Abstract
The state of intense peripancreatic inflammation in chronic pancreatitis can give rise to various vascular complications such as venous thrombosis and arterial pseudoaneurysms. Due to its intimate location with the pancreas, spleno-mesenteric-portal axis suffers the greatest blunt of thrombotic complications. Treatment modalities for such cases of chronic portal vein thrombosis have always been controversial and challenging. Medical management with anticoagulants is both risky and unsatisfactory due to presence of varices, hypersplenism, and persistence of the inflammatory pathology. Although endovascular techniques have been tried in various case reports, there are definite anatomical challenges in cases of long segment porto-mesenteric thrombosis with massive ascites. Surgical shunts have been historically described for cirrhotic and non-cirrhotic portal hypertensive patients. However, its use in patients with refractory ascites due to chronic pancreatitis induced portal vein thrombosis has not been reported in the medical literature. Here, we present a case of an extensive portal vein thrombosis with massive refractory ascites in a patient with alcohol-induced chronic pancreatitis successfully treated with a surgical mesocaval shunt using an interposition small diameter graft.
Chronic pancreatitis is a persistent inflammatory disease of pancreatic parenchyma characterized by irreversible morphologic changes and fibrosis resulting in pain and/or permanent functional deprivation [1]. This state of intense peripancreatic inflammation can give rise to various vascular complications such as venous thrombosis (VT) and arterial pseudoaneurysm. VT is a more frequent complication than pseudoaneurysm. It has strong associations with alcoholic chronic pancreatitis, pseudocyst, and the presence of an inflammatory head mass [2]. Although pharmacological management is the first-line treatment for recanalization of acute portal vein thromboses, treating a chronic long segment portal vein thrombosis (PVT) is always challenging, especially when there is a cavernomatous transformation along with portal hypertensive complications such as variceal bleeding and ascites. Here, we present a case of an extensive PVT with massive refractory ascites in a patient with alcohol-induced chronic pancreatitis treated with a surgical mesocaval shunt using an interposition small diameter graft.
A 41-year-old gentleman presented in our outpatient department with complaints of progressive abdominal distension associated with nausea and vomiting over two months. He had an alcohol-induced chronic calcific pancreatitis for the last five years. Abdominal distension was significant, causing respiratory distress, which had aggravated for two days. He had multiple acute episodes of pancreatitis in the past, for which he was managed conservatively with analgesics and hydration in other hospitals. He also had pancreatitis induced diabetes mellitus for the last two years, which was poorly controlled. He had no history of jaundice, hematemesis, or melena. Informed written consent and permission were obtained from the patient for the use of the clinical material and photographs for the purpose of study and publication.
On clinical examination, he was wholly conscious and oriented. He had a pulse rate of 92/min, blood pressure of 110/70 mmHg, respiratory rate of 20/min, and blood oxygen saturation of 97% in room air. He was not pale or icteric. There was no pedal oedema. His abdomen was distended with fluid thrill, prominent superficial veins, and centrifugal blood flow.
