Abstract
Backgrounds/Aims
The soft texture of the pancreas parenchyma may influence the incidence of pancreatic leakage after pancreaticoduodenectomy (PD). One possible method to assess pancreatic texture and atrophy, is via computed tomography (CT) scan of the abdomen. The purpose of our study was to evaluate the relation between the preoperative CT scan and the incidence of pancreatic fistula after PD.
Methods
A retrospective single-center study including patients who underwent PD for a benign and malignant tumor of the periampullary region between the years 2000 and 2016. Demographic and imaging data were analysed and a correlation with the post-operative leak was evaluated.
Results
Pancreatic leak was documented in 34 out of 154 (22.1%) patients. All the leakage cases occurred in the preserved pancreas group (33.1% of the total preserved pancreas group alone). No leak was documented in the atrophic pancreas group. This difference between the two groups was found to be statistically significant (p ≤ 0.00001).
Conclusions
Atrophic pancreas in the preoperative CT scan may be protective against leakage after PD. These findings may help the surgeon to risk stratify patients accordingly. In addition, the findings suggest that patients with a preserved pancreas may require more protective methods to prevent leakage.
Pancreaticoduodenectomy (PD) is the primary surgical treatment for tumors of the head of the pancreas, bile duct, duodenum, and the ampulla of Vater [1]. The procedure was first described in 1945 by Whipple as a modification of a prior procedure developed by Alessendro Codini villan in Italy and Walter Keusch in Germany [2]. Although postoperative mortality following PD has declined, the incidence of postoperative morbidity remains high [3]. Leakage from the pancreatojejunostomy anastomosis after PD is one of the most common and serious PD postoperative complications; intra-abdominal abscess, sepsis, or intra-abdominal bleeding are potentially devastating sequelae of pancreatic leakage [3-6]. Consequently, pancreatojejunostomy anastomosis leakage is one of the most significant causes of morbidity leading to a prolonged hospital stay, and even mortality after PD.
The soft texture of the pancreas parenchyma is one of the contributing factors of post-PD pancreatic leakage and fistula incidence and severity [7]. On the other hand, pancreatic atrophy may influence the pancreas texture; pancreatic atrophy is characterized by the disappearance of the acinar cells and to a lesser extent the islet of Langerhans, and a fatty replacement that may vary from mild to massive fatty infiltration [8]. Unfortunately, there are no clear preoperative or intra-operative objective tests to determine the two. Among the possible methods is the assessment of pancreatic texture and atrophy through computed tomography (CT) scan of the abdomen. Certain CT scans such as reduced pancreas tissue or diffuse fatty infiltration of the pancreas can help determine pancreatic atrophy and predict the texture of the pancreas. Therefore, a preoperative CT scan may help predict pancreatojejunostomy anastomosis leakage.
The purpose of this study was to evaluate the relationship between the atrophic pancreas in the preoperative CT scan and the pancreatic fistula incidence after PD.
This was a retrospective, observational single-center study including patients who underwent PD for a benign and malignant tumor of the periampullary region between the years 2000 and 2016 at our hospital. The perioperative data of the patients were collected from a computerized database of the hepatobiliary unit at. Patients who underwent major abdominal surgery in addition to the PD procedure (e.g., hepatectomy or colectomy) or those who had no pre-operative imaging results were excluded from the study. This study was approved by the Ethics Committee of Hadassah-Hebrew University Medical Center (No.0140-17-HMO). The IRB waived the need for informed consent for this retrospective study.
