Abstract
Lenvatinib prolongs the survival of patients with advanced thyroid cancer. At initiation of lenvatinib therapy, advanced thyroid cancer patients frequently have lung metastasis and are vulnerable to pulmonary complications due to concealed lung damage caused by previous therapies including radioactive iodine (RAI) therapy. Among 24 patients treated with lenvatinib, pulmonary events were observed in three patients with lung metastasis, including one with interstitial lung disease (ILD) and two with pneumothorax. One patient who was previously treated with 750 mCi RAI developed uncontrolled ILD after lenvatinib therapy and died of respiratory failure. Two pneumothorax cases had previous cavitation of metastatic lung nodules. Pneumothorax resolved spontaneously in both patients. Pulmonary events in patients with lung metastases treated with lenvatinib are uncommon and manageable in most cases, but may be fatal if detection and management are delayed. Special attention should be given to patients with lung metastasis treated with high cumulative dose of RAI therapy or cavitary changes that develop after lenvatinib therapy.
Lenvatinib is available for patients with locally advanced or metastatic radioactive iodine-refractory differentiated thyroid cancer (RR-DTC).1) Various adverse events (AEs) caused by lenvatinib therapy have been reported and the well-known lenvatinib-related AEs include hypertension, proteinuria, fatigue, decreased weight, nausea, and stomatitis.2,3) However, infrequent drug-related AEs can be overlooked and affect survival due to delayed intervention. The most common distant metastatic site of DTC is the lung and most such patients are previously treated with radioactive iodine (RAI) therapy.4) Therefore, lenvatinib therapy is frequently administered to RR-DTC patients who have vulnerable lungs due to RAI therapy or are susceptible to lung metastasis, which can cause uncommon pulmonary events including pulmonary hypertension, vascular events, pneumothorax, and interstitial lung disease (ILD).5) A small number of lenvatinib-related pneumothorax cases have been described in patients with rapidly growing anaplastic thyroid cancer (ATC),6,7) however, to the best of our knowledge, occurrence of ILD during lenvatinib therapy in a patient with thyroid cancer has not been reported to date. Herein, we describe a case-series of pulmonary events during lenvatinib therapy in patients with advanced thyroid cancer.
An 82-year-old male underwent total thyroidectomy due to the follicular variant of papillary thyroid cancer (FVPTC) 11 years prior and received RAI therapy with a total dose of 750 mCi. He was diagnosed with a RR-DTC with lung and bone metastases after the fourth RAI therapy. He was treated with sorafenib for 9 months; however, painful and aggravated bone metastases were observed. The serum thyroglobulin level was 2500 ng/mL. Radiation therapy to the lumbar spine was performed and lenvatinib therapy was started with a daily dose of 24 mg due to progressive bone metastases one year after sorafenib therapy withdrawal. He visited our emergency department complaining of chest pain, cough, and sputum production 2 months after lenvatinib initiation; chest CT revealed ILD with a severe cryptogenic organizing pneumonia (COP) pattern, which was not observed before lenvatinib therapy. Cardiac examination using 2D-echocardiogram revealed normal left ventricular ejection fraction (74.2% based on modified Simpson’s method) with mild mitral stenosis and intermediate atrial stenosis, which were not different from before lenvatinib therapy. He was a non-smoker, did not have a history of rheumatoid disease, and had not started any new drug history other than lenvatinib. All tests for other non-specific pneumonia including pneumocystis carinii, tuberculosis, and parasite were negative. Bronchoscopy demonstrated no endobronchial lesions and tests of bronchoalveolar lavage (BAL) fluid for the evaluation of other pneumonia were negative. Bronchoscopic biopsy and pulmonary function tests were not performed due to old age and frail condition. He died from acute respiratory failure after the ILD became aggravated, despite high-dose steroid therapy and discontinuation of lenvatinib (Fig. 1).