Regarding his blood parameters, he showed anemia (hemoglobin: 8.5 g/dL) with normal white blood cell and platelet counts. He had normal renal function, electrolytes, and liver function except raised alkaline phosphate (507 IU/dL) and gamma-glutamyl transferase (201 IU/L). His blood amylase and lipase were within their normal limits (amylase: 45 IU/L; lipase: 46 IU/L). Ascitic fluid analysis showed albumin 0.5 g/dL, with serum ascites-albumin gradient of 1.7 g/dL, suggestive of a transudative or a portal-hypertensive pattern.
He underwent an ultrasound of the abdomen, which showed normal liver parenchyma but an extensive portal thrombus with cavernomatous transformation. The pancreas showed multiple intraparenchymal and intraductal calcifications. Also, there were borderline splenomegaly and massive ascites. Acoustic radiation force impulse scan of the liver showed a mean shear velocity of the liver of 1.37 m/sec, suggesting a non-cirrhotic liver. Contrast-enhanced computed tomography (CECT) showed that the portal vein was markedly attenuated with thinning of the splenic vein close to the confluence with non-visualization of the distal superior mesenteric vein (SMV). Additionally, multiple periportal, peri-splenic, peri-gastric, intra- and extra-hepatic collaterals, hepatic artery appeared hypertrophied. There was also a pseudocyst in the caudate lobe of the liver, which was compressing the hilum of the liver. Pancreas appeared atrophic. Multiple parenchymal and intraductal calcifications diffusely involving the pancreas with beaded dilatation of main pancreatic duct were noted (Fig. 1). An upper gastrointestinal endoscopy showed grade 2 esophageal varices. Prophylactic band ligation was done.
Diuretics were started. Their doses were increased subsequently. However, they failed to relieve the distension. High volume therapeutic paracentesis was done thrice. However, it only resulted in minor symptomatic relief for a few days. Due to symptomatic refractory ascites, surgical shunt was planned to relieve the mesenteric venous hypertension. After a multidisciplinary review of his portomesenteric (PM) venous anatomy, only a mesocaval shunt was deemed feasible (Fig. 2).
The surgery was done via a midline laparotomy. Due to extensive inflammatory adhesions caused by chronic pancreatitis, the lesser sac was not explored. The small bowel mesentery in the infracolic compartment was exposed by retracting the transverse colon superiorly and the small bowel loops inferiorly. On retracting the transverse colon, the middle colic vein was identified. It was traced toward its junction with the SMV. Superior mesenteric artery (SMA) pulsations were also felt and the site of SMV was identified (anterior and to the right of the SMA). The overlying peritoneum was divided with extreme caution to expose around 3 cm length of SMV which was free of major venous tributaries. This SMV segment was dissected free and encircled. A segment of the inferior vena cava (IVC) was then dissected out from the retroperitoneum, inferior to the duodenum. Following sufficient dissection, the site for anastomosis was selected after reasonable space for partial side clamping was obtained. An 8 mm expanded polytetrafluoroethylene (e-PTFE) graft was then anastomosed in an end to side fashion to the IVC first using running 5-0 polypropylene sutures. The other end was then anastomosed to the SMV in the same end to side fashion using running 5-0 polypropylene sutures. Graft occlusion test showed good flow between SMV to IVC (Fig. 3).
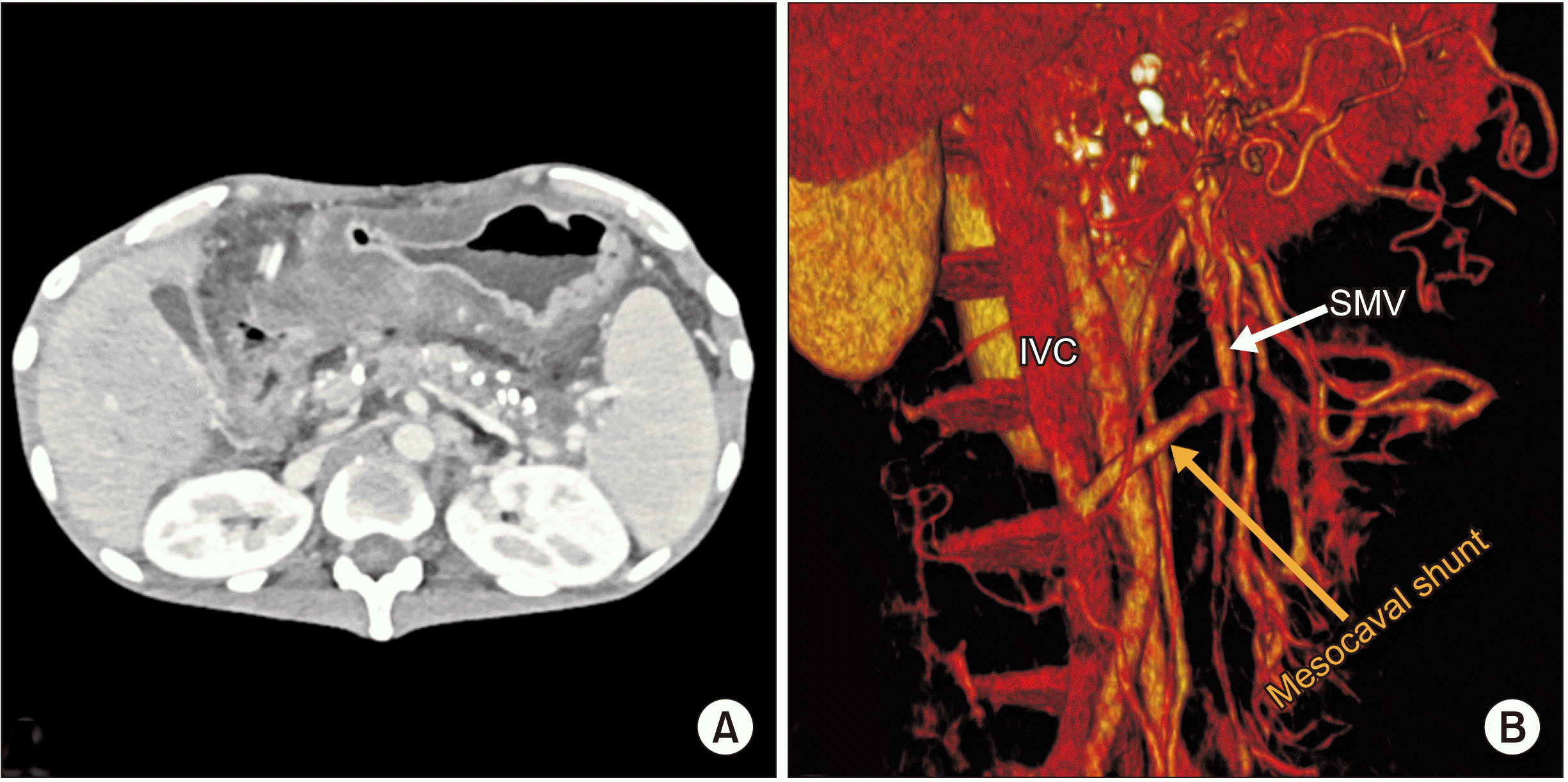
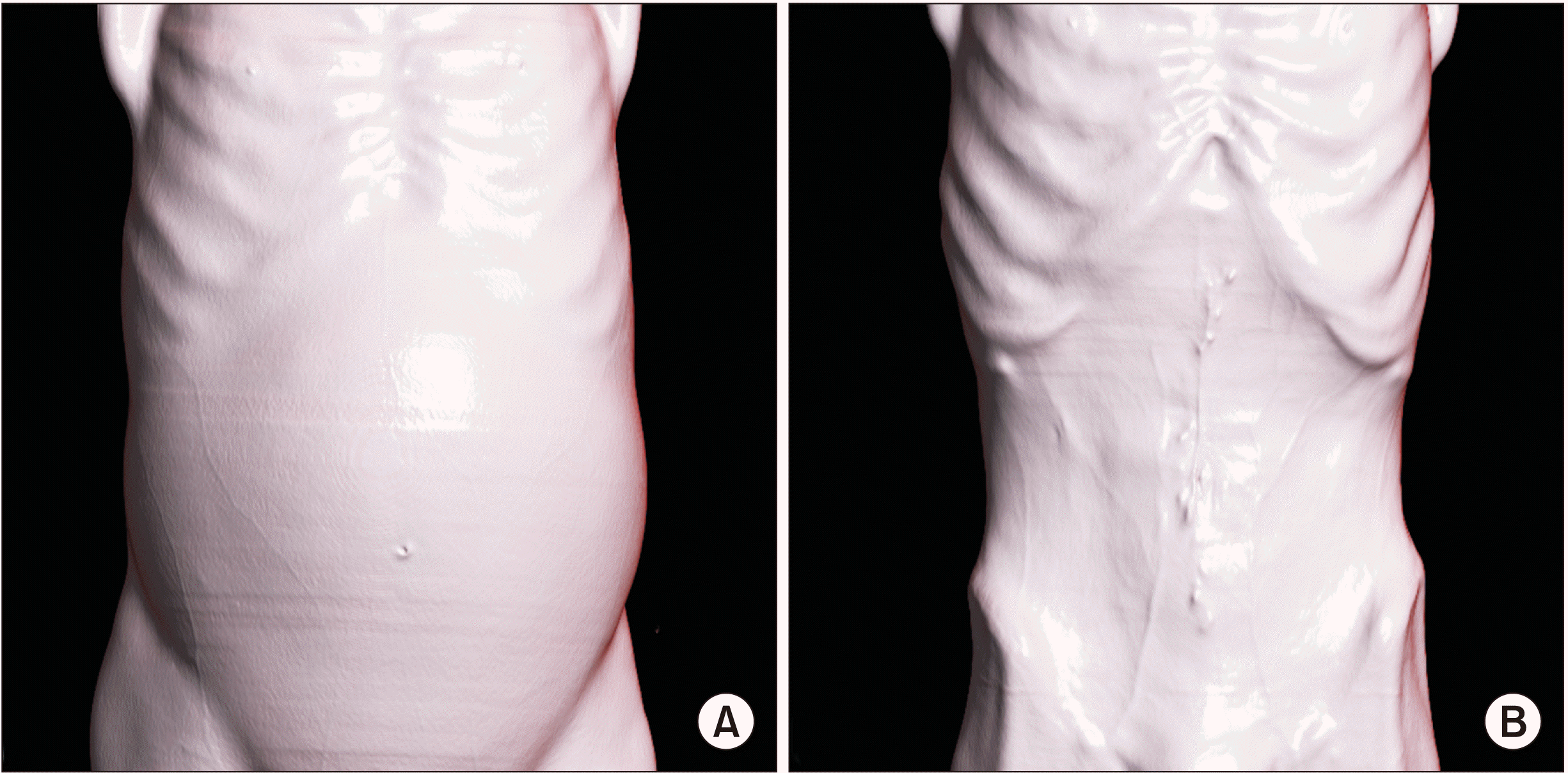