The patients were treated with a 2nd generation cephalosporin as a prophylactic preoperative antibiotic unless there were previous positive cultures that necessitated different antibiotics. All the PD surgical procedures were performed by a senior hepatobiliary surgeon in the hepatobiliary unit at our hospital; an open technique was solely used. A two-layer end-to-side duct to mucosa type pancreaticojejunostomy was performed using a polydioxanone suture (PDS) 7-0 or PDS 6-0 in all cases. The omega loop that was used for the pancreaticojejunostomy was also used to perform a one-layer end-to-side hepaticojejunostomy and two-layer side-to-side gastroenterostomy or an end-to-side duodenojejunostomy. Two drains were routinely left next to the anastomotic sites.
Post-operatively, the patients were monitored for at least 24 hours in the intensive care unit before being transferred to the general surgical ward. Nasogastric tubes were removed after at least five days, after the patients had a bowel movement and when the nasogastric tube drained less than 300 milliliters. All the patients were routinely treated with a somatostatin drip. At least two Jackson prat drains were placed and were then removed when the drained fluid had a normal amylase level and the collected amount of fluid was less than 15 milliliters.
Data including demographics, etiology of the patients who underwent PD, laboratory results, imaging studies, surgical pathology results, TNM staging of disease (when relevant), and perioperative data were retrospectively collected. Data collected from the surgical database was confirmed by reviewing the individual computerized medical records. When computerized medical records did not suffice, hard copy medical records were viewed. All the imaging studies evaluated were CT scans. Since there are no clear criteria to define atrophic pancreas, we defined pancreatic atrophy as reduced tissue mass of the tail and body of the pancreas, lack of continuity of the pancreatic tail towards the spleen hilum, highly diminished pancreas, or marked diffuse fat infiltration of the pancreas (Fig. 1). All the CT scans were blindly reviewed by a senior radiologist specialized in abdominal imaging in an attempt to reduce the bias by focusing on the pancreatic tissue. The accepted definition of a pancreatic fistula in our institution is, as defined by the International Study Group on Pancreatic Surgery, the drainage of any measurable volume of fluid with an amylase level greater than three times the upper limit of the institutional normal serum amylase activity [9].
Statistical analysis of the data in this study was performed using IBM SPSS ver. 20 (IBM Corp., Armonk, NY, USA). We defined the independent variable as the presence of atrophic pancreas on preoperative imaging and was documented as a binary value—preserved pancreas or atrophic pancreas. The dependent variable was the incidence of a pancreatic fistula or leak after PD during the postoperative period (30 days post-operation) and it was also documented as a binary value; either yes or no.
To test the study’s main hypothesis and possible confounders, multiple statistical tests were performed, and parametric statistics were used to reduce assumptions on which the study relied, and to increase the study reliability. Univariate analysis was conducted in two ways; the Fisher’s exact test was used to test the association between dichotomous variables (pancreas structure and pancreatic leak) and the nonparametric Mann–Whitney U test was used to evaluate continuous variables. To calculate the prediction strength of the rates of a pancreatic leak while controlling for possible confounders, a binary multivariable logistic regression model was performed and for each parameter entered in the model an odds ratio value was calculated. A p-value of ≤ 0.05 was considered statistically significant; significance was calculated by the Wald test.
For statistical analysis (binary logistic regression analysis of the effect of clinical factors on the postoperative pancreatic leak) that did not require imaging information about the pancreatic atrophy (such as age, sex, tumor size) we also used the 41 patients with inadequate imaging, but had full relevant clinical data and outcome. This improved the statistical reliability of the sample size, as the main assessed outcome in this test was the postoperative pancreatic leak that was available for the 44 patients.
