Abstract
Background and Objectives
Mesenchymal stem cells (MSCs) have immunomodulatory function and participate in the pathogenesis of many immunoregulation-related diseases, including psoriasis. Previously, we found that MSCs from psoriatic lesions overexpress the proinflammatory microRNA, miR-155 and exhibit a decreased immunosuppressive capacity. But the origin of these aberrant characteristics is still not clear. To investigate whether inflammatory cytokines in serum and peripheral blood mononuclear cells (PBMCs) from psoriatic patients can regulate the expression patterns of immunoregulation-related cytokines and the immunoregulation function of MSCs.
Methods and Results
Normal dermal mesenchymal stem cells (nDMSCs) were treated with serum or PBMCs derived from patients with psoriasis or healthy donors. Expression of miR-155 and immunoregulation-related genes in each MSCs were measured using real-time PCR or western-blot. Meanwhile, the immunosuppressive capacity of DMSCs was evaluated by its inhibitory ability on proliferation of activated PBMCs. Compared to control serum, psoriatic serum significantly increased the expression levels of miR-155 (27.19±2.40 vs. 3.51±1.19, p<0.001), while decreased TAB2 expression (0.28±0.04 vs. 0.72±0.20, p<0.01) in DMSCs. Expression levels of immunoregulation-related genes such as PGE2, IL-10, and TLR4 were also markedly down-regulated following the psoriatic serum treatment. Those DMSCs treated with healthy serum could inhibit PBMC proliferation, while those psoriatic serum-treated DMSCs could not inhibit PBMC proliferation effectively.
Psoriasis is an immune-mediated disorder with coexistence of inflammation and autoimmune responses (1). Although the pathogenesis of psoriasis is inconclusive, evidence indicates that activation of immune system by pro-inflammatory cytokines provokes the helper T cells to differentiate into Th1/Th17 direction, consequently resulting in excessive proliferation and inflammation at the psoriasis-involved sites (2).
Mesenchymal stem cells (MSCs) are a kind of fibroblast-like cells expressing CD29, CD73, CD90, CD105 and other surface markers. These cells come from a wide range of tissues such as bone marrow, fat, placenta, skin and so on, and have multi-directional differentiation potential (3). MSCs have caught much attention because of its potent immunomodulatory effect. Although the underlying mechanisms of its immunomodulatory effect are not clear, studies have demonstrated that MSCs can inhibit the proliferation and function of T cells, B cells, and NK cells by releasing extracellular vesicles and anti-inflammatory cytokines such as interleukin-10(IL-10), prostaglandin E2 (PGE2), indoleamine 2,3-dioxygenase (IDO), and transforming growth factor-β (TGF-β) (4, 5). In addition, TLR4 expressed by MSCs also participates in this process, and its activation regulates the immunoregulation function of MSCs (6). Dermal MSCs (DMSCs) from psoriatic lesions display high expression level of vascular endothelial growth factor (VEGF), and low levels of interleukin-1β (IL-1β), GATA-6 and CXCL14, along with decreased immunosuppressive effect on T cells (7, 8). Additionally, MSCs from psoriatic lesions secrete more VEGF and nitric oxide than that from normal MSCs (9). Taken together, this evidence suggests a pathogenic role of DMSCs in psoriasis.
MicroRNA is short non-coding RNA, regulating multiple functions of cells and/or tissues, such as cell proliferation, organ development and metabolism, by influencing expression of genes and proteins (10). Recently studies showed that some microRNAs such as miR-146, miR-223 and miR-155 regulated immune responses (11). For example, miR-155 deficient mice exhibit immunode-ficiency due to the dysfunction of B cells, T cells and dendritic cells (12). And we previously found that MSCs from psoriatic lesions overexpress miR-155 and exhibit a decreased immunosuppressive capacity on T cells. But the origin of these aberrant characteristics is still not clear. Recent study has shown that inflammatory cytokines TNF-α, IL-1β, and IFN-γ induce miR-155 expression in MSCs. Moreover, miR-155 can target TGF-Beta Activated Kinase 1 (MAP3K7) Binding Protein 2 (TAB2), leading to reduced expression levels of inducible nitric oxide synthase (iNOS) and immunosuppressive effect of MSCs (13, 14). These data indicate that miR-155 plays an important role in regulating immune responses.
Therefore, we speculated that the inflammatory cytokines in the psoriatic circulation can regulate the expression of miR-155 in DMSCs, and may influence the immunosuppressive ability of DMSCs. To test this hypothesis, here we treated DMSCs with serum or PBMCs derived from patients with psoriasis or healthy donors. Expression of miR-155 and immunoregulation-related genes PGE2, IL-10, and TLR4 in each MSCs were measured using real-time PCR or western-blot. Meanwhile, the immunosuppressive capacity of DMSCs was evaluated by its inhibitory ability on proliferation of activated PBMCs.
Blood samples were taken from 12 psoriatic patients (6 males and 6 females, aged 19∼55 years) and 12 healthy volunteers (8 males and 4 females, aged range 16∼54 years), while skin samples were collected from 5 healthy volunteers (3 males and 2 females, aged 18∼50 years). All participants provided informed consent, and no topical or systemic immunosuppressants, glucocorticoids, tretinoin were received within three months prior to the study. The protocol was approved by the Medical Ethics Committee of the Taiyuan central hospital (ID:2016005).
DMSCs were isolated and cultured according to methods described previously (7). After 72-hour culture, the non-adherent cells were discarded, and half of medium was changed every 3∼4 days. The adherent DMSCs were passaged at 80% confluence. The cell surface markers, including CD29, CD44, CD34, CD105, CD45 and HLA-DR (Becton, Dickinson and Company, New York, USA), were detected by flow cytometry (Beckman Coulter, Inc., CA, USA). The osteogenic and adipogenic differentiation of MSCs in vitro was also performed according to published protocols (7).
Blood from 6 patients and 6 controls was collected in 5 ml promoting coagulating tubes. After centrifugation at 4000 r/min for 5 min, the serum was filtered and stored at −80℃. Five ml of peripheral blood was obtained from each subject (6 patients and 6 controls), and PBMCs were isolated by Ficoll-Hypaque gradient separation (8).
The passage 3 DMSCs at 70% confluence were cultured in DMEM/F12 medium, containing 50% human serum, for 24 hours at 37℃ in a humidified atmosphere supplemented with 5% CO2. Meanwile, after washing with PBS, PBMCs were co-cultured with DMSCs at a ratio of 5:1 for 24 h at 37℃. Normal DMSCs (nDMSCs) cultured with 10 ng/ml TNF-α and 10 ng/ml IL-1β served as positive control. Afterwards, cells were digested with trypsin and harvested. DMSCs treated with psoriatic and normal serum were abbreviated as PS-DMSCs and HS-DMSCs. And DMSCs treated with psoriatic PBMCs, normal PBMCs and cytokines were abbreviated as PMNC-DMSCs, HMNC-DMSCs and Cy-DMSCs, respectively.