Postoperatively, he was started on intravenous heparin injections 5,000 units every 8th hourly. The dose was further titrated to keep activated partial thromboplastin time values double of the control. He started orals on POD 3, following which he was prescribed warfarin tablet 5 mg once daily. The dose was further titrated to keep international normalized ratio (INR) between 2–3. The same was advised to continue after discharge. Radiological follow-up of the shunt patency was done with CECT on postoperative day 14 which showed a significant reduction in ascites with a patent shunt (Fig. 4). He had three episodes of infected abdominal collections which were managed by ultrasound-guided percutaneous pigtail drainage and antibiogram guided antibiotics. There was no hepatic encephalopathy noted post-surgery. He was discharged on postoperative day 40 with a plan of 3 monthly follow-ups. Follow-up on postoperative day 90 with CT imaging showed functional shunt with complete resolution of ascites (Fig. 5). A three-dimensional surface reconstruction of CTs taken preoperatively and on postoperative day 90 showed result of the surgery in decreasing refractory ascites (Fig. 6). On postoperative day 90, he underwent a screening upper gastrointestinal endoscopy. It showed grade 2 esophageal varices that were prophylactically banded. Although there was complete resolution of ascites, there was no significant improvement with esophageal varices as PM thrombosis was also involved the region where the left gastric vein and the splenic vein joined the PM system as shown in Fig. 2. Thus, the thrombus practically disconnected the esophago-gastric venous system from the mesenteric venous system, resulting in no improvement or decompression of varices following surgery. Hence, he was planned to be under endoscopic surveillance for esophago-gastric varices every three months.
The pancreas shares a strategically important location in the abdomen at the crossroads of the portal venous system. Thrombosis of the portal vein is one of the most important vascular complications following chronic pancreatitis. Probable mechanisms for PVT are either prolonged venous compression due to fibrosis in chronic calcific pancreatitis, or compression by pancreatic pseudocysts or enlarged pancreatic parenchyma, or intimal injury and resulting thrombosis due to acute on chronic pancreatitis [3]. These will ultimately result in the activation of components of Virchow’s triad (venous stasis, intimal injury, or hypercoagulability), leading to thrombosis.
With current imaging modalities, a recognition of an early PVT is possible, even before cavernous transformation or variceal bleeding, both of which represent chronicity of thrombus. Ultrasonography is the investigation of choice for identification of portal thrombosis. Doppler imaging sensitivity and specificity ranges from 66% to 100% [4]. CECT helps in accurately assessing the extent of thrombus with other important information such as complete evaluation of the rest of mesenteric vasculature, the status of the pancreas and potential complications like bowel ischemia and even perforation, thus helping the decision about the mode of management [5].
Treatment of symptomatic PVT has always been controversial with limited options [6]. Although spontaneous resolution of PVT has been reported in the literature [5], the goal of treatment of PVT in chronic pancreatitis is to prevent progression of thrombus extension and re-establish splanchnic circulation to prevent complications of portal hypertension such as hypersplenism, development of varices, ascites, and portal cholangiopathy. The initial step in managing PVT is to consider anticoagulation for both acute and chronic PVT. After six months of therapy, a complete recanalization was reported in approximately 50% of acute PVT patients with better results in mesenteric venous involvement. However, 10% of cases of PVT were resistant to anticoagulation [7]. Anticoagulant use in chronic PVT is controversial due to the presence of varices, low platelet counts, and existing coagulation dysfunctions. It is only limited to those with known hypercoagulability to prevent thrombosis or any new onset PVT. The risk of progression of the PVT has to be weighed against that of variceal bleeding [8,9].
