Introduction
Materials and Methods
Animals
Isolation, expansion, and identification of BMSCs
Isolation and identification of BMSC exosomes
Isolation and identification of EPCs
Lentivirus construction and infection
Tube formation assay
Rat model of diabetic wound healing
Histological analysis
Western blot analysis
Immunohistochemistry
ELISAs
Statistical analysis
Results
BMSC exosomes were successfully harvested and identified
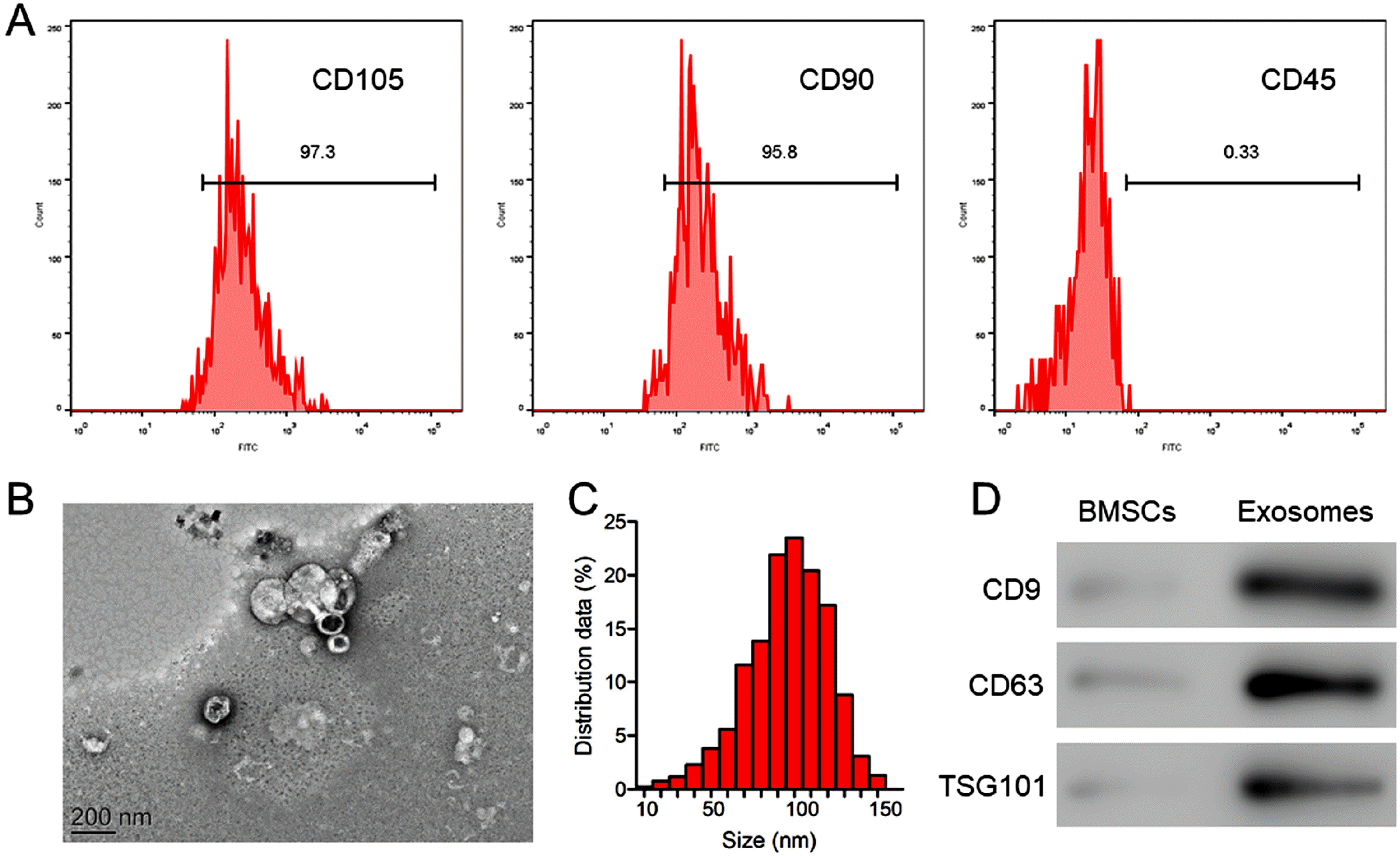 | Fig. 1Identification of BMSCs and BMSC exosomes. (A) The detection of the surface markers CD105, CD90, and CD45 in isolated BMSCs with flow cytometry. (B) A TEM image of BMSC exosomes. Scale