To assess the paracrine effects of HS-DMSCs and PS-DMSCs on PBMCs, PBMCs (peripheral blood from 3 normal subjects) were co-cultured with DMSCs in transwell (Corning, NY, USA). DMSCs were added to the upper chamber at a cell density of 2×105/well in DMEM/F12 medium, and PBMCs stimulated by 50 μg/ml Phy-tohemagglutinin (PHA; Solarbio, Beijing, China) were added to the lower chamber at a density of 1×106/well in RPMI 1640 medium. Experiment included the following five groups: a. Normal DMSCs group: nDMSCs+PBMCs; b. Normal serum group: HS-DMSCs+PBMCs; c. Psoriatic serum group: PS-DMSCs+PBMCs; d. Cytokines group: Cy-DMSCs+PBMCs, and e. Control: PBMCs cultured alone. All groups were co-cultured for 72 h in 12-well transwell plates with 0.4 μm filter at 37℃ in a humidified atmosphere supplemented with 5% CO2. The suspended cells were collected for subsequent assay.
After co-cultured with PBMCs or serum, total RNA was extracted from DMSCs, using Trizol (Invitrogen, Carlsbad, CA, USA) and miRNeasy mini kit (Qiagen, Hilden, Germany). Quantitative real-time PCR (qRT-PCR) reaction contained 2 μl cDNA, 10 μl SYBR premix EX TaqII (TaKaRa, Dalian, China), 0.4 μl ROX Reference Dye (TaKaRa), and 0.4 μl primers (Table 1). The relative gene expression levels were calculated by the 2−ΔΔCt method and normalized to U6 RNA (miRNA) and β-actin mRNA (mRNA).
Western blot assay was performed, using Wes system (ProteinSimple, Silicon Valley, CA, USA) according to manufacturer’s instructions described in published protocols (15). The β-actin protein was used as internal reference, and the image was analyzed by Compass software (ProteinSimple).
PBMC proliferation was measured using a CCK-8 assay kit (Boster, Wuhan, China) according to manufacturer’s instructions. The cells (5∼10×105 cells/ml) were seeded into 96-well plates (100 μl/well). After incubation for 24 h at 37℃, 10 μl of CCK-8 solution was added to each well, and incubated for 4 h. The absorbance at 450 nm (OD value) was detected with Type 352 automatic microplate reader (Labsystems, Helsinki, Finland), and the proliferation of PBMCs was reflected by OD value. Afterwards, total RNA was extracted from PBMCs using Trizol (Invitrogen) for qRT-PCR. The β-actin mRNA was used as internal reference. Primers were shown in Table 1.
Although co-culture of DMSCs with either psoriatic serum or psoriatic PBMCs did not induce noticeable changes in the morphology of DMSCs (Fig. 1a), psoriatic serum markedly up-regulated expression levels of miR-155 (27.19±2.40 vs. 3.51±1.19, p<0.001), while down-regulating TAB2 mRNA (Fig. 1b) (0.28±0.04 vs. 0.72±0.20, p< 0.01) and protein (Fig. 1c, 1d and Fig. S1) (0.03±0.00 vs. 0.62±0.00, p<0.01) in comparison to normal serum-trea-ted DMSCs. In contrast, normal PBMCs (HMNC) and psoriatic PBMCs (PMNC) exhibited a comparable effect on expression levels of miR-155 and TAB2 (Fig. 1e). Notably, expression levels of mRNA for SMAD2 and SOCS-1 were also comparable between HS-DMSCs and PS-DMSCs, HMNC-DMSCs and PMNC-DMSCs (Fig. 1b and 1e). These results demonstrate that psoriatic serum up-regulate miR-155 expression, while inhibiting TAB2 expression in DMSCs.
Next, we aimed to determine whether PBMCs and serum regulate expression of other immunoregulation-related genes such as IL-10, PGE2, TLR4 in DMSCs. As shown in Fig. 2a, mRNA expression of IL-10, PGE2, and TLR4 were significantly lower in PS-DMSCs than in HS-DMSCs (p<0.05). However, the expression levels of these genes were not significantly different between PMNC-DMSCs and HMNC-DMSCs (Fig. 2b, p>0.05). In accordance with the results of mRNA, the protein levels of TLR4 and IL-10 were obviously lower in PS-DMSCs than in HS-DMSCs (Fig. 2c, 2d, and Fig. S1, p<0.001). These results demonstrate that in comparison to normal serum, psoriatic serum down-regulate expression of IL-10, PGE2, and TLR4 in DMSCs, while PBMCs from normal and psoriatic subjects exhibit a comparable effect on expression levels of these biomarkers. The raw images of western blot are shown in the supplementary Fig. S1.
Because psoriatic serum decrease expression of various immunoregulation-related biomarkers in DMSCs. In order to further explore whether the abnormal expression of these immunoregulation-related genes can affect the immunosuppressive function of MSCs, we evaluated the inhibitory effect of each MSCs on the proliferation of activated PBMCs. As shown in Fig. 3a and 3b, either nDMSCs or HS-DMSCs inhibited PBMC proliferation (p<0.001 vs. PBMCs cultured alone). In contrast, PS-DMSCs lost their ability to inhibit PBMC proliferation (2.59±0.03 in PS-DMSCs vs. 1.92±0.06 in HS-DMSCs, p<0.001) (Fig. 3b). Similarly, PS-DMSCs displayed less potency in inhibiting expression of mRNA for cytokines in PBMCs in comparison to HS-DMSCs (p<0.05 for all) (Fig. 3c). The results indicate that psoriatic serum not only induce abnormal expression patterns of immunoregulation-related cytokines, but also impair the immunosuppressive function of DMSCs.
Psoriasis is a Th1-Th17-mediated cutaneous inflamma-tory disease, accompanied by elevations in circulatory levels of inflammatory cytokines (16-18). It has been postulated that psoriatic lesions are caused by abnormal interaction of the cutaneous cells and immune system, and activation of dendritic cells by inflammation, resulting in increased pro-inflammatory cytokines such as IFN-γ, TNF-α, and IL-23, consequently leading to stimulation of differentiation and proliferation of Th1 and Th17, and release of TNF-α, IL-17, and IL-22. Increased cytokines can further induce cutaneous inflammation (19, 20). However, how cutaneous cells such as DMSCs are involved in the cutaneous inflammation in psoriasis is still not completely understood although some of their immunomodulatory properties have been demonstrated.
In addition to the multi-directional differentiation potential and high plasticity, MSCs also possess the capacities of tissue remodeling/repair, support of hematopoiesis, angiogenesis, migration and chemotaxis, and immunomo-dulatory properties (21). As an important part of the dermis, DMSCs significantly regulate the skin microenvironment through secreting cytokines which play a crucial role in the pathogenesis of psoriasis. Studies have suggested the following possible mechanisms. First, DMSCs can enhance proliferation and inhibit apoptosis in HaCaT cells, and eventually result in epidermis hyperplasia (22). Second, DMSCs can promote dermal angiogenesis by regulating angiogenesis-related factors (23). Third, DMSCs show a greater regulation of the imbalance between Th1-Th17 and Th2 inflammatory axes via secreting immunoregulation-related genes and anti-inflammatory cytokine (20). These mechanisms constitute the typical histological changes of psoriasis include epidermal hyperplasia, neovascularization, inflammatory infiltrates composed of neutrophils, T cells, and macrophages.