Various endovascular techniques have been successfully tried for recanalization of chronic PM thrombosis through a transjugular route or a transhepatic route [6]. However, such techniques have always been challenging in cases of long segment chronic PM thrombus with cavernous transformation, especially when there is a lack of enough patent intrahepatic portal branches with huge ascites [10] as in the present case. Although novel percutaneous endovascular techniques of mesocaval shunt creation in cases of PM thrombosis have been described in a few case reports [11], such options were infeasible in this patient due to massive refractory ascites and long segment proximal SMV thrombosis.
Surgical mesovcaval shunts were first described in medical literature by Santy and Marion [12] and Clatworthy et al. [13]. Clatworthy et al. [13] have experimented this technique in dogs. They then successfully performed it in a pediatric patient with portal hypertension where the IVC was divided above its bifurcation and anastomosed of the to the side of the SMV. Later, due to massive lower extremity oedema and operative difficulty because extensive dissection is needed to expose vena cava, interposition grafts, first described by Drapanas [14], came into use. Although such surgical mesocaval shunt has been mostly described in the literature for variceal bleeding in cirrhotic patients [15] and non-cirrhotic portal hypertensive patients [16], application of such a surgical shunting technique in extensive PM thrombosis due to chronic pancreatitis is very rare. In our extensive literature search using three search engines, Pubmed, Google Scholar, and Ovid, we could find just one such case of pancreatitis induced SMV thrombosis being treated by a mesocaval shunt [17]. However, the patient suffered from upper gastrointestinal bleeding from isolated duodenal varices, not refractory ascites as in the present case.
Graft length should be only 3 to 6 cm to minimize kinking [18]. The diameter of the graft used for a mesocaval shunt has been a topic of significant debate historically. After his initial publication on the technique of interposition mesocaval graft, Drapanas et al. [19] has published a follow-up series on the hemodynamics of mesocaval shunts where he used large diameter (18–22 mm) Dacron grafts. Although such large diameter grafts had excellent patency rates, subsequent studies by other authors showed a significant reduction in hepatic portal perfusion and increased rates of encephalopathy. Mercado et al. [20], in 2000 have reported their 10-year experience with 10-mm PTFE mesocaval shunts and shown excellent long-term patency rates (81%). In this case, we achieved excellent short-term results with a small diameter (8 mm) mesocaval shunt in decompressing a hypertensive mesenteric venous circuit and relieving refractory ascites.
As seen in the current case, mesocaval shunt was a successful treatment modality for pancreatitis induced long segment PM thrombosis, with good short-term outcomes. With more adoption of this technique to such cases with long-term follow-up studies, better standardization of the surgery can be done in future.
REFERENCES
1. Etemad B, Whitcomb DC. 2001; Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology. 120:682–707. DOI: 10.1053/gast.2001.22586. PMID: 11179244.