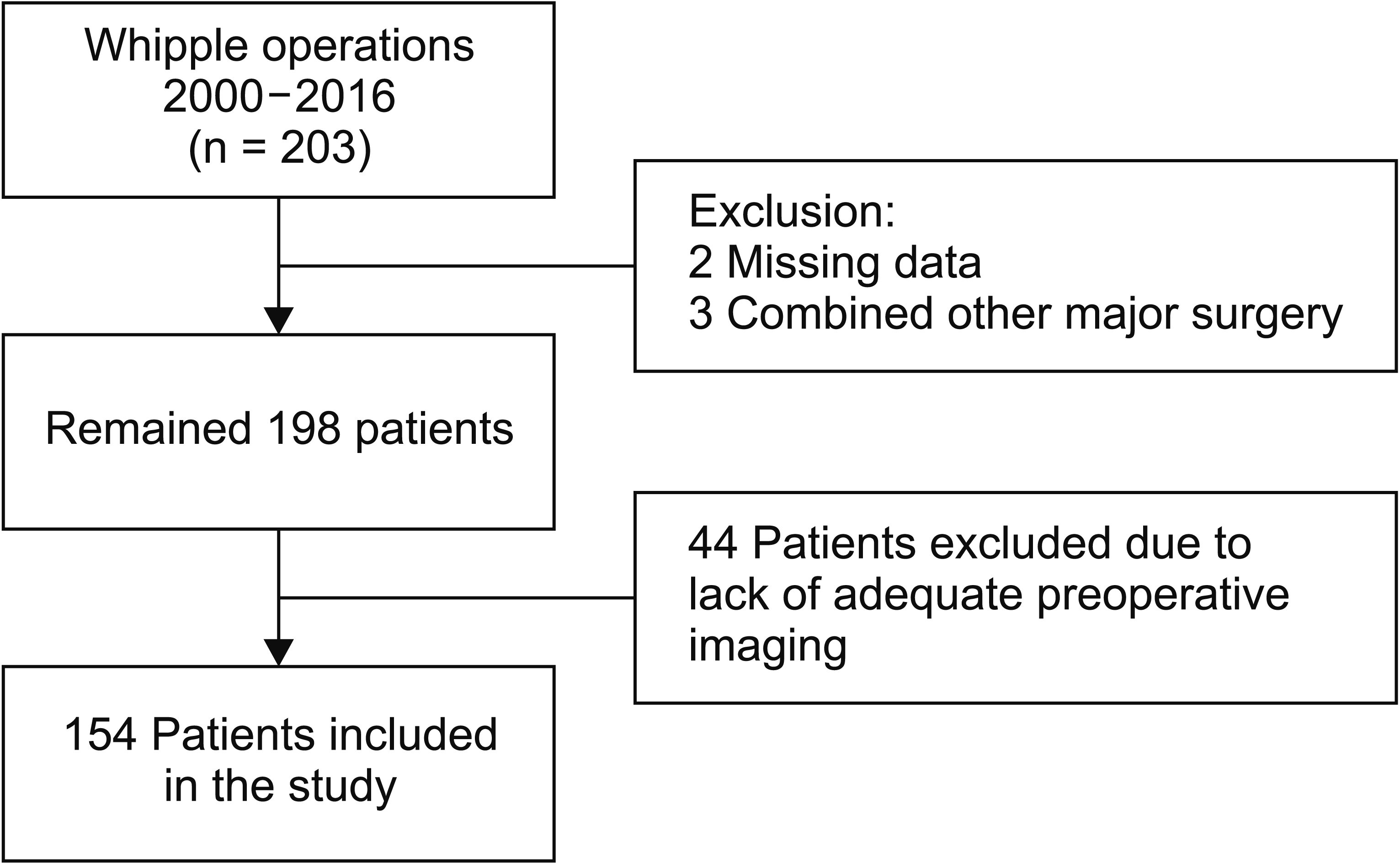
Between the years 2000 and 2016, 203 patients underwent PD. Forty-nine patients out of the 203 patients in the cohort were excluded from the study due to the previously mentioned exclusion criteria; 44 patients did not have any imaging or did not have adequate preoperative CT scan imaging results, while the remaining five patients had a concomitant major surgery along with the PD or their data was missing (Fig. 2). Finally 154 patients were included in this study. For the statistical analysis that did not require imaging information of the pancreas structure, 41 cases (that had full adequate clinical data and reported leak outcome) from the excluded 44 cases with absent or inadequate imaging were included. The majority of the patients were males 89 (57.8%) with a mean age of 61.8 years (range, 26–84 years) (Table 1).
Table 1 depicts the sample pathological characteristics. The majority of the procedures were due to a malignant neoplasm in the head of the pancreas 86 (55.8%), followed by Ampullary adenocarcinoma 22 (14.3%).
Of the 154 patients that were included in the study, 103 (66.9%) patients had a preserved pancreas while 51 patients (33.1%) were shown to have an atrophic pancreas on pre-operative imaging. The atrophic pancreas was found to be more common among males (36.0% in males, 29.2% in females) (Table 2). The mean age was 61.65 years in the preserved pancreas group as compared to 62.1 years in the atrophic pancreas group.
The pancreatic leak was documented in 34 out of 154 (22.1%) patients. All the leakage cases occurred in the preserved pancreas group (33.1% of the total preserved pancreas group alone). No leak was documented in the atrophic pancreas group. The difference between the two groups was found to be statistically significant (p ≤ 0.00001) (Table 2).
We also compared the age between intact and atrophied pancreas groups, and we found that the average age was 61.65 and 62.1 years old respectively, in addition to this we compared the age between positive and negative pancreatic leak groups, the mean age was 63.07 and 61.2 years old respectively, however; these results were not statistically significant.
We evaluated the relationship between the mean tumor size as found in pathology and preoperative imaging of pancreatic structure. A correlation was found between the large tumors and an atrophic pancreas and was shown to be statistically significant (p = 0.04). The average maximal tumor size was 3.08 cm in the preserved pancreas group and 3.52 cm in the atrophic pancreas group (Table 2).
In order to exclude possible confounding variables, logistic regression models were used. Age, sex, and tumor size were included in the model. Forty-one patients with no imaging documentation were included in the final logistic regression model (total n = 195). No statistical significance was found for any of the variables tested, (Table 3). Although there were no significant differences was it is worth noting that there was a correlation between maximal tumor size and pancreas leak rates with an odds ratio of 0.83 (p-value = 0.09).
There was a strong relationship between the pancreas structure as seen in preoperative imaging and incidence of a pancreatic leak in both male and female (Table 4).
This study result showed a dismal rate of leakage and fistula formation in patients with atrophic pancreas as shown in the preoperative CT scan, however, the rate was 33% in those with the preserved structure of the pancreas. It’s worth mentioning that pancreatic fistula formation remains the most important cause of morbidity following PD [3,4]. Therefore, preoperative risk stratification is essential in predicting the risk of leakage and fistula formation post PD. In prior studies, the presence of a fibrotic pancreas has been shown to be protective against leaks. This may be explained by the fact that atrophic pancreas on imaging may reflect a fibrotic pancreas intraoperatively [10].