A 49-year-old female was diagnosed with ATC with lung and bone metastasis. She was administered lenvatinib therapy with starting dose of 24 mg. She had lung metastasis-related symptoms including cough, mild dyspnea, and blood-tinged sputum; the metastasis had thin-walled cavities that were evident in chest CT prior to pneumothorax emergence. The pneumothorax was detected via regular follow-up CT 1 year after starting lenvatinib therapy; however, her symptoms did not change (Fig. 2). The pneumothorax resolved spontaneously after temporary withdrawal of lenvatinib for 1 week and supportive care including oxygen therapy.
An 80-year-old female with Graves’ disease was diagnosed as poorly differentiated thyroid cancer (PDTC) with bone metastasis. She underwent total thyroidectomy and received RAI therapy with a 100 mCi dose. Post-therapeutic 131I scan showed no uptake although her serum levels of thyroglobulin level were elevated to 1730 ng/mL. Three years later, lenvatinib therapy was started due to aggravated lung metastasis with invasion of the right pulmonary vein by metastatic lymph nodes. She exhibited marked reduction of lung metastasis with newly observed cavitary changes in metastatic tumors. A small asymptomatic pneumothorax during regular CT follow-up approximately 10 months after lenvatinib initiation was observed and resolved without lenvatinib interruption (Fig. 3).
Two patients with lung metastases developed drug-related pneumothorax that resolved with only supportive care. The clinical characteristics of three pneumothorax cases previously reported and our cases are summarized in Table 1.
Lenvatinib therapy is associated with a variety of AEs that affect almost all patients and all are manageable and require lenvatinib dose reductions or pauses in most cases.8-10) However, rare AEs such as thromboembolisms, aero-digestive tract fistulae, biliary tract disorders, and pulmonary toxicities have not been well-described.11-13) Delayed AE detection could be fatal; thus, early detection and appropriate management are crucial. Unlike other tyrosine kinase inhibitor (TKI) therapies for lung cancer and secondary lung metastasis, lenvatinib related pulmonary AEs in patients with advanced thyroid cancer have not been well-described; only a few cases of pneumothorax in ATC patients developing during lenvatinib therapy have been reported.5)
In a previous study, the evaluation of drug induced ILD in early phase oncology clinical trials reported diverse clinical and radiological findings with variable onset, which were mild grade in most cases and improved with early treatment interruption.14) The ILD incidence associated with the use of various TKIs ranges from 1-5% and some are life-threatening.15) Three cases of sorafenib-induced ILDs with acute interstitial pneumonia (AIP) have been reported in hepatocellular carcinoma (HCC) patients and all patients developed ILD within a few days to months after sorafenib administration.16) TKI-associated ILDs vary in frequency, severity, and time from TKI administration.17-20) The mechanism of drug-induced ILD remains unclear but several theories have been proposed. TKIs may directly induce pulmonary fibrosis, act on the immune system, increase the levels of inflammatory cytokines, and/or trigger phospholipidosis in alveolar macrophages. A diagnosis of TKI-caused ILD is based on drug history, chest CT data, and the absence of infection and cancer progression.18) The most common ILD symptoms are dyspnea, cough, fever, and hypoxemia (the latter in severe cases). A diagnosis of drug-induced ILD prompts discontinuance of the drug and high-dose steroid therapy. However, most advanced cancer patients that respond to TKIs have no other treatment choice; the only possible options are dose reduction or a switch to another TKI.21) Two cases of lenvatinib-related ILD have been reported.22,23) In a patient, who had HCC, a reticular shadow induced by previous regorafenib therapy