2. Anand A, Gunjan D, Agarwal S, Kaushal K, Sharma S, Gopi S, et al. 2020; Vascular complications of chronic pancreatitis: a tertiary center experience. Pancreatology. 20:1085–1091. DOI: 10.1016/j.pan.2020.07.005. PMID: 32800648.

3. Bernades P, Baetz A, Lévy P, Belghiti J, Menu Y, Fékété F. 1992; Splenic and portal venous obstruction in chronic pancreatitis. A prospective longitudinal study of a medical-surgical series of 266 patients. Dig Dis Sci. 37:340–346. DOI: 10.1007/BF01307725. PMID: 1735356.
4. Chawla Y, Duseja A, Dhiman RK. 2009; Review article: the modern management of portal vein thrombosis. Aliment Pharmacol Ther. 30:881–894. DOI: 10.1111/j.1365-2036.2009.04116.x. PMID: 19678814.

5. Webster GJ, Burroughs AK, Riordan SM. 2005; Review article: portal vein thrombosis -- new insights into aetiology and management. Aliment Pharmacol Ther. 21:1–9. DOI: 10.1111/j.1365-2036.2004.02301.x. PMID: 15644039.

6. Stein M, Link DP. 1999; Symptomatic spleno-mesenteric-portal venous thrombosis: recanalization and reconstruction with endovascular stents. J Vasc Interv Radiol. 10:363–371. DOI: 10.1016/S1051-0443(99)70044-8. PMID: 10102204.

7. Plessier A, Darwish-Murad S, Hernandez-Guerra M, Consigny Y, Fabris F, Trebicka J, et al. 2010; Acute portal vein thrombosis unrelated to cirrhosis: a prospective multicenter follow-up study. Hepatology. 51:210–218. DOI: 10.1002/hep.23259. PMID: 19821530.

8. Loffredo L, Pastori D, Farcomeni A, Violi F. 2017; Effects of anticoagulants in patients with cirrhosis and portal vein thrombosis: a systematic review and meta-analysis. Gastroenterology. 153:480–487.e1. DOI: 10.1053/j.gastro.2017.04.042. PMID: 28479379.

9. Orr DW, Harrison PM, Devlin J, Karani JB, Kane PA, Heaton ND, et al. 2007; Chronic mesenteric venous thrombosis: evaluation and determinants of survival during long-term follow-up. Clin Gastroenterol Hepatol. 5:80–86. DOI: 10.1016/j.cgh.2006.09.030. PMID: 17142105.

10. Han G, Qi X, He C, Yin Z, Wang J, Xia J, et al. 2011; Transjugular intrahepatic portosystemic shunt for portal vein thrombosis with symptomatic portal hypertension in liver cirrhosis. J Hepatol. 54:78–88. DOI: 10.1016/j.jhep.2010.06.029. PMID: 20932597.

11. Moriarty JM, Kokabi N, Kee ST. 2012; Transvenous creation of a mesocaval shunt: report of use in the management of extrahepatic portal vein occlusion. J Vasc Interv Radiol. 23:565–567. DOI: 10.1016/j.jvir.2011.09.023. PMID: 22464719.

12. Santy P, Marion P. 1953; [Technics of portacaval derivations]. Sem Hop. 29:2758–2767. French. PMID: 13113234.
13. Clatworthy HW, Wall T, Watman RN. 1955; A new type of portal-to-systemic venous shunt for portal hypertension. AMA Arch Surg. 71:588–599. DOI: 10.1001/archsurg.1955.01270160114014.

14. Drapanas T. 1972; Interposition mesocaval shunt for treatment of portal hypertension. Ann Surg. 176:435–448. DOI: 10.1097/00000658-197210000-00001. PMID: 4263236. PMCID: PMC1355426.