The immune-regulation function of MSCs mainly depend on a serious of cytokines they secreted and their direct contact with immune cells. Recent studies demonstrated that psoriatic DMSCs secret increased proliferation-related molecules such as epidermal growth factor (EGF), stem cell factor (SCF) and decreased negative regulatory factors transforming growth factor-β (TGF-β), basic fibroblast growth factor (bFGF), which resulted in promotion of keratinocyte (KC) proliferation and inhibition of KC apoptosis (7). The up-regulation of epidermal growth factor-like repeats and discoidin I-like domains3 (EDIL3), angiomotin (AMOT), extracellular matrix protein1 (ECM1) and the down-regulation of tumor necrosis factor superfamily15 (TNFSF15), transcription factor GATA6, thrombospondins (THBS) in psoriatic DMSCs result in the excessive vasodilation and angiogenesis (23). Besides, studies have also shown that psoriatic DMSCs express high level of human leucocyte antigen-I, exhibit decreased differentiation ability as well as weakened immunosuppressive capacity (24). MSCs exert its immunosuppressive properties by expressing immunoregulation-related genes TLR4 and releasing serious of anti-inflammatory cytokines such as PGE2, IL-10, and IDO, restraining the immune response to increased levels of cytokines, including IFN-γ, TNF-α, and IL-1β (25). Numerous studies found that miR-155 can regulate the expression of many inflammatory cytokines, so it is considered to be pro-inflammatory miRNA (26). We have previously found that MSCs from psoriatic lesions overexpress miR-155 (14). So, the purpose of this study is to explore the reason of increased miR-155 expression in psoriatic DMSCs and its effect on MSC function.
In the present study, we show that expression levels of miR-155 were up-regulated in MSCs when treated by psoriatic serum, which is consistent with previous results (13, 14). At the same time, the miR-155 target gene TAB2 was down-regulated following the treatment of psoriatic serum, while the expression of the other two miR-155 target gene SOCS-1 and SMAD2 were not influenced by psoriatic serum. So, we consider that miR-155 is more likely to involved in the psoriatic MSCs dysfunction by targeting TAB2 rather than SOCS-1 or SMAD2. Our data also found that expression levels of some immunomodulation-related genes PGE2, IL-10, TLR4 were down-regulated in DMSCs following the treatment with psoriatic serum. Psoriatic serum can induce the abnormal expression of immunoregulation-related genes. It is generally known that the gene expression and function of MSCs are regulated by the inflammatory cytokines of the microenvironment, and inflammatory cytokines TNF-α, IL-1β and IFN-γ are increased mainly in psoriatic serum (27). Therefore, we inferred that the increased inflammatory cytokines TNF-α, IL-1β in the psoriatic circulation may induce the abnormal expression of miR-155 and immunoregulation-related genes. As shown in our results, psoriatic serum displayed a comparable effect on regulating of DMSCs gene expression with the cytokine combination TNF-α and IL-1β, which also confirmed our conjecture that the increased inflammatory cytokines such as TNF-α and IL-1β in psoriatic circulation can induce an abnormal immunoregulation-related genes phenotype in DMSCs.
As a target gene of miR-155 and a multifunctional signaling molecule, TAB2 can promote activation of NF-κB, JNK, TLR/IL-1 signaling pathway and ubiquitination of IL-1-dependent TNF receptor-associated factor 6 (TRAF6) (28). Previous study suggested that RNF4 negatively regulates NF-κB pathway through down‐regulating TAB2 (29). And activated TLR4 triggers NF‐κB pathway and in turn causes inflammatory responses (30). Furthermore, a recent study shows that TLR4 activation of MSCs promotes the induction of Treg (6). NF-kB signaling pathway participates in the immunoregulatory functions of MSCs on T cell activation and proliferation, and the function was markedly decreased after inhibiting the pathway (31). Gutierrez indicated that Petiveria alliacea regulate the production of inflammatory cytokines such as PGE2 and IL-10 by down-regulating NF-κB signaling pathway (32). Our present study also found a significant decreased expression of TLR4, PGE2, and IL-10 along with the TAB2 inhibition. PGE2 has been found to stimulate the secretion of IL-10, promote Tregs differentiation, and decrease the proliferation of T cells (33). IL-10 can down-regulate the expression of Th1 cytokines, stimulate the secretion of HLA-G5 and inhibit the ability of DCs (34). So, it is reasonable to speculate that down-regulated TAB2 may cause the inhibition of MSC immunoregulation through NF‐κB signaling pathway. Our results also confirmed that the inhibition of DMSCs on PBMC proliferation were weakened along with the down-regulation of PGE2, IL-10, and TLR4 after psoriatic serum treatment. These results illustrated that the up-regulated miR-155, the down-regulated target gene TAB2 and immunoregulation-related genes TLR4 may cause the decrease of PGE2, IL-10 by inhibition of NF-κB signaling pathway, and resulted in a decreased immunosuppressive function of MSCs.
In conclusion, we found in this study that serum from psoriatic patients induce an abnormal inflammatory phenotype and a decreased immunosuppressive function of mesenchymal stem cells. The elevated inflammatory cytokines such as TNF-α and IL-1β in the psoriatic serum likely contribute to the up-regulation of miR-155 and the down-regulation of its target gene TAB2 in DMSCs. Moreover, the levels of immunoregulation-related genes were decreased in DMSCs after treated by psoriatic serum, along with decreased immunosuppressive function of DMSCs. This process may be mediated by NF‐κB signaling pathway (Fig. 4). However, further studies are still needed to clarify the specific mechanisms by which inflammatory cytokines in the serum alter the function of DMSCs and the role of miR-155 in this process.
Supplementary data including one figure can be found with this article online at https://doi.org/10.15283/ijsc20210.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 81602768), the Department of Science and Technology of Shanxi Province, China (no. 201801D221441), and the Health and Family Planning Commission of Shanxi Province, China (no. 2015153). We would like to thank Professor Maoqiang Man for language editing.
References
1. Liang Y, Sarkar MK, Tsoi LC, Gudjonsson JE. 2017; Psoriasis: a mixed autoimmune and autoinflammatory disease. Curr Opin Immunol. 49:1–8. DOI: 10.1016/j.coi.2017.07.007. PMID: 28738209. PMCID: PMC5705427.