became exacerbated during lenvatinib therapy and was treated by discontinuation of lenvatinib and steroid pulse therapy.23) In the other patient who had squamous cell carcinoma of unknown origin, 1 month after the drug was administered, lenvatinib-induced ILD developed and improved only after drug discontinuation.22) Lenvatinib therapy was discontinued for our FVPTC patient because of his poor general condition when lenvatinib-induced ILD exhibiting the COP pattern developed in both lungs; he died from respiratory failure despite high-dose steroid therapy (Fig. 1). To the best of our knowledge, this is the first case of lenvatinib-induced ILD reported in thyroid cancer patient. Thyroid cancer patients receiving high cumulated doses of RAI therapy might be vulnerable to pulmonary fibrosis and pneumonitis.24)
Most cases of pneumothorax induced by anti-cancer drugs have been described only in case reports. The mechanism remains poorly understood. The various theories include rupture of a subpleural bleb or bulla that existed prior to drug therapy or developed via a check-valve effect mediated by tumor growth accompanied by alveolar overdistension; rupture of an emphysematous bulla during lung overexpansion caused by partial bronchial obstruction by the tumor; and bronchopleural fistulation attributable to tumor invasion per se or tumor-associated necrosis.25,26) Lenvatinib-induced pneumothorax is rare, and all such patients reported to date have had lung metastases. Among the many lenvatinib toxicity studies, only two cases of uncontrolled, fatal lenvatinib-induced pneumothorax in 75 RR-DTC patients have been reported in one real world study.5) Three cases of lenvatinib-induced pneumothorax in ATC patients with lung metastases have been described as case reports; two cases were treated using chest tubing and pleurodesis6,7) and the other case underwent wedge resection due to uncontrolled pneumothorax despite chest tubing.27) In one study, when TKI-induced pneumothorax required intervention, chest tubing and pleurodesis were favored (compared to thoracotomy) to avoid pauses in TKI therapy and bleeding.28) We recorded two cases of lenvatinib-induced pneumothorax, and both achieved spontaneous reabsorption using best supportive care alone. Prior to pneumothorax development, chest CT of an ATC patient lacking any bulla or bleb prior to lenvatinib therapy revealed changes in the lung mass; thin-walled cavities developed after lenvatinib administration (Fig. 2). Marked reductions in the extents of metastatic lung nodules were associated with tumor necrosis and cavitary lesions, culminating in bronchopleural fistula. In our study, we decided to maintain lenvatinib therapy if pneumothorax did not increase due to no symptom and small amount of pneumothorax. A small pneumothorax in a PDTC patient improved even when lenvatinib was continued and a pneumothorax in an ATC patient resolved after temporary interruption of lenvatinib therapy and resumption of such therapy at a lower dose. A Japanese case exhibiting symptomatic recurrent pneumothorax resolved after chest tubing and pleurodesis; lenvatinib was continued at a lower dose.7) Multi-targeted TKI therapy tends to cause lung cavitation in thyroid cancer patients with lung metastasis.29,30)
Pulmonary complications in RR-DTC patients might be easily overlooked during lenvatinib therapy. Early detection is critical because potentially fatal complications might develop in severe cases. Patients with lung metastasis previously treated with previous large doses of RAI therapy or who exhibit new cavitary changes after lenvatinib treatment should be closed monitored.
Acknowledgments
This work was supported by a grant from the Chonnam National University Hwasun Hospital Institute for Biomedical Science (HCRI 21009).
References
1. Fugazzola L, Elisei R, Fuhrer D, Jarzab B, Leboulleux S, Newbold K, et al. 2019; 2019 European Thyroid Association guidelines for the treatment and follow-up of advanced radioiodine-refractory thyroid cancer. Eur Thyroid J. 8(5):227–45. DOI: 10.1159/000502229. PMID: 31768334. PMCID: PMC6873012.

2. Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. 2015; Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 372(7):621–30. DOI: 10.1056/NEJMoa1406470. PMID: 25671254.

3. Oh HS, Shin DY, Kim M, Park SY, Kim TH, Kim BH, et al. 2019; Extended real-world observation of patients treated with sorafenib for radioactive iodine-refractory differentiated thyroid carcinoma and impact of lenvatinib salvage treatment: a Korean multicenter study. Thyroid. 29(12):1804–10. DOI: 10.1089/thy.2019.0246. PMID: 31592739.

4. Ruegemer JJ, Hay ID, Bergstralh EJ, Ryan JJ, Offord KP, Gorman CA. 1988; Distant metastases in differentiated thyroid carcinoma: a multivariate analysis of prognostic variables. J Clin Endocrinol Metab. 67(3):501–8. DOI: 10.1210/jcem-67-3-501. PMID: 3410936.

5. Berdelou A, Borget I, Godbert Y, Nguyen T, Garcia ME, Chougnet CN, et al. 2018; Lenvatinib for the treatment of radioiodine-refractory thyroid cancer in real-life practice. Thyroid. 28(1):72–8. DOI: 10.1089/thy.2017.0205. PMID: 29048237.

6. Kazzaz FI, Cabanillas ME, Bashoura L, Shannon VR, Faiz SA. 2019; Bilateral spontaneous pneumothoraces in anaplastic thyroid cancer. Respir Med Case Rep. 26:197–9. DOI: 10.1016/j.rmcr.2019.01.006. PMID: 30705818. PMCID: PMC6348391.

7. Yamazaki H, Iwasaki H, Suganuma N, Toda S, Masudo K, Nakayama H, et al. 2019; Anaplastic thyroid carcinoma diagnosed after treatment of lenvatinib for papillary thyroid carcinoma. Endocrinol Diabetes Metab Case Rep. 2019:19–0085. DOI: 10.1530/EDM-19-0085. PMID: 31600721. PMCID: PMC6790905.

8. Lorusso L, Pieruzzi L, Biagini A, Sabini E, Valerio L, Giani C, et al. 2016; Lenvatinib and other tyrosine kinase inhibitors for the treatment of radioiodine refractory, advanced, and progressive thyroid cancer. Onco Targets Ther. 9:6467–77. DOI: 10.2147/OTT.S84625. PMID: 27799794. PMCID: PMC5079697.

9. Ancker OV, Kruger M, Wehland M, Infanger M, Grimm D. 2019; Multikinase inhibitor treatment in thyroid cancer. Int J Mol Sci. 21(1):10. DOI: 10.3390/ijms21010010. PMID: 31861373. PMCID: PMC6982227.

10. Aydemirli MD, Kapiteijn E, Ferrier KRM, Ottevanger PB, Links TP, van der Horst-Schrivers ANA, et al. 2020; Effectiveness and toxicity of lenvatinib in refractory thyroid cancer: Dutch real-life data. Eur J Endocrinol. 182(2):131–8. DOI: 10.1530/EJE-19-0763. PMID: 31751307.

11. Blevins DP, Dadu R, Hu M, Baik C, Balachandran D, Ross W, et al. 2014; Aerodigestive fistula formation as a rare side effect of antiangiogenic tyrosine kinase inhibitor therapy for thyroid cancer. Thyroid. 24(5):918–22. DOI: 10.1089/thy.2012.0598. PMID: 24635127. PMCID: PMC4026371.

12. Guan Y, Meng J, Zhao H, Hu Y, Yan X, Zhao SH, et al. 2014; Fatal interstitial lung disease after addition of sorafenib to a patient with lung adenocarcinoma who had failed to improve with erlotinib alone. Case Rep Oncol. 7(1):273–6. DOI: 10.1159/000362402. PMID: 24926256. PMCID: PMC4036133.

13. Nervo A, Ragni A, Gallo M, Ferraris A, Fonio P, Piovesan A, et al. 2020; Symptomatic biliary disorders during lenvatinib treatment for thyroid cancer: an underestimated problem. Thyroid. 30(2):229–36. DOI: 10.1089/thy.2019.0355. PMID: 31854230.

14. Terbuch A, Tiu C, Candilejo IM, Scaranti M, Curcean A, Bar D, et al. 2020; Radiological patterns of drug-induced interstitial lung disease (DILD) in early-phase oncology clinical trials. Clin Cancer Res. 26(18):4805–13. DOI: 10.1158/1078-0432.CCR-20-0454. PMID: 32332017.

