
Fig. 1C). She was referred to an oncologist and started receiving a combination of XELoda
® (capecitabine) and OXaliplatin (XELOX) chemotherapy.
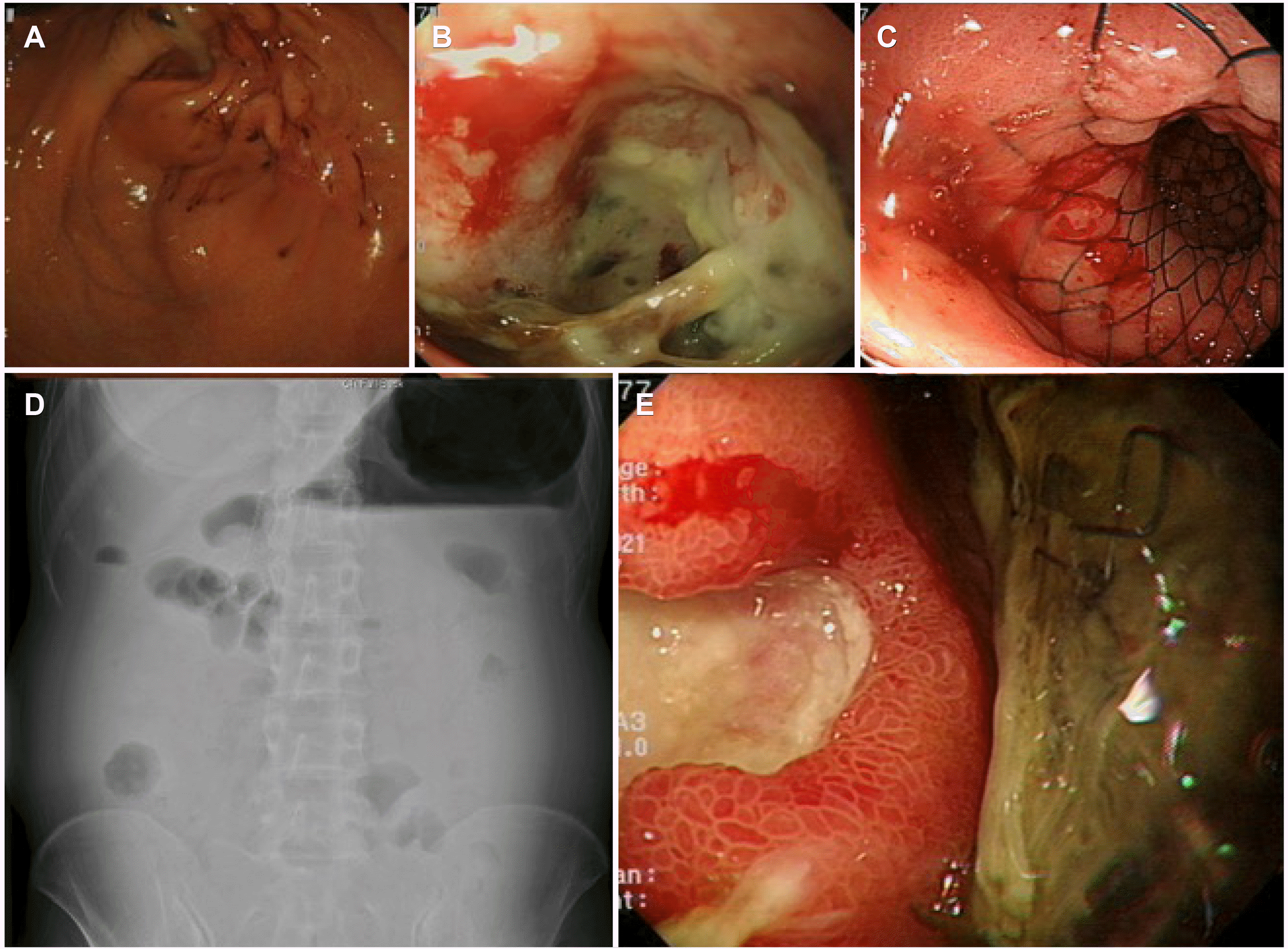 | Fig. 1Pyloric mass and self-expandable metal stent (SEMS) insertion. (A, B) Endoscopic view of the infiltrating mass at the pylorus. (C) Pyloric SEMS insertion. (D) Simple abdominal radiography indicating gastric outlet obstruction. (E) Previous SEMS dysfunction. 
|
Approximately 40 days later, she visited the outpatient clinic with the chief complaint of abdominal distension. Simple abdominal radiography showed an air-fluid level in the stomach, and gastroscopy revealed a pyloric stent dysfunction (
Fig. 1D, E). Stent revision or additional stent insertion was not possible due to cancer infiltration.