2. Zhang P, Wu MX. 2018; A clinical review of phototherapy for psoriasis. Lasers Med Sci. 33:173–180. DOI: 10.1007/s10103-017-2360-1. PMID: 29067616. PMCID: PMC5756569.

3. Zhao L, Chen S, Yang P, Cao H, Li L. 2019; The role of mesenchymal stem cells in hematopoietic stem cell transplantation: prevention and treatment of graft-versus-host disease. Stem Cell Res Ther. 10:182. DOI: 10.1186/s13287-019-1287-9. PMID: 31227011. PMCID: PMC6588914. PMID: 3502f2e3c8e548ee8714319d24a999c4.

4. Andrukhov O, Behm C, Blufstein A, Rausch-Fan X. 2019; Immunomodulatory properties of dental tissue-derived mesenchymal stem cells: implication in disease and tissue regeneration. World J Stem Cells. 11:604–617. DOI: 10.4252/wjsc.v11.i9.604. PMID: 31616538. PMCID: PMC6789188.

5. Harrell CR, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. 2019; Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells. 8:1605. DOI: 10.3390/cells8121605. PMID: 31835680. PMCID: PMC6952783.

6. Rashedi I, Gómez-Aristizábal A, Wang XH, Viswanathan S, Keating A. 2017; TLR3 or TLR4 activation enhances mesenchymal stromal cell-mediated Treg induction via Notch signaling. Stem Cells. 35:265–275. DOI: 10.1002/stem.2485. PMID: 27571579.

7. Hou R, Yan H, Niu X, Chang W, An P, Wang C, Yang Y, Yan X, Li J, Liu R, Li X, Zhang K. 2014; Gene expression profile of dermal mesenchymal stem cells from patients with psoriasis. J Eur Acad Dermatol Venereol. 28:1782–1791. DOI: 10.1111/jdv.12420. PMID: 24593802.

8. Liu R, Wang Y, Zhao X, Yang Y, Zhang K. 2014; Lymphocyte inhibition is compromised in mesenchymal stem cells from psoriatic skin. Eur J Dermatol. 24:560–567. DOI: 10.1684/ejd.2014.2394. PMID: 25445090.

9. Orciani M, Campanati A, Salvolini E, Lucarini G, Di Benedetto G, Offidani A, Di Primio R. 2011; The mesenchymal stem cell profile in psoriasis. Br J Dermatol. 165:585–592. DOI: 10.1111/j.1365-2133.2011.10438.x. PMID: 21623755.

10. Wojciechowska A, Braniewska A, Kozar-Kamińska K. 2017; MicroRNA in cardiovascular biology and disease. Adv Clin Exp Med. 26:865–874. DOI: 10.17219/acem/62915. PMID: 29068585.

11. Testa U, Pelosi E, Castelli G, Labbaye C. 2017; miR-146 and miR-155: two key modulators of immune response and tumor development. Noncoding RNA. 3:22. DOI: 10.3390/ncrna3030022. PMID: 29657293. PMCID: PMC5831915.

12. Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, van Dongen S, Grocock RJ, Das PP, Miska EA, Vetrie D, Okkenhaug K, Enright AJ, Dougan G, Turner M, Bradley A. 2007; Requirement of bic/microRNA-155 for normal immune function. Science. 316:608–611. DOI: 10.1126/science.1139253. PMID: 17463290. PMCID: PMC2610435.

13. Xu C, Ren G, Cao G, Chen Q, Shou P, Zheng C, Du L, Han X, Jiang M, Yang Q, Lin L, Wang G, Yu P, Zhang X, Cao W, Brewer G, Wang Y, Shi Y. 2013; miR-155 regulates immune modulatory properties of mesenchymal stem cells by targeting TAK1-binding protein 2. J Biol Chem. 288:11074–11079. DOI: 10.1074/jbc.M112.414862. PMID: 23449975. PMCID: PMC3630877.

14. Hou RX, Liu RF, Zhao XC, Jia YR, An P, Hao ZP, Li JQ, Li XH, Yin GH, Zhang KM. 2016; Increased miR-155-5p expression in dermal mesenchymal stem cells of psoriatic patients: comparing the microRNA expression profile by microarray. Genet Mol Res. 15:gmr.15038631. DOI: 10.4238/gmr.15038631. PMID: 27706699.

15. Li X, Li J, Lu F, Cao Y, Xing J, Li J, Hou R, Yin G, Zhang K. 2020; Role of SPRED1 in keratinocyte proliferation in psoriasis. J Dermatol. 47:735–742. DOI: 10.1111/1346-8138.15369. PMID: 32396270.

16. Niu X, Li J, Zhao X, Wang Q, Wang G, Hou R, Li X, An P, Yin G, Zhang K. 2019; Dermal mesenchymal stem cells: a resource of migration-associated function in psoriasis? Stem Cell Res Ther. 10:54. DOI: 10.1186/s13287-019-1159-3. PMID: 30760317. PMCID: PMC6375130. PMID: ab2d99bcde9044cab53d07a1c5c40c43.

17. Michalak-Stoma A, Pietrzak A, Szepietowski JC, Zalewska-Janowska A, Paszkowski T, Chodorowska G. 2011; Cytokine network in psoriasis revisited. Eur Cytokine Netw. 22:160–168. DOI: 10.1684/ecn.2011.0294. PMID: 22236965.