In our study, we used a relatively simple parameter of atrophic pancreas, as diagnosed on preoperative imaging, and found that it is considered protective against pancreatic leakage. Pancreatic atrophy is characterized by the loss of acinar cells and to a lesser extent the islet of Langerhans [8]. This may contribute to the low rate of leakage in the atrophied pancreas, as the reduced number of exocrine cells may, in turn, decrease the volume of secretions and ultimately decrease the chance of leakage.
There are important preoperative information clinical values that would help to assess surgical risk. First, in an older patient population who are considered to be at a higher risk due to their age and comorbidities, an atrophic pancreas on CT scan may promote the decision to operate on this group of patients as the risks of surgery may be less. Second, in patients with a normal pancreas on preoperative imaging, additional preventive methods may be undertaken to help prevent leaks following PD. Third, this information is useful to the patients and provides more accurate evidence regarding the risk of leakage. Fourth, it may help the patient’s treatment team to calculate the risk versus the benefits of the procedure before deciding on the appropriate treatment for benign lesions that necessitate PD.
There are other studies that have evaluated the relationship between pancreas as shown on pre-operative imaging leakage and rate [11-13]. Kim et al. [11] compared preoperative MRI of the pancreas with the leakage rate and showed that the signal intensity ratio between the pancreas and different intrabdominal organs such as the liver and spleen may predict the leakage rate. The main limitation of this method is the use of MRI in the preoperative evaluation. Frozanpor et al. [12] evaluated the incidence of leakage based on two criteria of the preoperative findings of the CT scan; the volume of the pancreas and the duct size. The complexity of measuring the pancreatic volume in this study may limit its routine use by both radiologists and surgeons. Hong et al. [13] studied the relation between the density of the pancreas and its texture and found that when the density is low, the pancreas is fatty and soft, and in turn, the leakage rate is higher.
In our study, we found that a larger tumor size on the pathology specimen is associated with atrophic pancreas in the preoperative imaging and in turn a decrease in leakage rate. We believe that the larger the tumor size, the more aggressive the local inflammatory response will be, and this leads to a chronic inflammatory response that eventually results in atrophy and fibrosis. Likewise, the inflammatory signals in the microenvironment level of pancreatic cancer have been described in multiple studies. These inflammatory signals may affect the environment of the tumor itself and lead to fibrosis [14,15]. Furthermore, lobular atrophy may be seen in pancreatic malignancies including adenocarcinoma, pancreatic intraepithelial neoplasia, and IPMN [16-18]. Another study has shown that pancreatic parenchymal atrophy on CT scans is one of the secondary signs of pancreatic cancer [19]. Nevertheless, all this data didn’t take into consideration the effect of the tumor size on the degree of pancreatic fibrosis or atrophy.
Our study had several limitations. First, although, a standardized surgical technique was used, four different hepatobiliary surgeons performed the procedures. Secondly, there were varying indications for PD, which may affect the pancreatic texture and affect the incidence of leakage. Third, there were no intraoperative descriptions of the pancreas texture. A prospective study including preoperative imaging, intraoperative findings, and the pathological finding is necessary to validate our findings.
In conclusion, atrophic pancreas as shown in the preoperative CT scan may be protective against leakage after PD. These findings may help surgeons to risk stratify patients accordingly. In addition, the findings suggest that patients with a preserved pancreas may require more protective methods to prevent leakage.
REFERENCES
1. Pallisera A, Morales R, Ramia JM. 2014; Tricks and tips in pancreatoduodenectomy. World J Gastrointest Oncol. 6:344–350. DOI: 10.4251/wjgo.v6.i9.344. PMID: 25232459. PMCID: PMC4163732.