15. Kashiwabara K, Semba H, Fujii S, Tsumura S. 2017; Outcome in advanced non-small cell lung cancer patients with successful rechallenge after recovery from epidermal growth factor receptor tyrosine kinase inhibitor-induced interstitial lung disease. Cancer Chemother Pharmacol. 79(4):705–10. DOI: 10.1007/s00280-017-3261-5. PMID: 28258422.

16. Takeda H, Nishikawa H, Iguchi E, Matsuda F, Kita R, Kimura T, et al. 2012; Sorafenib-induced acute interstitial pneumonia in patients with advanced hepatocellular carcinoma: report of three cases. Clin J Gastroenterol. 5(4):407–12. DOI: 10.1007/s12328-012-0339-9. PMID: 24672586. PMCID: PMC3961597.

17. Hotta K, Kiura K, Takigawa N, Yoshioka H, Harita S, Kuyama S, et al. 2010; Comparison of the incidence and pattern of interstitial lung disease during erlotinib and gefitinib treatment in Japanese patients with non-small cell lung cancer: the Okayama Lung Cancer Study Group experience. J Thorac Oncol. 5(2):179–84. DOI: 10.1097/JTO.0b013e3181ca12e0. PMID: 20101144.

18. Schwaiblmair M, Behr W, Haeckel T, Markl B, Foerg W, Berghaus T. 2012; Drug induced interstitial lung disease. Open Respir Med J. 6:63–74. DOI: 10.2174/1874306401206010063. PMID: 22896776. PMCID: PMC3415629.

19. Sakurada T, Kakiuchi S, Tajima S, Horinouchi Y, Okada N, Nishisako H, et al. 2015; Characteristics of and risk factors for interstitial lung disease induced by chemotherapy for lung cancer. Ann Pharmacother. 49(4):398–404. DOI: 10.1177/1060028014566446. PMID: 25565405.

20. Komada F. 2018; Analysis of time-to-onset of interstitial lung disease after the administration of small molecule molecularly-targeted drugs. Yakugaku Zasshi. 138(2):229–35. DOI: 10.1248/yakushi.17-00194. PMID: 29386436.

21. Koma Y, Matsuoka H, Yoshimatsu H, Suzuki Y. 2012; Successful treatment with erlotinib after gefitinib-induced interstitial lung disease: a case report and literature review. Int J Clin Pharmacol Ther. 50(10):760–4. DOI: 10.5414/CP201759. PMID: 22853866.

22. Kimura-Tsuchiya R, Sasaki E, Nakamura I, Suzuki S, Kawana S, Okouchi C, et al. 2018; A case of squamous cell carcinoma of unknown primary that responded to the multi-tyrosine kinase inhibitor lenvatinib. Case Rep Oncol. 11(1):75–80. DOI: 10.1159/000486569. PMID: 29515414. PMCID: PMC5836208.

23. Kotani K, Enomoto M, Okada M, Yoshida K, Motoyama H, Fujii H, et al. 2019; Interstitial pneumonia suspected during regorafenib administration and exacerbated by subsequent therapy with lenvatinib for unresectable hepatocellular carcinoma. Clin J Gastroenterol. 12(4):355–60. DOI: 10.1007/s12328-019-00983-x. PMID: 31020569.

24. Rall JE, Alpers JB, Lewallen CG, Sonenberg M, Berman M, Rawson RW. 1957; Radiation pneumonitis and fibrosis: a complication of radioiodine treatment of pulmonary metastases from cancer of the thyroid. J Clin Endocrinol Metab. 17(11):1263–76. DOI: 10.1210/jcem-17-11-1263. PMID: 13475467.

25. Maniwa T, Nakagawa K, Isaka M, Ohde Y, Okumura T, Kondo H. 2011; Pneumothorax associated with treatment for pulmonary malignancy. Interact Cardiovasc Thorac Surg. 13(3):257–61. DOI: 10.1510/icvts.2011.273201. PMID: 21700592.