18. Michalak-Stoma A, Bartosińska J, Kowal M, Juszkiewicz-Borowiec M, Gerkowicz A, Chodorowska G. 2013; Serum levels of selected Th17 and Th22 cytokines in psoriatic patients. Dis Markers. 35:625–631. DOI: 10.1155/2013/856056. PMID: 24288431. PMCID: PMC3832981.

19. Boehncke WH. 2015; Etiology and pathogenesis of psoriasis. Rheum Dis Clin North Am. 41:665–675. DOI: 10.1016/j.rdc.2015.07.013. PMID: 26476225.

20. Campanati A, Orciani M, Consales V, Lazzarini R, Ganzetti G, Di Benedetto G, Di Primio R, Offidani A. 2014; Characterization and profiling of immunomodulatory genes in resident mesenchymal stem cells reflect the Th1-Th17/Th2 imbalance of psoriasis. Arch Dermatol Res. 306:915–920. DOI: 10.1007/s00403-014-1493-3. PMID: 25160906.

21. Mareschi K, Castiglia S, Sanavio F, Rustichelli D, Muraro M, Defedele D, Bergallo M, Fagioli F. 2016; Immunoregulatory effects on T lymphocytes by human mesenchymal stromal cells isolated from bone marrow, amniotic fluid, and placenta. Exp Hematol. 44:138–150.e1. DOI: 10.1016/j.exphem.2015.10.009. PMID: 26577566.

22. Liu RF, Wang F, Wang Q, Zhao XC, Zhang KM. 2015; Research Note Mesenchymal stem cells from skin lesions of psoriasis patients promote proliferation and inhibit apoptosis of HaCaT cells. Genet Mol Res. 14:17758–17767. DOI: 10.4238/2015.December.21.49. PMID: 26782421.

23. Niu X, Chang W, Liu R, Hou R, Li J, Wang C, Li X, Zhang K. 2016; mRNA and protein expression of the angiogenesis-related genes EDIL3, AMOT and ECM1 in mesenchymal stem cells in psoriatic dermis. Clin Exp Dermatol. 41:533–540. DOI: 10.1111/ced.12783. PMID: 26644074.

24. Castro-Manrreza ME, Bonifaz L, Castro-Escamilla O, Monroy-García A, Cortés-Morales A, Hernández-Estévez E, Hernández-Cristino J, Mayani H, Montesinos JJ. 2019; Mesen-chymal stromal cells from the epidermis and dermis of psoriasis patients: morphology, immunophenotype, differentiation patterns, and regulation of T cell proliferation. Stem Cells Int. 2019:4541797. DOI: 10.1155/2019/4541797. PMID: 31885608. PMCID: PMC6914887. PMID: 65e75cdeb3774761bdf98d969592c11b.

25. Zhou Y, Yamamoto Y, Xiao Z, Ochiya T. 2019; The immuno-modulatory functions of mesenchymal stromal/stem cells mediated via paracrine activity. J Clin Med. 8:1025. DOI: 10.3390/jcm8071025. PMID: 31336889. PMCID: PMC6678920.

26. Sonkoly E, Pivarcsi A. 2009; Advances in microRNAs: implications for immunity and inflammatory diseases. J Cell Mol Med. 13:24–38. DOI: 10.1111/j.1582-4934.2008.00534.x. PMID: 19175698. PMCID: PMC3823034.

27. Kong Y, Zhang S, Wu R, Su X, Peng D, Zhao M, Su Y. 2019; New insights into different adipokines in linking the pathophysiology of obesity and psoriasis. Lipids Health Dis. 18:171. DOI: 10.1186/s12944-019-1115-3. PMID: 31521168. PMCID: PMC6745073. PMID: 8bafd9624fe8458fa2fdc24d1fa69d76.

28. Ceppi M, Pereira PM, Dunand-Sauthier I, Barras E, Reith W, Santos MA, Pierre P. 2009; MicroRNA-155 modulates the interleukin-1 signaling pathway in activated human monocyte-derived dendritic cells. Proc Natl Acad Sci U S A. 106:2735–2740. DOI: 10.1073/pnas.0811073106. PMID: 19193853. PMCID: PMC2650335.

29. Tan B, Mu R, Chang Y, Wang YB, Wu M, Tu HQ, Zhang YC, Guo SS, Qin XH, Li T, Li WH, Zhang XM, Li AL, Li HY. 2015; RNF4 negatively regulates NF-κB signaling by down-regulating TAB2. FEBS Lett. 589(19 Pt B):2850–2858. DOI: 10.1016/j.febslet.2015.07.051. PMID: 26299341.

30. Alhusaini A, Fadda LM, Ali HM, Hasan IH, Ali RA, Zakaria EA. 2019; Mitigation of acetamiprid - induced renotoxicity by natural antioxidants via the regulation of ICAM, NF-kB and TLR 4 pathways. Pharmacol Rep. 71:1088–1094. DOI: 10.1016/j.pharep.2019.06.008. PMID: 31629938.

31. Dorronsoro A, Ferrin I, Salcedo JM, Jakobsson E, Fernández-Rueda J, Lang V, Sepulveda P, Fechter K, Pennington D, Trigueros C. 2014; Human mesenchymal stromal cells modulate T-cell responses through TNF-α-mediated activation of NF-κB. Eur J Immunol. 44:480–488. DOI: 10.1002/eji.201343668. PMID: 24307058.

32. Gutierrez RMP, Hoyo-Vadillo C. 2017; Anti-inflammatory potential of Petiveria alliacea on activated RAW264.7 murine macrophages. Pharmacogn Mag. 13(Suppl 2):S174–S178. DOI: 10.4103/pm.pm_479_16. PMID: 28808377. PMCID: PMC5538151.
33. Rodríguez M, Domingo E, Municio C, Alvarez Y, Hugo E, Fernández N, Sánchez Crespo M. 2014; Polarization of the innate immune response by prostaglandin E2: a puzzle of receptors and signals. Mol Pharmacol. 85:187–197. DOI: 10.1124/mol.113.089573. PMID: 24170779.

34. Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, Borg C, Saas P, Tiberghien P, Rouas-Freiss N, Carosella ED, Deschaseaux F. 2008; Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 26:212–222. DOI: 10.1634/stemcells.2007-0554. PMID: 17932417.