bar=100 nm. (C) The particle size distribution of BMSC exosomes determined with light scattering. (D) The detection of the exosome markers CD9, CD63, and TSG101 in BMSC exosomes with western blot analysis. |
EPCs were successfully isolated and identified
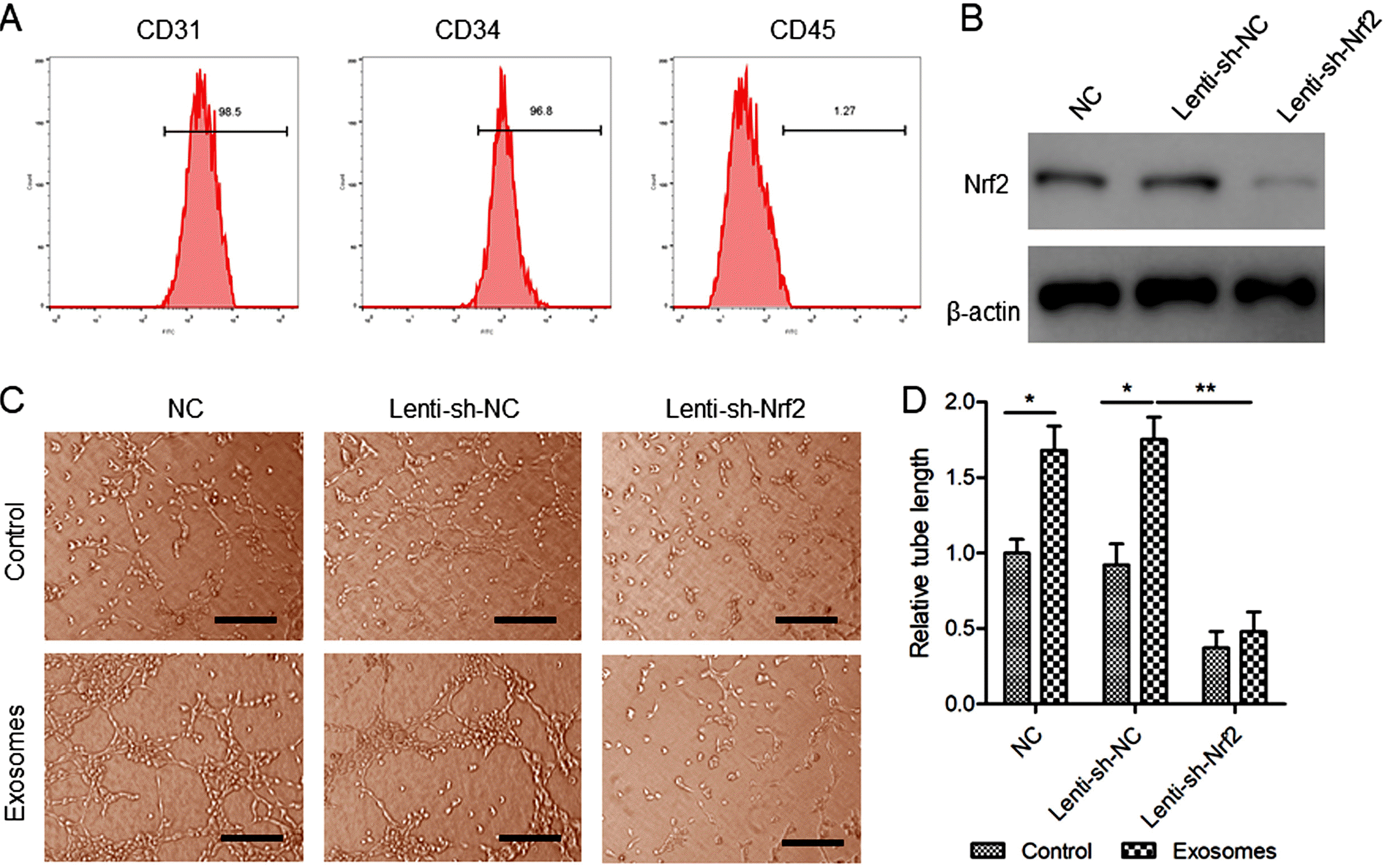 | Fig. 2The effects of BMSC exosomes and Nrf2 knockdown, alone or in combination, on EPC tube formation. (A) The detection of the surface markers CD31, CD34, and CD45 in isolated EPCs with flow cytometry. (B) EPCs were transfected with Lenti-sh-Nrf2 or Lenti-sh-NC for 48 hours. The Nrf2 protein levels were determined with western blot analysis. (C, D) EPCs stably expressing