26. Hendarsih E, Fadjari TH, Oehadian A. 2016; Chemotherapy-induced spontaneous pneumothorax: case series. Acta Med Indones. 48(2):134–8. PMID: 27550883.
27. Lee HN, Chung WS, Jang HJ, Jee S, Tae K. 2020; Bilateral pneumothorax in a patient with anaplastic thyroid carcinoma and lung metastasis during lenvatinib therapy: a case report. Gland Surg. 9(5):1579–83. DOI: 10.21037/gs-20-462. PMID: 33224834. PMCID: PMC7667081.

28. Xie L, Xu J, Sun X, Tang X, Yan T, Yang R, et al. 2020; Anorexia, hypertension, pneumothorax, and hypothyroidism: potential signs of improved clinical outcome following apatinib in advanced osteosarcoma. Cancer Manag Res. 12:91–102. DOI: 10.2147/CMAR.S232823. PMID: 32021426. PMCID: PMC6956393.
29. Datar S, Cabanillas M, Dadu R, Ost D, Grosu HB. 2020; Pulmonary cavitation in patients with thyroid cancer receiving antiangiogenic agents. BMC Cancer. 20(1):1181. DOI: 10.1186/s12885-020-07693-5. PMID: 33267782. PMCID: PMC7709335.

30. Kawanishi Y, Kuwahara M, Utsunomiya M, Ishida N, Ishikawa Y, Hiroi M, et al. 2021; Pneumothorax during lenvatinib treatment for hepatocellular carcinoma with lung metastasis. Clin J Gastroenterol. 14(1):288–92. DOI: 10.1007/s12328-020-01273-7. PMID: 33108567.