2. Whipple AO. 1945; Pancreaticoduodenectomy for islet carcinoma: a five-year follow-up. Ann Surg. 121:847–852. DOI: 10.1097/00000658-194506000-00008. PMID: 17858621. PMCID: PMC1618156.
3. Cameron JL, Pitt HA, Yeo CJ, Lillemoe KD, Kaufman HS, Coleman J. 1993; One hundred and forty-five consecutive pancreaticoduodenectomies without mortality. Ann Surg. 217:430–435. discussion 435–438. DOI: 10.1097/00000658-199305010-00002. PMID: 8098202. PMCID: PMC1242815.

4. Trede M, Schwall G. 1988; The complications of pancreatectomy. Ann Surg. 207:39–47. DOI: 10.1097/00000658-198801000-00009. PMID: 3276272. PMCID: PMC1493257.

5. Strasberg SM, Drebin JA, Soper NJ. 1997; Evolution and current status of the Whipple procedure: an update for gastroenterologists. Gastroenterology. 113:983–994. DOI: 10.1016/S0016-5085(97)70195-1. PMID: 9287993.

6. Yang YM, Tian XD, Zhuang Y, Wang WM, Wan YL, Huang YT. 2005; Risk factors of pancreatic leakage after pancreaticoduodenectomy. World J Gastroenterol. 11:2456–2461. DOI: 10.3748/wjg.v11.i16.2456. PMID: 15832417. PMCID: PMC4305634.