15. Cameron JL, Zuidema GD, Smith GW, Harrington DP, Maddrey WC. 1979; Mesocaval shunts for the control of bleeding esophageal varices. Surgery. 85:257–262. DOI: 10.1016/s0022-3468(79)80179-7. PMID: 311525.
16. Mangla V, Pal S, Sahni P. 2016; Surgery for non-cirrhotic portal hypertension: current status. Trop Gastroenterol. 37:152–155. DOI: 10.7869/tg.348.

17. Bommana V, Shah P, Kometa M, Narwal R, Sharma P. 2010; A case of isolated duodenal varices secondary to chronic pancreatitis with review of literature. Gastroenterology Res. 3:281–286. DOI: 10.4021/gr249w. PMID: 27942309. PMCID: PMC5139857.

18. Moore WS. 2018. Vascular and endovascular surgery e-book: a comprehensive review. 9th ed. Elsevier;Philadelphia: DOI: 10.7869/tg.348.
19. Drapanas T, LoCicero J 3rd, Dowling JB. 1975; Hemodynamics of the interposition mesocaval shunt. Ann Surg. 181:523–533. DOI: 10.1097/00000658-197505000-00004. PMID: 124159. PMCID: PMC1345527.

20. Mercado MA, Orozco H, Guillén-Navarro E, Acosta E, López-Martínez LM, Hinojosa C, et al. 2000; Small-diameter mesocaval shunts: a 10-year evaluation. J Gastrointest Surg. 4:453–457. DOI: 10.1016/S1091-255X(00)80085-2. PMID: 11077318.