The decision was made to use another route between the stomach and jejunum endoscopically. A 0.025-inch guidewire was passed through a cannula (RX ERCP Cannula Standard Tip; Boston Scientific, Natick, MA, USA). An endoscopic nasobiliary drainage (ENBD) tube (7-Fr; Boston Scientific) was placed in the proximal jejunum along the guidewire (
Fig. 2A). A linear echoendoscope (GF-UCT 240; Olympus Medical Systems, Tokyo, Japan) was inserted into the stomach. While contrast medium mixed with saline solution and methylene blue was injected continuously through the ENBD tube, the proximal jejunum adjacent to the stomach body was found. A 19-gauge needle (EchoTip Ultra; COOK Medical, Bloomington, IN, USA) was used to puncture the jejunum under the EUS guided view (
Fig. 2B). The jejunal fluid was aspirated and checked for methylene blue-colored fluid, confirming the needle inside the jejunal lumen. After a 0.025-inch guidewire was delivered into the jejunum far enough (
Fig. 2C), a 16 mm×31 mm Niti-S™ HOT SPAXUS™ (TaeWoong Medical) was inserted into the jejunum through the guidewire (AUTOCUT mode, 100 Watts, effect 3; VIO 200s, ERBE, Tübingen, Germany), and the distal flange was first deployed inside the jejunum under the fluoroscopic and EUS views and pulled back against the jejunal wall. The proximal flange was then deployed in the stomach (