sh-Nrf2 or sh-NC were treated with BMSC exosomes (200 μg/ml) for 72 hours. (C) Tube formation was observed under a microscope. Scale bar=100 μm. (D) The relative tube length in each treatment group was determined. N=3, *p<0.05, **p< 0.01. |
Nrf2 knockdown inhibited EPC tube formation promoted by BMSC exosomes
Pharmaceutical activation of Nrf2 enhanced wound healing in diabetic rats by BMSC exosomes
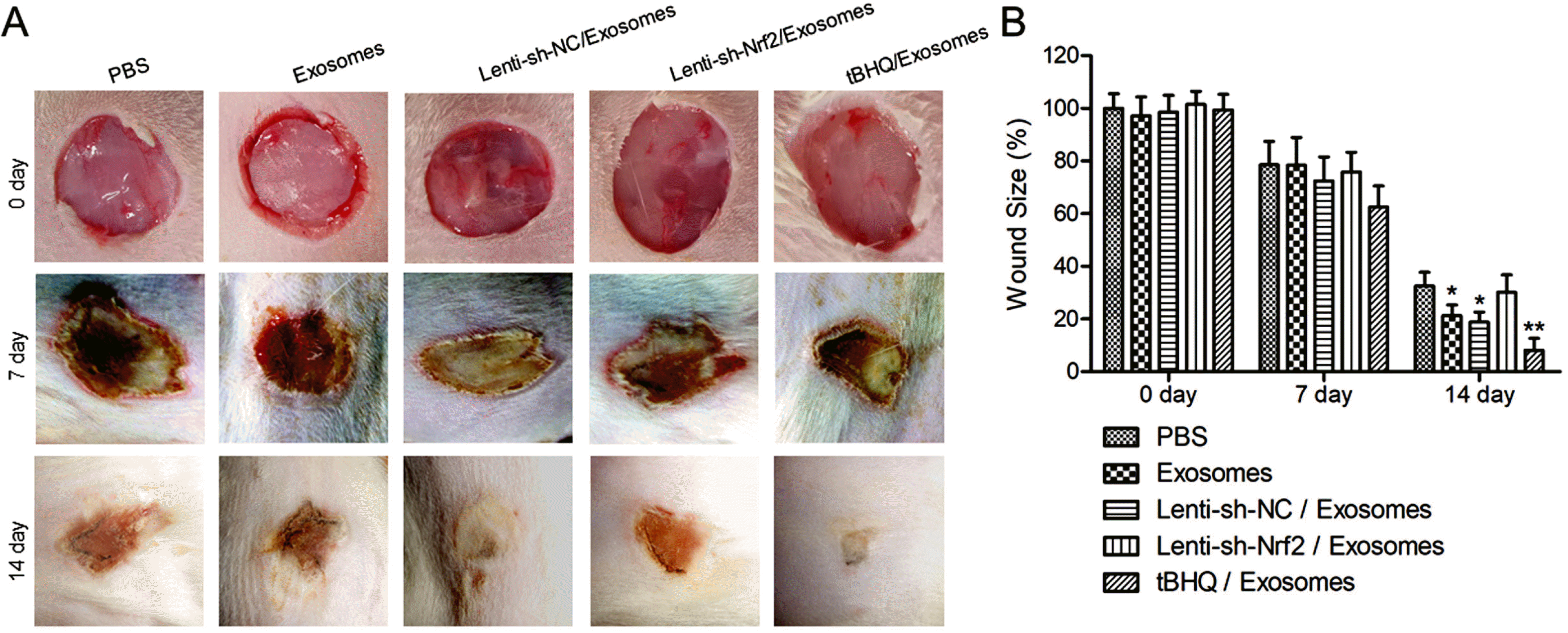 | Fig. 3The effects of BMSC exosomes alone, or in combination with knockdown or pharmaceutical activation of Nrf2 on wound closure in diabetic rats. STZ-induced diabetic rats received 100 μg/ml subcutaneous BMSC exosomes