Fig. 1
Psoriatic serum up-regulate miR-155, while inhibiting TAB2 expression in DMSCs. (a) Cell morphology of untreated DMSCs, PS-DMSCs, PMNC-DMSCs (scale bar=100 μm); (b) The mRNA levels of miR-155 were up-regulated and TAB2 were down-regulated in PS-DMSCs and Cy-DMSCs than in HS-DMSCs; (c, d) The protein levels of TAB2 were down-regulated in PS-DMSCs and Cy-DMSCs than in HS-DMSCs; (e) The mRNA levels of miR-155 and TAB2 were similar between PMNC-DMSCs and HMNC-DMSCs. *Represents the difference between each group and nDMSCs. *p<0.05, **p<0.01, ***p<0.001, N=6. Abbreviation: nDMSCs: untreated DMSCs; HS-DMSCs: DMSCs treated with normal serum; PS-DMSCs: DMSCs treated with psoriatic serum; HMNC-DMSCs: DMSCs treated with normal PBMCs; PMNC-DMSCs: DMSCs treated with psoriatic PBMCs; Cy-DMSCs: DMSCs treated with cytokines (TNF-α and IL-1β).
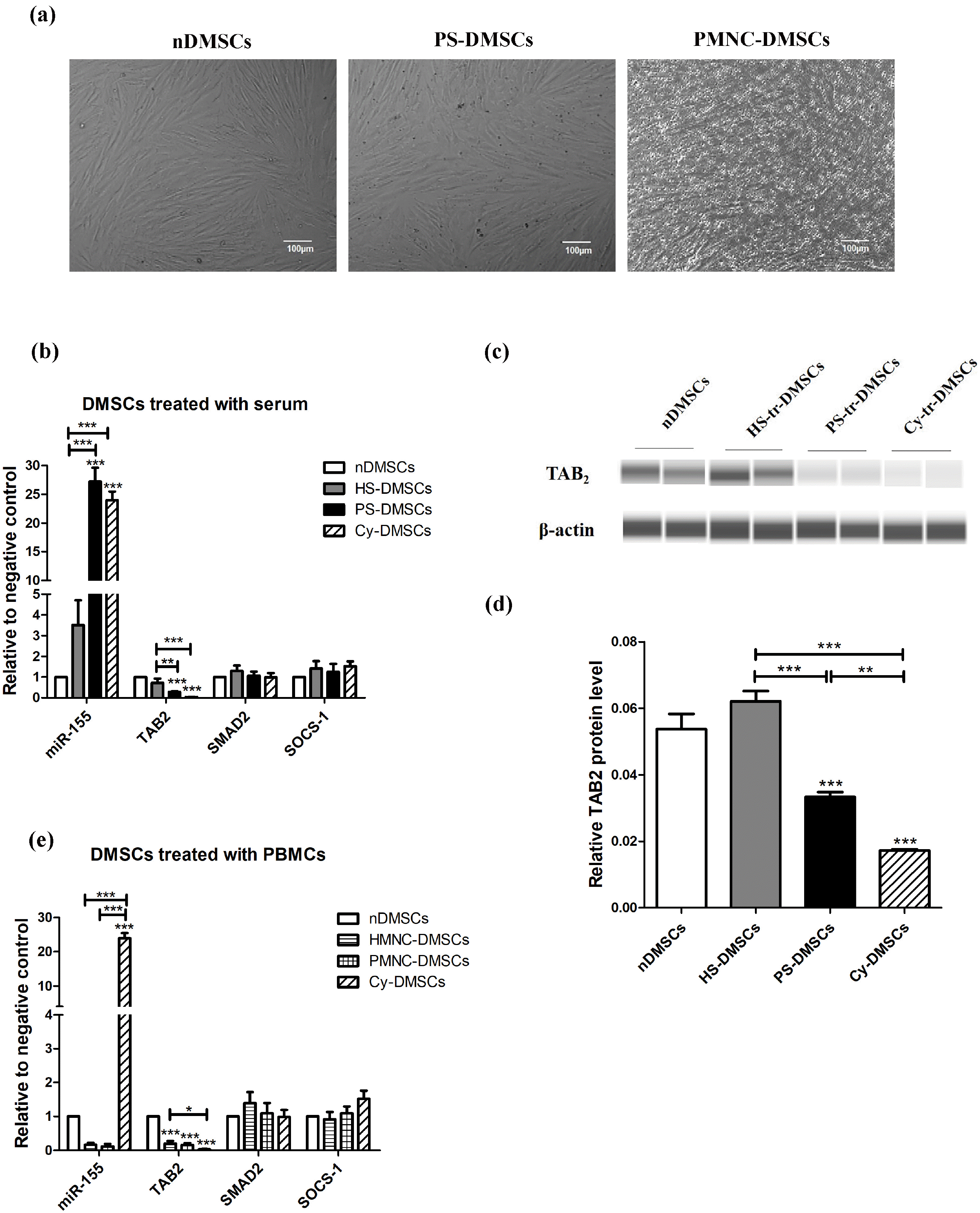
Fig. 2
Psoriatic serum suppress the mRNA expression of immunoregula-tion-related genes in DMSCs. (a) Expression levels of mRNA for IL-10, PGE2 and TLR4 were down-regulated in PS-DMSCs and Cy-DMSCs than in HS-DMSCs; (b) The mRNA levels of IL-10, PGE2, TLR4 