Fig. 2D). Balloon dilatation (CRE™ 12-15 mm; Boston Scientific) was performed to secure the passage patency for food (
Fig. 2E), and a conventional gastroscope (GIF-H 180; Olympus Medical Systems) was inserted into the jejunum through the lumen apposing metal stent (LAMS) (
Fig. 2F).
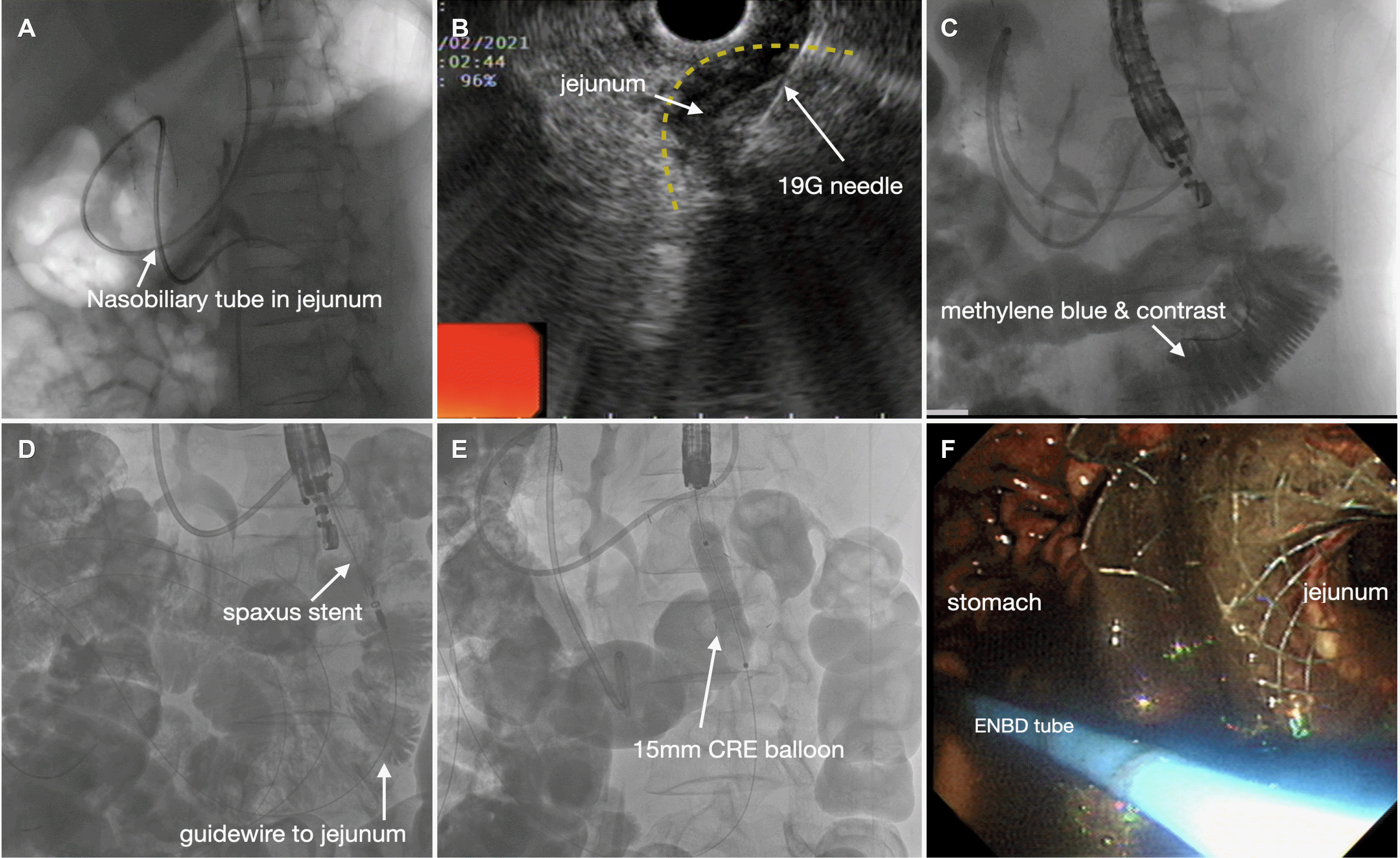 | Fig. 2Endoscopic nasobiliary drainage (ENBD) tube-assisted endoscopic ultrasonography-guided gastrojejunostomy. (A) Fluoroscopic view of the ENBD tube placed in the proximal jejunum. (B) Echoendoscopic view showing a 19-gauge needle punctured into the jejunum. (C) Fluoroscopic view of the contrast medium and guidewire in the jejunum. (D) SPAXUS stent insertion into the jejunum along the guidewire. (E) CRE balloon dilatation inside the SPAXUS stent. (F) Endoscopic direct view of the jejunum from the stomach. 
|
After the procedure, the patient had a good oral intake for more than 7 months. Follow-up abdominal radiography and CT showed a well-functioning LAMS between the stomach and jejunum (
Fig. 3).
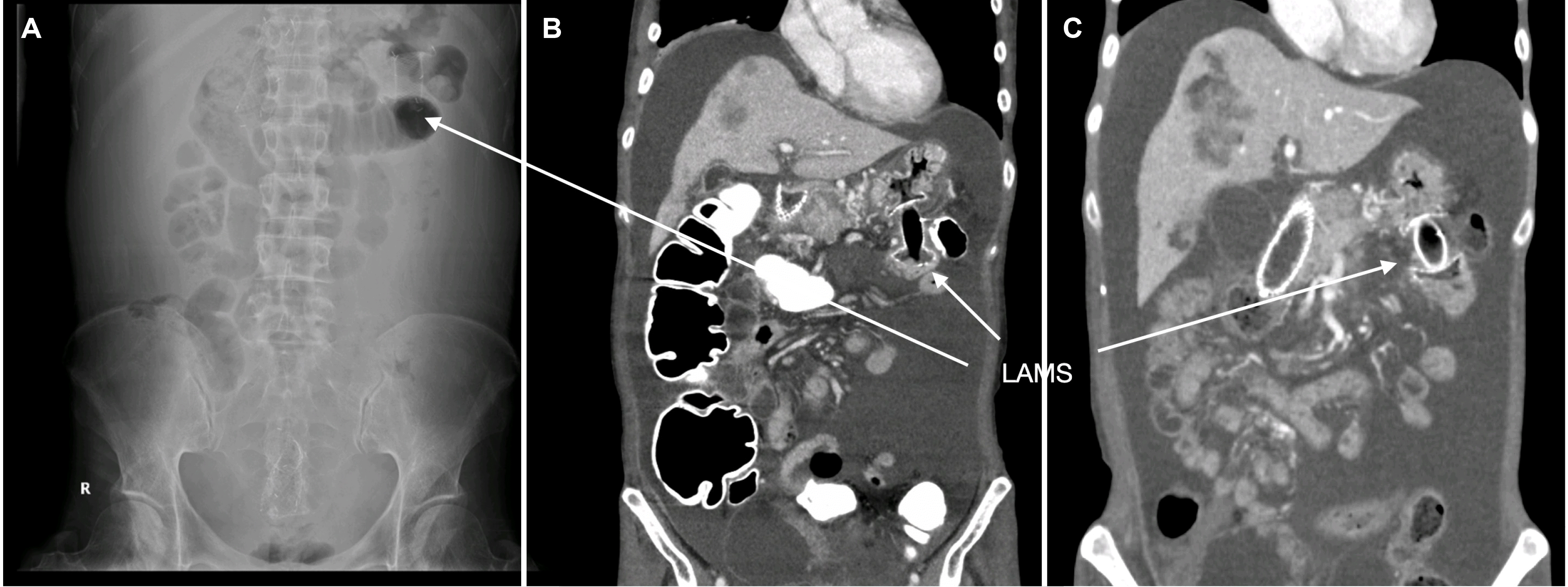 | Fig. 3Gastrojejunostomy with LAMS. (A) Abdominal radiograph. (B) CT scan. (C) f/u CT scan (4 months later). LAMS, lumen apposing metal stent; CT, computed tomography. 
|
The patient provided consent for the publication of this case with the removal of all identifying information to ensure anonymity and retain her privacy.