Fig. 1
Sections from preoperative contrast-enhanced computed tomography. (A) Axial section showing a pseudocyst along the caudate lobe of liver (white arrow). (B) Axial section showing thrombosed portal vein with periportal collaterals (black arrow). (C, D) Axial sections showing chronic calcific pancreatitis changes in the entire pancreas (yellow arrows). (E, F) Axial and coronal sections showing massive ascites.
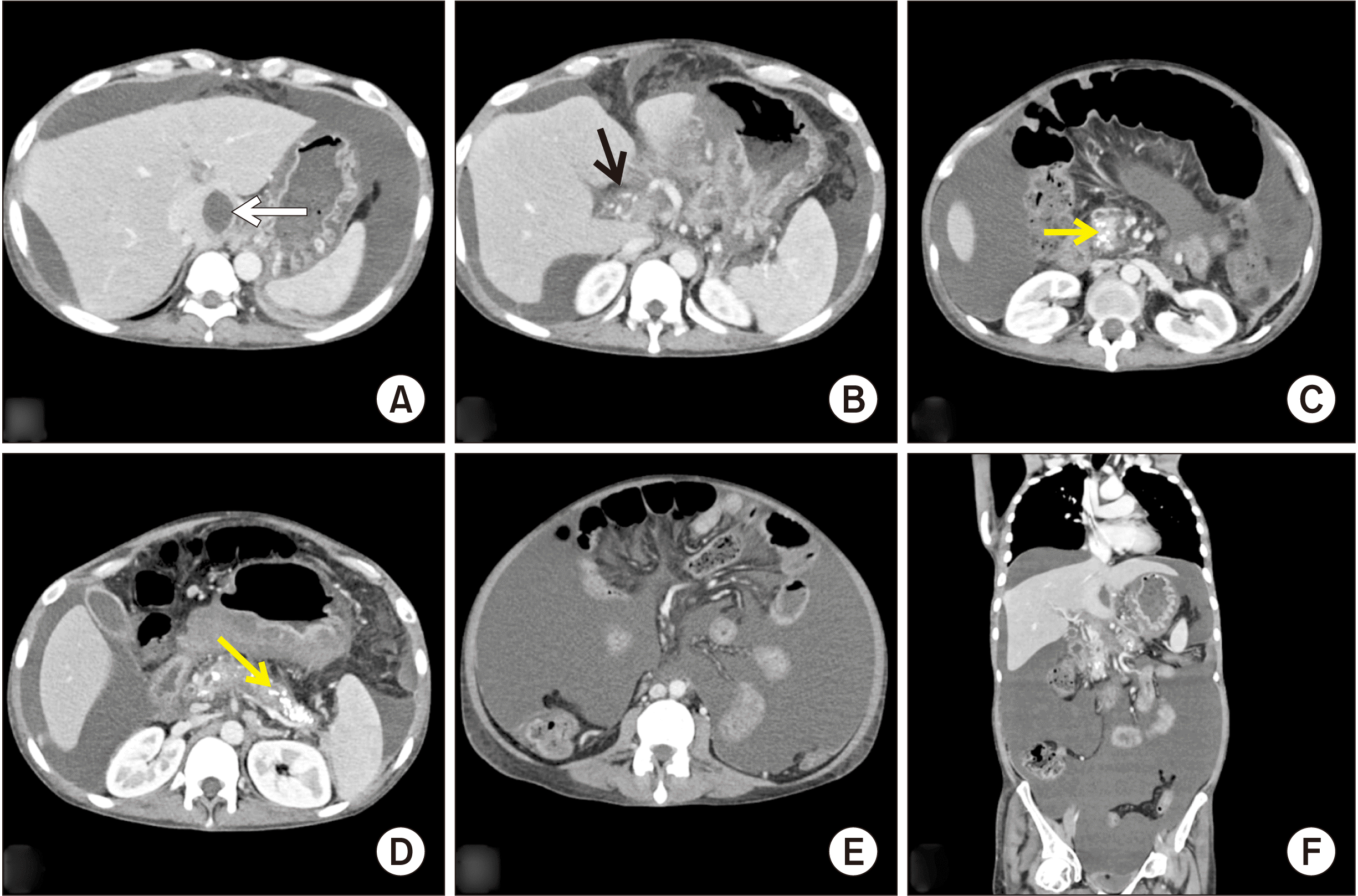
Fig. 2
Schematic diagram showing the surgical plan to decompress the hypertensive SMV with a mesocaval shunt. SMV, superior mesenteric vein; IVC, inferior vena cava.
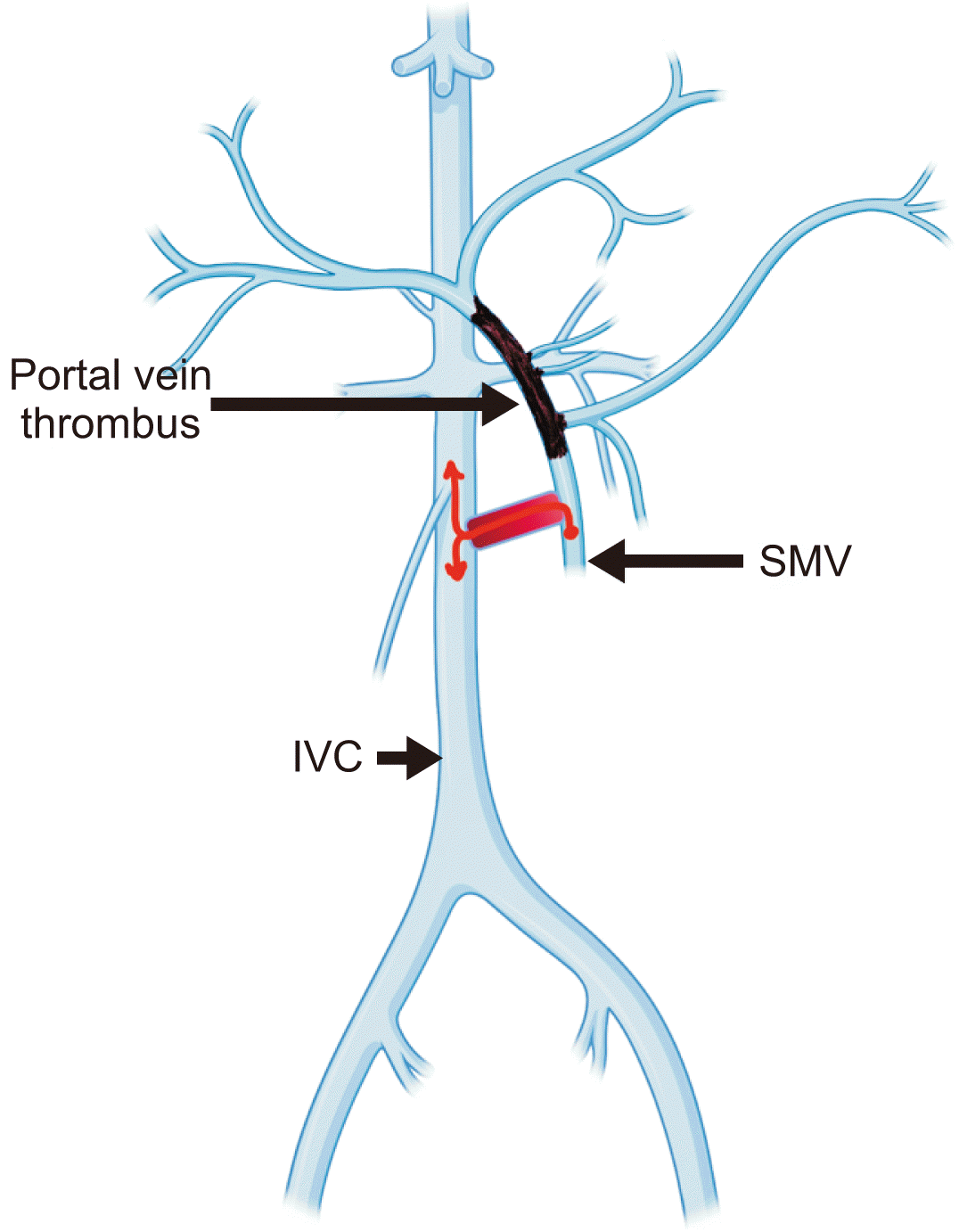
Fig. 3
Intraoperative photos. (A) After complete dissection of the superior mesenteric vein (SMV). (B) Infrarenal inferior vena cava (IVC) dissected from the retroperitoneum. (C) After completion of IVC-graft anastomosis. (D) After completion of anastomoses on both sides of graft with IVC and SMV. White arrows indicate SMV; black arrows, IVC.