were similar between PMNC-DMSCs and HMNC-DMSCs; (c, d) The protein levels of IL-10 and TLR4 were down-regulated in PS-DMSCs and Cy-DMSCs than in HS-DMSCs. *Re-presents the difference between each group and nDMSCs. *p<0.05, **p< 0.01, ***p<0.001, N=6. Abbrevia-tion: nDMSCs: untreated DMSCs; HS-DMSCs: DMSCs treated with normal serum; PS-DMSCs: DMSCs treated with psoriatic serum; HMNC-DMSCs: DMSCs treated with normal PBMCs; PMNC-DMSCs: DMSCs treated with psoriatic PBMCs; Cy-DMSCs: DMSCs treated with cytokines (TNF-α and IL-1β).
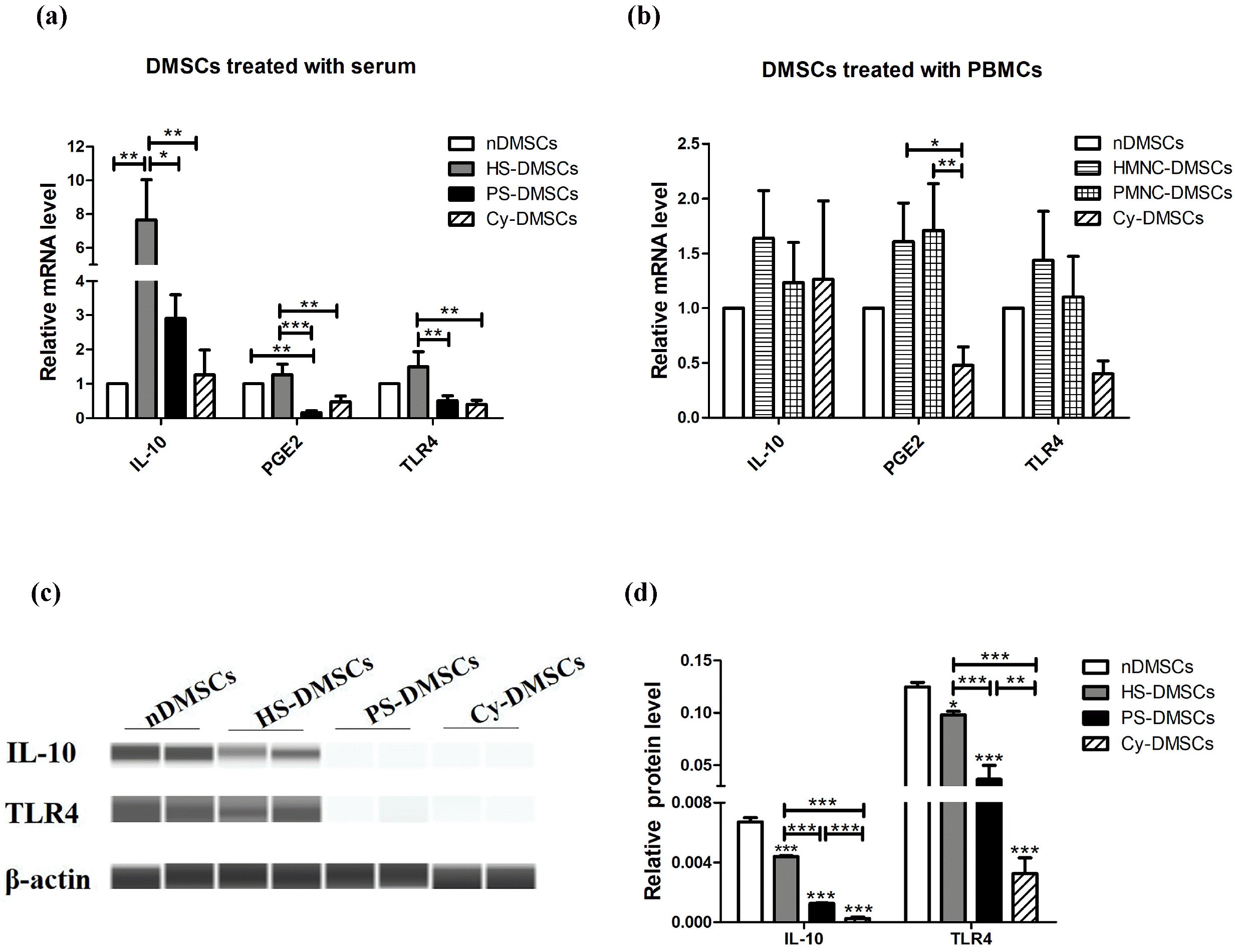
Fig. 3
Psoriatic serum inhibit the immunosuppression of DMSCs. (a) Morphology of PBMCs cultured alone and co-cultured with either HS-DMSCs or PS-DMSCs or Cy-DMSCs for 3 days; (b) Proliferation of PBMCs cultured alone and co-cultured with either HS-DMSCs or PS-DMSCs or Cy-DMSCs for 3 days, *Represents the difference between each group and nDMSCs, ***p<0.001; (c) Expression levels of mRNA for IL-17A, IL-23, and IL-8 in PBMCs cultured alone and co-cultured with either HS-DMSCs or PS-DMSCs or Cy-DMSCs, a represents p<0.05 vs. nDMSCs, b represents p<0.001 vs. nDMSCs, c