alone at the wound site, or in combination with 200 μl intravenous Lenti-sh-Nrf2 or Lenti-sh-NC, or 50 mg/kg intraperitoneal tBHQ immediately after the wounding and again a week after. Rats that received PBS were included as control. (A) The photo images of the wounds at day 0, 7, and 14 after the wounding. (B) The wound size at day 0, 7, and 14 after the wounding. N=5; *p<0.05, **p<0.01 vs. PBS control. |
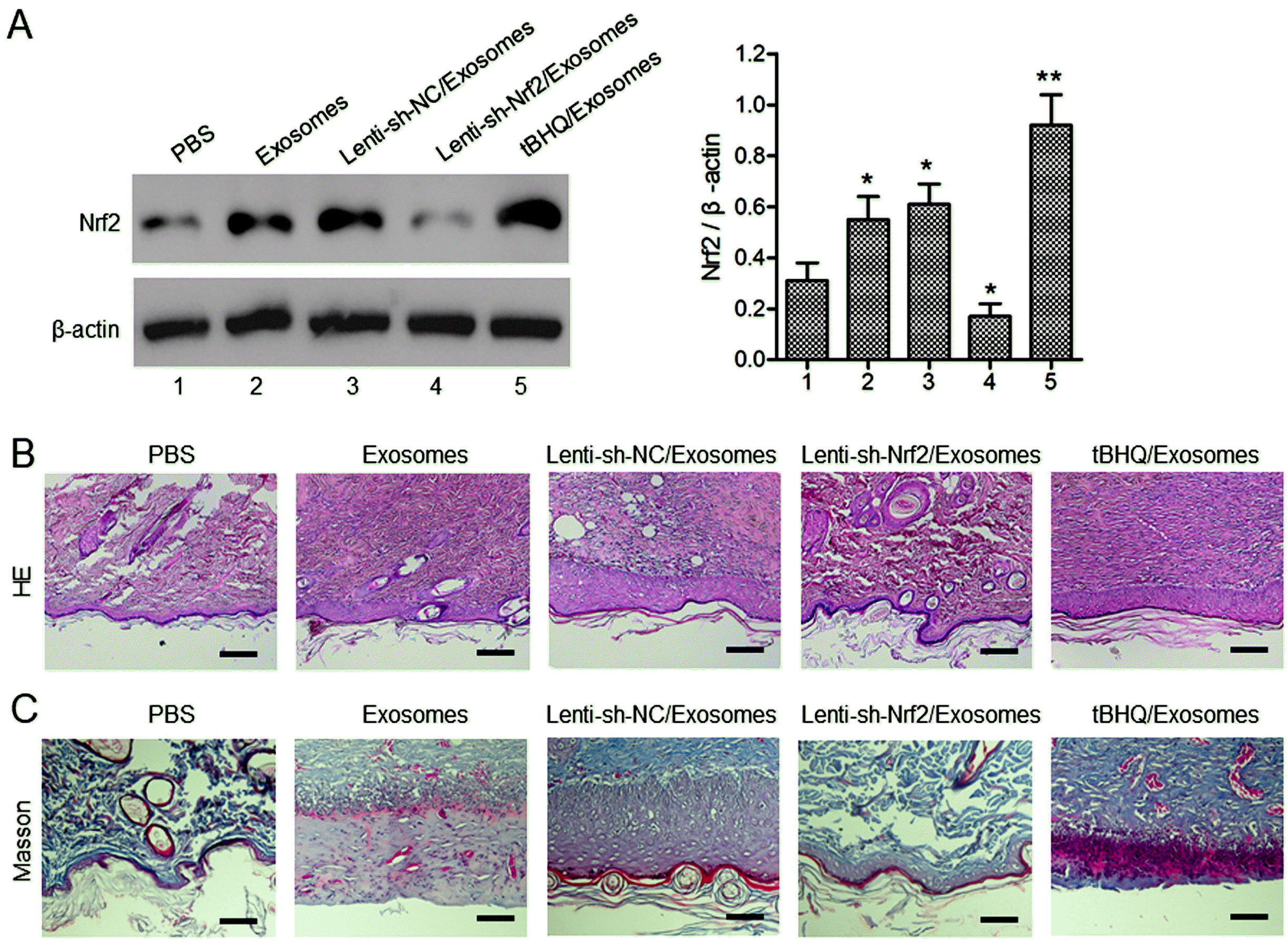 | Fig. 4The effects of BMSC exosomes alone, or in combination with knockdown or pharmaceutical activation of Nrf2 on wound tissue regeneration in diabetic rats. (A) The Nrf2 protein levels in the wound tissues at day 