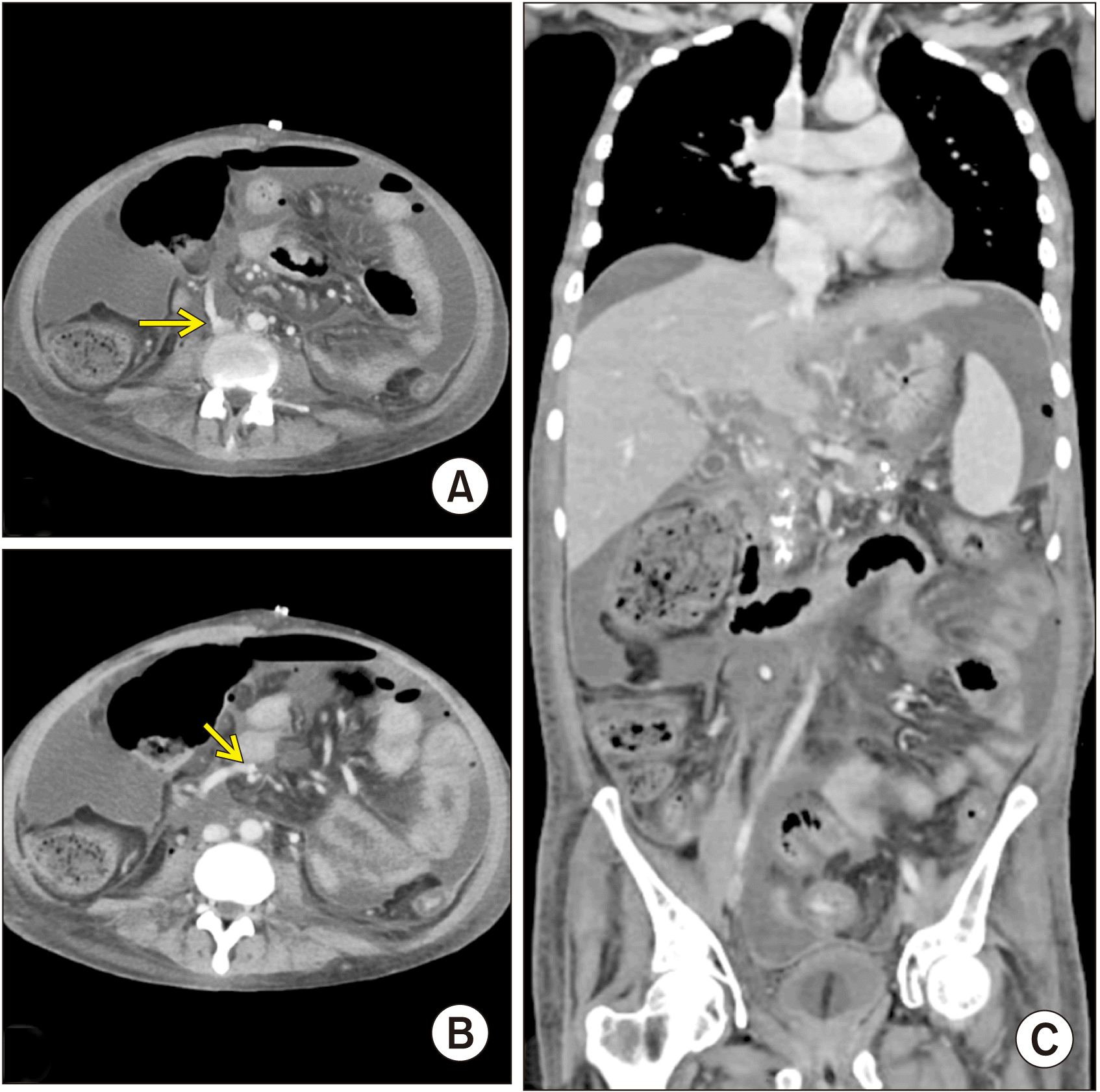
Fig. 4
Contrast-enhanced computed tomography images (portal phase) on postoperative day 14. (A) Axial section showing one end of the graft anastomosing with inferior vena cava (arrow). (B) Axial section showing the other end of the graft anastomosing with superior mesenteric vein (arrow). Both images represent patent shunt with proper contrast opacification along its entire length. (C) Coronal section representing decreased ascites in the postoperative period.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download