represents p<0.05 vs. HS-DMSCs, d represents p<0.001 vs. HS-DMSCs, ***p<0.001, N=6. Abbreviation: nDMSCs+PBMCs: PBMCs co-cultured with nDMSCs (untreated DMSCs); HS-DMSCs+PBMCs: PBMCs co-cultured with HS-DMSCs (DMSCs treated with normal serum); PS-DMSCs+PBMCs: PBMCs co-cultured with PS-DMSCs (DMSCs treated with psoriatic serum); Cy-DMSCs+PBMCs: PBMCs co-cultured with Cy-DMSCs (DMSCs treated with cytokines [TNF-α and IL-1β]); PBMCs: PBMCs cultured alone.
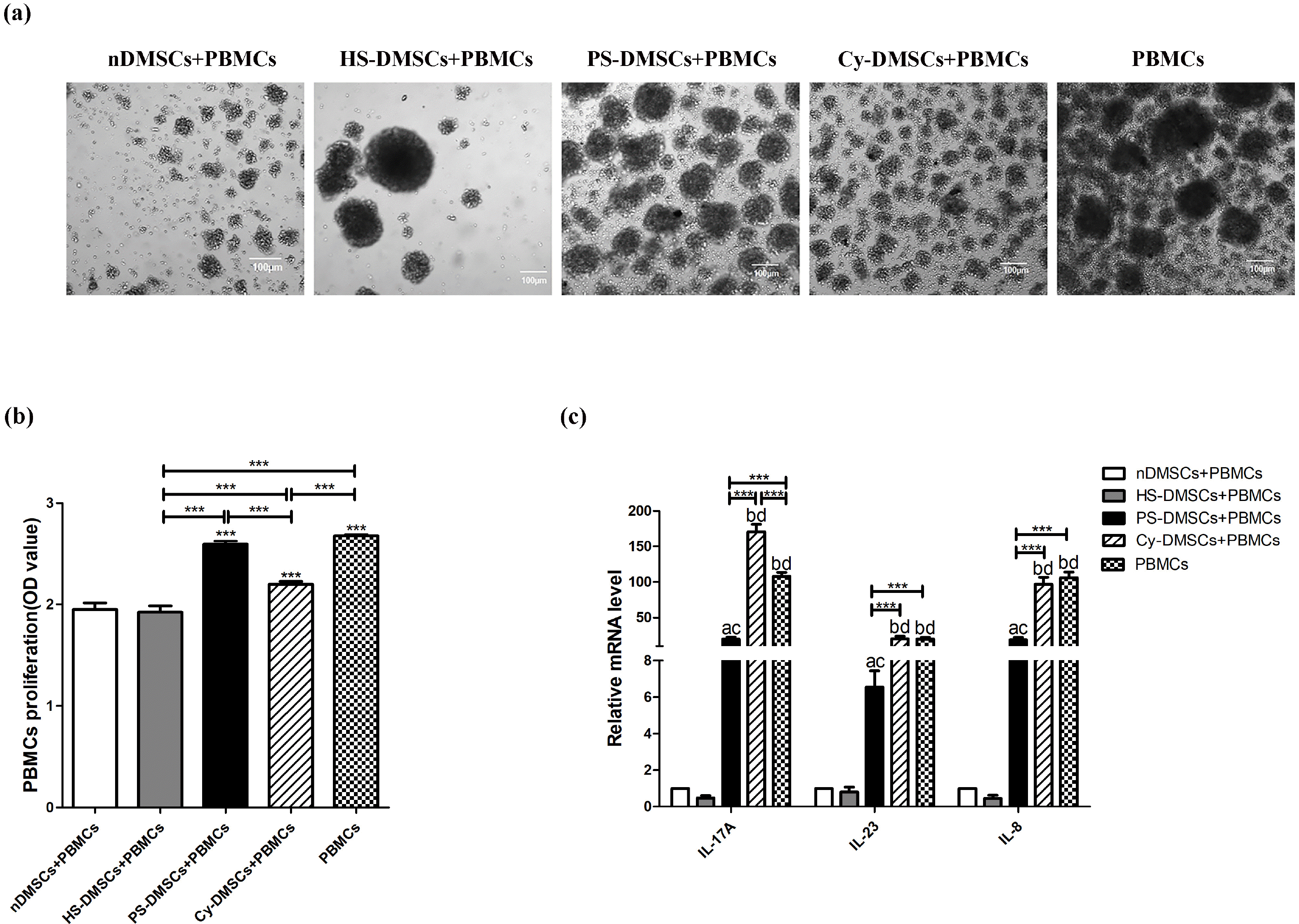
Fig. 4
The effect of psoriatic serum on dermal mesenchymal stem cells. The increased TNF-α and IL-1β in the psoriatic serum up-regulate the expression of miR-155 and down-regulate the expression of TAB2 in DMSCs, along with the down-regulation of expression of PGE2, IL-10 and TLR4 and decreasing immuno-suppressive ability of DMSCs. As a result, the proliferation and expression of inflammatory cytokines IL-17A, IL-23, IL-8 of PBMCs were increased, which in turn, further aggravate cutaneous inflammation.
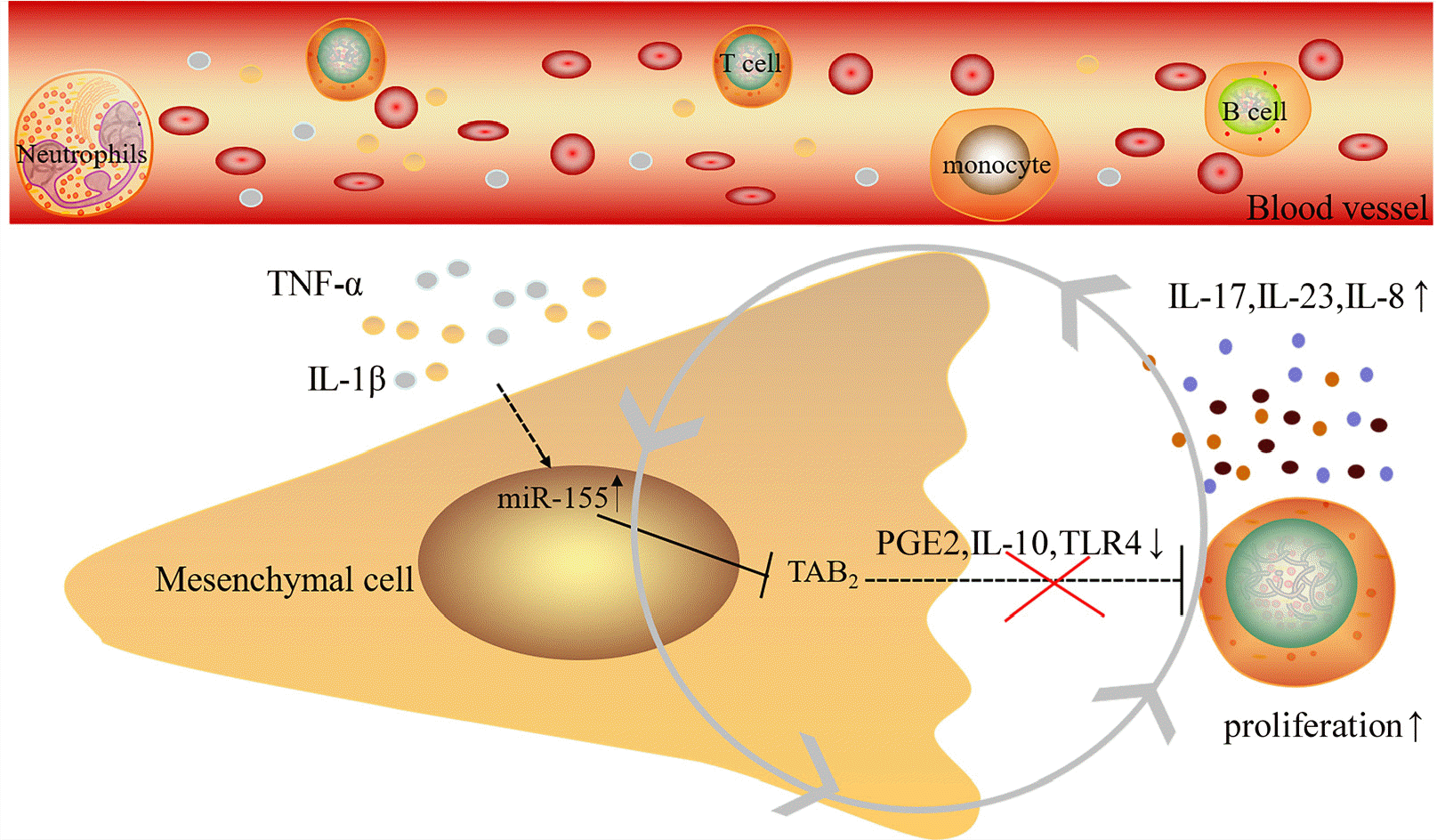
Table 1
Primers used for RT-qPCR




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download