Go to :

DISCUSSION
GOO is one of the most common complications of the pancreatic head, advanced distal gastric, and periampullary cancers.
4,5 Patients with GOO experience significant weight loss, vomiting, and poor oral intake. Most patients have a poor prognosis and usually do not survive more than 6 months.
6 Endoscopic SEMS and surgical GJJ are well-established treatment options. According to Fiori et al.
7, there were no significant differences between the two treatment options regarding morbidity, mortality, and delayed gastric emptying. On the other hand, endoscopic SEMS insertion was more effective in terms of operative time, restoration of oral intake, and median hospitalization duration.
7 Endoscopic SEMS insertion can be selected as the first-line treatment option for GOO because of its procedural simplicity and convenience under sedation.
4
Pyloric SEMS dysfunction is often encountered in patients with GOO because of cancer growth or stent migration.
4,8 On the other hand, these patients usually have a poor condition from the terminal stage of cancer and are unsuitable for surgery because of the risk of general anesthesia. Therefore, alternative treatment options are needed.
The EUS techniques have progressed substantially in recent decades with advances in scope devices and imaging resolutions. Recently, the main indication for EUS has shifted to therapeutic interventions similar to ERCP.
9 The EUS-guided procedures, such as hepaticogastrostomy, choledochoduodenostomy, gallbladder drainage, and pancreatic duct drainage, have developed remarkably along with EUS improvement and stent development.
9,10 The EUS-guided procedures can be used in cases where the papilla or target point over the obstructive lesion is inaccessible.
11 In particular, LAMS brought about a new era, in which two luminal structures can be communicated by securely anchoring the stent and preventing its migration.
12,13 Moreover, electrocautery-enhanced LAMS, such as Niti-S™ HOT SPAXUS™ (TaeWoong Medical) and Hot AXIOS™ (Boston Scientific), reduce the procedural failure rates by simplifying the procedural steps.
14 Most procedural errors come from multiple steps in exchanging accessory devices, such as cystotomes, dilation balloons, and metal stents, through the guidewire.
EUS-GJ is defined as the insertion of a LAMS from the stomach to the jejunum, distal to the obstructive lesion.
15 The concept of EUS-GJ was first introduced by Fritscher-Ravens et al.
16 Perez-Miranda et al.
17 reported that EUS-GJ has similar efficacy to laparoscopic GJ and significantly lower adverse effects, even though the EUS-GJ group had more complex patients.
Several techniques are available for performing EUS-GJs.
1,5,18 Among these, device-assisted EUS-GJ was performed using an ENBD tube. This technique offers several advantages. First, it is safer than direct EUS-GJ because these devices, such as a dilation balloon or an ENBD tube, can delineate the jejunum to find the optimal site for puncture and help avoid mislocation of LAMS. Second, many centers are equipped with an ENBD tube, whereas a unique double-balloon enteric tube (Create Medic Co. Ltd., Yokohama, Japan), used in the EUS-guided double-balloon-occluded GJ bypass technique, is not yet commercially available in many countries.
18 EUS-GJ is a technically challenging procedure, and many hurdles need to be overcome.
5 First, approaching the duodenum or jejunum is quite difficult because of the pyloric mass or stricture. Second, endoscopy of the duodenum and jejunum requires air insufflation, which disturbs the clear ultrasound images. Third, a large amount of fluid infusion into the small bowel can cause adverse events, such as hyponatremia and volume overload. EUS-GJ can also have adverse effects, such as bleeding and stent dislodgement, and sometimes requires rescue therapy with a bridging stent.
17 Thus, EUS-GJ should be performed carefully to avoid these problems. CO2 gas should be used instead of room air because complications, such as perforation, can occur. Care should be taken not to use too much fluid infusion in the small bowel.
There is a possibility of mislocating the LAMS during the EUS-guided procedures. The most important step is to secure the guidewire until all the procedural steps are finished. When the distal or proximal flange is deployed in the peritoneal cavity, rescue therapy can be performed by inserting an additional LAMS along the previous guidewire.
17,19
The EUS-GJ technique can also be used in cases of afferent loop syndrome, a rare complication that occurs after pancreaticoduodenectomy.
3 A multicenter retrospective study on EUS-GJ for 18 afferent loop syndrome patients reported clinical and technical success rates of 89% and 100%, respectively.
20 In conclusion, EUS-guided GJ is a feasible treatment option instead of surgery for patients with dysfunctional pyloric metal stents, gastric outlet obstruction, and afferent loop syndrome.
Go to :






 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download