14 after the wounding were evaluated with western blot analysis. The STZ-induced diabetic rats were treated after skin wounding as described in Fig. 3. (A, B) Wound re-epithelization and collagen deposition were evaluated with H&E (B) and Masson (C) staining of the wound tissues at day 14 after the wounding. Scale bar=100 μm. N=5; *p<0.05, **p<0.01 vs. PBS control. |
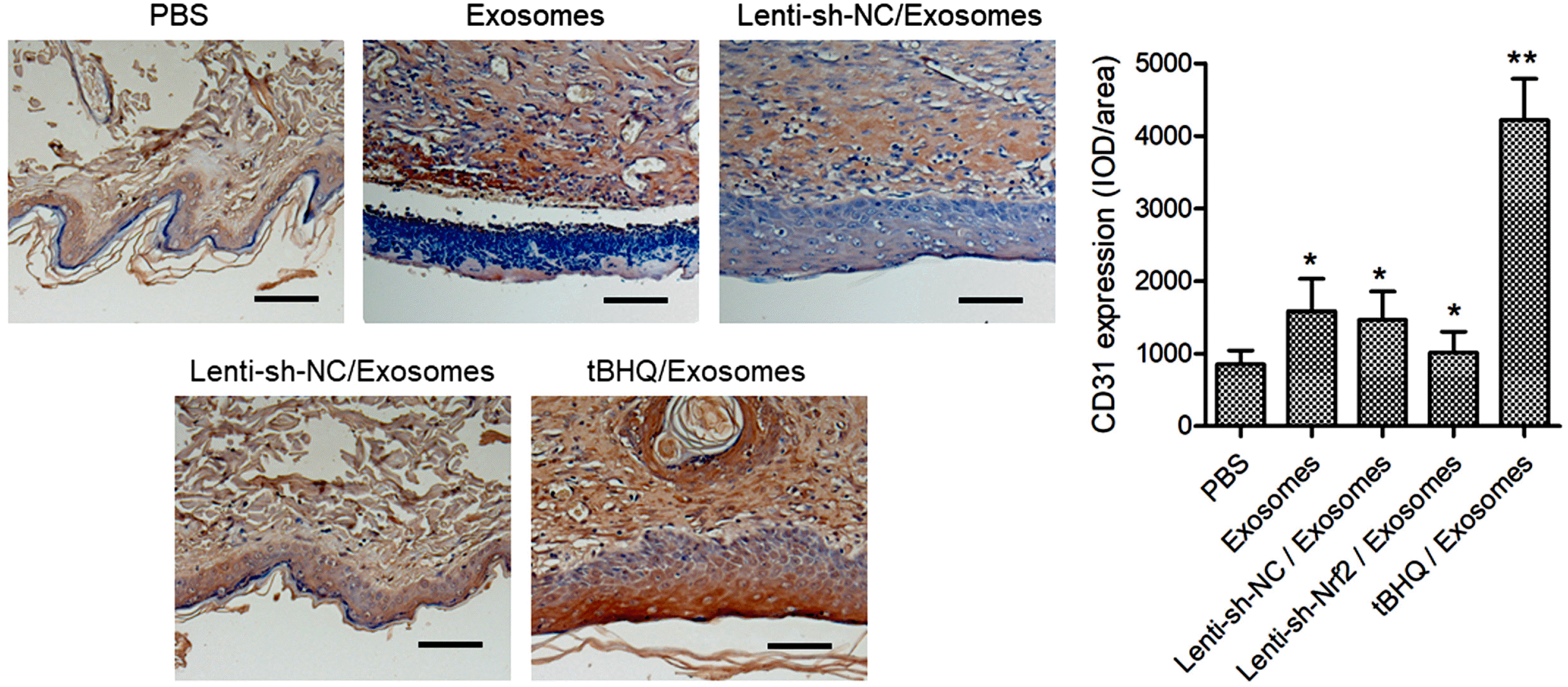 | Fig. 5The effects of BMSC exosomes alone, or in combination with knockdown or pharmaceutical activation of Nrf2 on wound neovascularization in diabetic rats. The STZ-induced diabetic rats were treated after skin wounding as described in Fig. 3. The CD31 