Fig. 1
Chest CT scan of 82-year-old male with lung metastasis from follicular variant papillary thyroid cancer (Case 1). (A) Chest CT scan before lenvatinib therapy. (B) Chest CT scan at 2 months after starting lenvatinib therapy. (C) Chest CT of the time that a patient had acute respiratory failure (10 days after chest CT [B]).
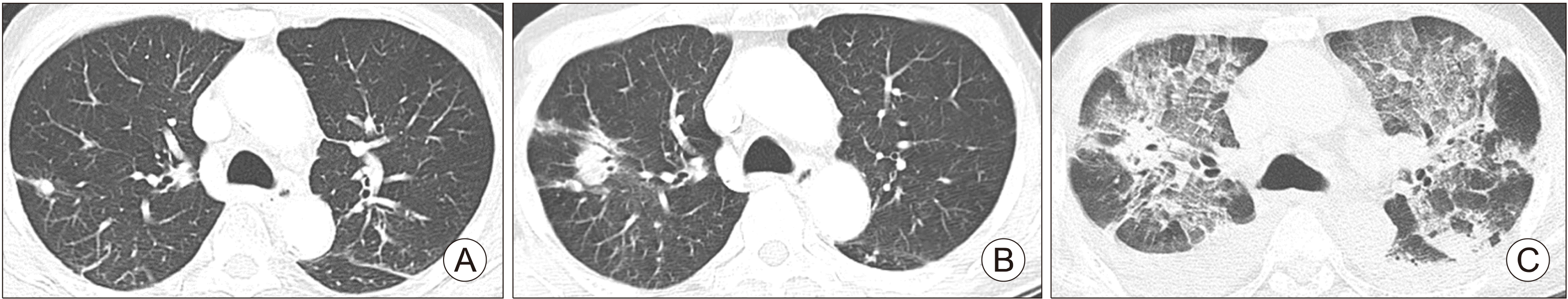
Fig. 2
Chest CT scan of 49-year-old female with lung metastasis from anaplastic thyroid cancer (Case 2). (A) Chest CT scan before lenvatinib therapy. (B) Chest CT scan at 3 months after lenvatinib therapy. (C) Chest CT scan of the time pneumothorax occurred at 353 days after lenvatinib administration.
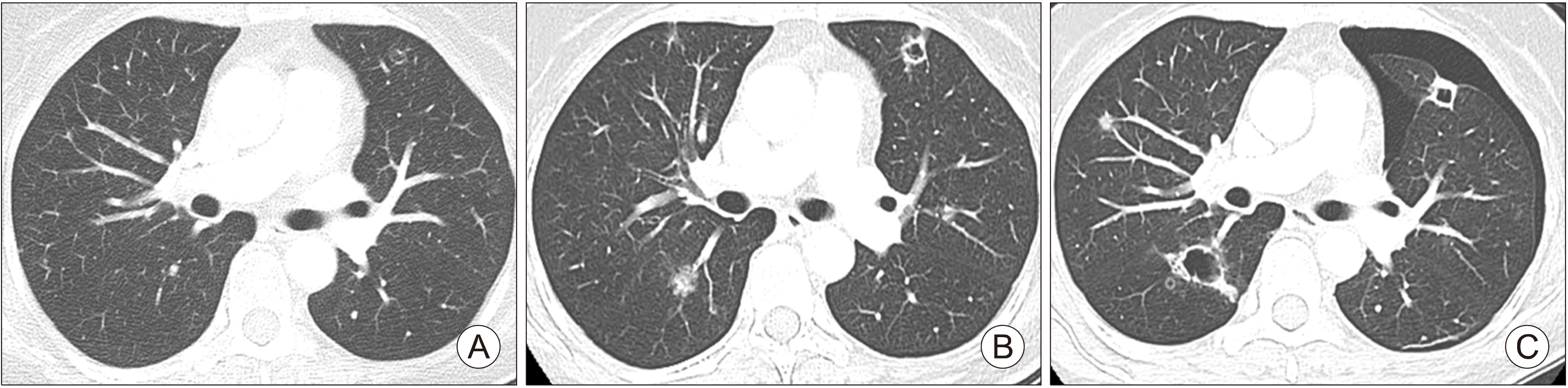
Fig. 3
Chest CT scan of 80-year-old female with lung metastasis from poorly differentiated thyroid cancer (Case 3). (A) Post-radioactive therapy with 131I (RAI) whole body scan. (B) 18FDG positron emission tomography/computed tomography (PET/CT). (C) Chest CT scan before lenvatinib therapy. (D) Chest CT scan of the time pneumothorax occurred at 10 months after lenvatinib therapy. (E) Chest CT scan at 3 months after lenvatinib related pneumothorax occurrence.
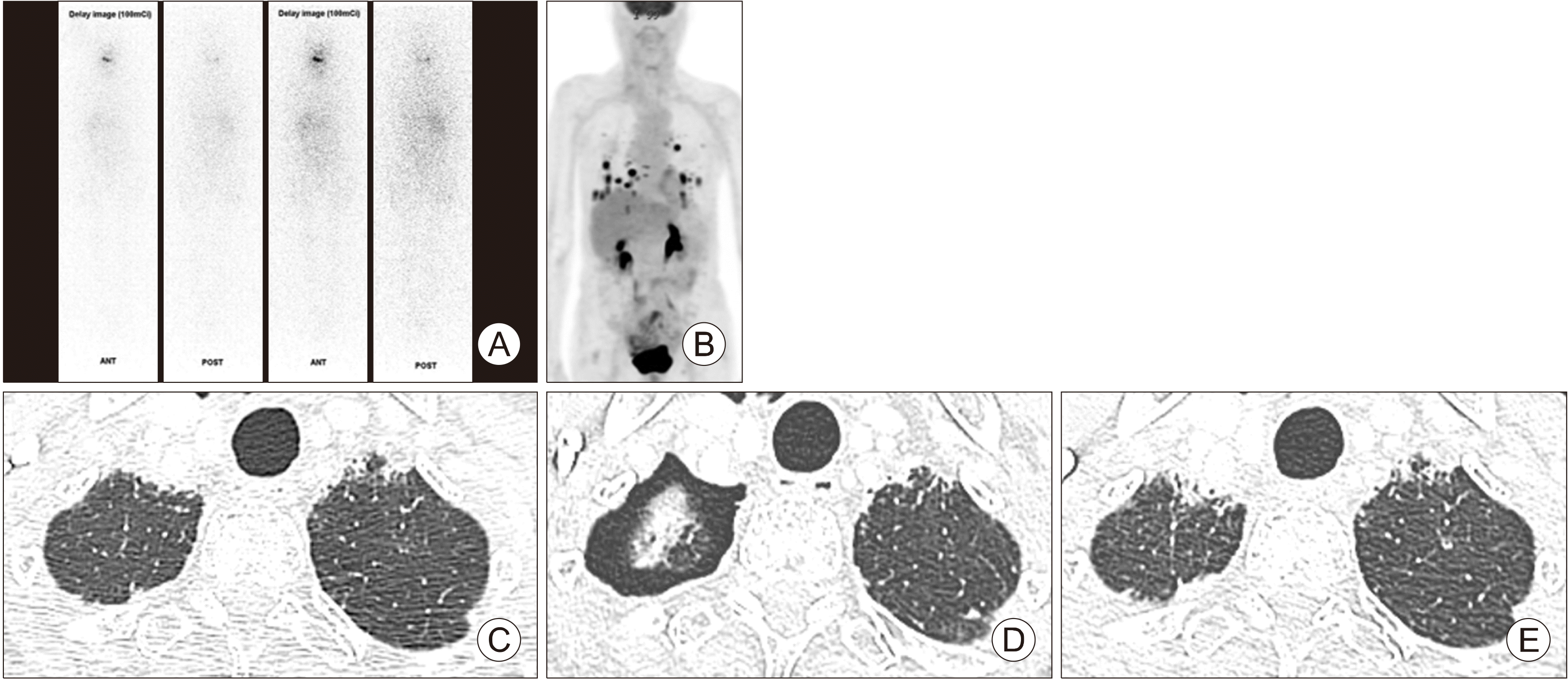
Table 1
The summary of clinical characteristics of lenvatinib induced pneumothorax in the literature with our cases
| Age/sex | Cancer type | Surgery | Lung metastasis | Other distant metastases | Cavitations of metastatic lung nodules after lenvatinib | Pneumothorax | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||
| Symptom | Time from lenvatinib initiation | Bilaterality | Lenvatinib dose at the moment of pulmonary event | Treatment | Lenvatinib therapy | Disease response to lenvatinib | |||||||
| Kazzaz et al.6) | 73/M | ATC | TT | + | Mediastinal lymph nodes | + | Mild dyspnea | 2 months | Bilateral | NA | Chest tubing and chemical pleurodesis | Temporary cessation and restart with lower dose | Progression during lenvatinib withhold. |
| Yamazaki et al.7) | 63/M | ATC | None | + | NA | + | None | 34 days | Unilateral | 20 mga | Chest tubing | Maintenance | SD |
| Lee et al.27) | 77/M | ATC | None | + | None | Dyspnea | 50 days | Bilateral | 24 mga | Chest tubing and wedge resection | Temporary cessation and restart with lower dose (20 mg) | SD → patient died before 1 month after lenvatinib restarting | |
| Kawanishi et al.30) | 71/M | HCC | None | + | None | + | Dyspnea, back pain | 2 months | Unilateral | 12 mga | Thoracoscopic wedge resection and chest tubing | Cessation | PD → patients died ten days after discharge |
| Our case 1 (case 2) | 49/F | ATC | None | + | Bone, liver | + | None | 353 days | Unilateral | 14 mga | Supportive care | Temporary cessation and restart at the same dose | PD |
| Our case 2 (case 3) | 80/F | PDTC | TT | + | Bone | + | None | 292 days | Unilateral | 10 mga | Supportive care | Maintenance | SD |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download