expression in the wound tissues at day 14 after the wounding was detected with immunohistochemistry. Scale bar=100 μm. N=5; *p<0.05, **p<0.01 vs. PBS control. |
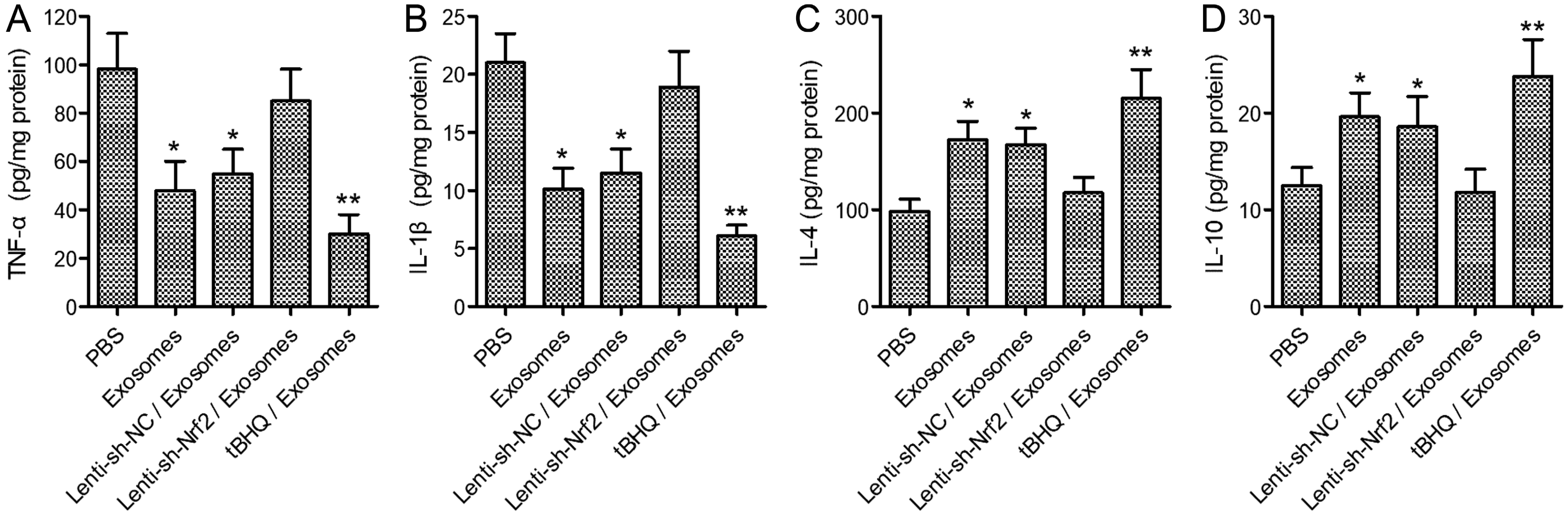 | Fig. 6The effects of BMSC exosomes alone, or in combination with knockdown or pharmaceutical activation of Nrf2 on wound inflammation in diabetic rats. The STZ-induced diabetic rats were treated after skin wounding as described in Fig. 3. The levels of TNF-α, IL-1β, IL-4, and IL-10 in the wound tissues at day 14 after the wounding were determined with ELISA. N=5; *p<0.05, **p<0.01 vs. PBS control. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download