7. Mathur A, Pitt HA, Marine M, Saxena R, Schmidt CM, Howard TJ, et al. 2007; Fatty pancreas: a factor in postoperative pancreatic fistula. Ann Surg. 246:1058–1064. DOI: 10.1097/SLA.0b013e31814a6906. PMID: 18043111.
8. Bartholomew LG, Baggenstoss AH, Morlock CG, Comfort MW. 1959; Primary atrophy and lipomatosis of the pancreas. Gastroenterology. 36:563–572. DOI: 10.1016/S0016-5085(59)80023-8. PMID: 13653276.

9. Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. 2005; Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 138:8–13. DOI: 10.1016/j.surg.2005.05.001. PMID: 16003309.

10. Felix K, Schuck A, Gaida MM, Hinz U, Dovzhanskiy D, Werner J. 2014; Objective parameters aid the prediction of fistulas in pancreatic surgery. Exp Ther Med. 8:719–726. DOI: 10.3892/etm.2014.1829. PMID: 25120588. PMCID: PMC4113644.

11. Kim Z, Kim MJ, Kim JH, Jin SY, Kim YB, Seo D, et al. 2009; Prediction of post-operative pancreatic fistula in pancreaticoduodenectomy patients using pre-operative MRI: a pilot study. HPB (Oxford). 11:215–221. DOI: 10.1111/j.1477-2574.2009.00011.x. PMID: 19590650. PMCID: PMC2697900.

12. Frozanpor F, Loizou L, Ansorge C, Lundell L, Albiin N, Segersvärd R. 2014; Correlation between preoperative imaging and intraoperative risk assessment in the prediction of postoperative pancreatic fistula following pancreatoduodenectomy. World J Surg. 38:2422–2429. DOI: 10.1007/s00268-014-2556-5. PMID: 24711156.

13. Hong W, Ha HI, Lee JW, Lee SM, Kim MJ. 2019; Measurement of pancreatic fat fraction by CT histogram analysis to predict pancreatic fistula after pancreaticoduodenectomy. Korean J Radiol. 20:599–608. DOI: 10.3348/kjr.2018.0557. PMID: 30887742. PMCID: PMC6424834.

14. Yadav D, Lowenfels AB. 2013; The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 144:1252–1261. DOI: 10.1053/j.gastro.2013.01.068. PMID: 23622135. PMCID: PMC3662544.

15. Hausmann S, Kong B, Michalski C, Erkan M, Friess H. 2014; The role of inflammation in pancreatic cancer. Adv Exp Med Biol. 816:129–151. DOI: 10.1007/978-3-0348-0837-8_6. PMID: 24818722.

16. Meckler KA, Brentnall TA, Haggitt RC, Crispin D, Byrd DR, Kimmey MB, et al. 2001; Familial fibrocystic pancreatic atrophy with endocrine cell hyperplasia and pancreatic carcinoma. Am J Surg Pathol. 25:1047–1053. DOI: 10.1097/00000478-200108000-00009. PMID: 11474289.

17. Del Chiaro M, Segersvärd R, Lohr M, Verbeke C. 2014; Early detection and prevention of pancreatic cancer: is it really possible today? World J Gastroenterol. 20:12118–12131. DOI: 10.3748/wjg.v20.i34.12118. PMID: 25232247. PMCID: PMC4161798.

18. Hruban RH, Maitra A, Goggins M. 2008; Update on pancreatic intraepithelial neoplasia. Int J Clin Exp Pathol. 1:306–316. DOI: 10.1007/978-0-387-69252-4_3. PMID: 18787611. PMCID: PMC2480542.
19. Chu LC, Goggins MG, Fishman EK. 2017; Diagnosis and detection of pancreatic cancer. Cancer J. 23:333–342. DOI: 10.1097/PPO.0000000000000290. PMID: 29189329.

Fig. 1
Axial computed tomography shows intact pancreatic architecture (A) and axial tomography (B) showed atrophic pancreas.
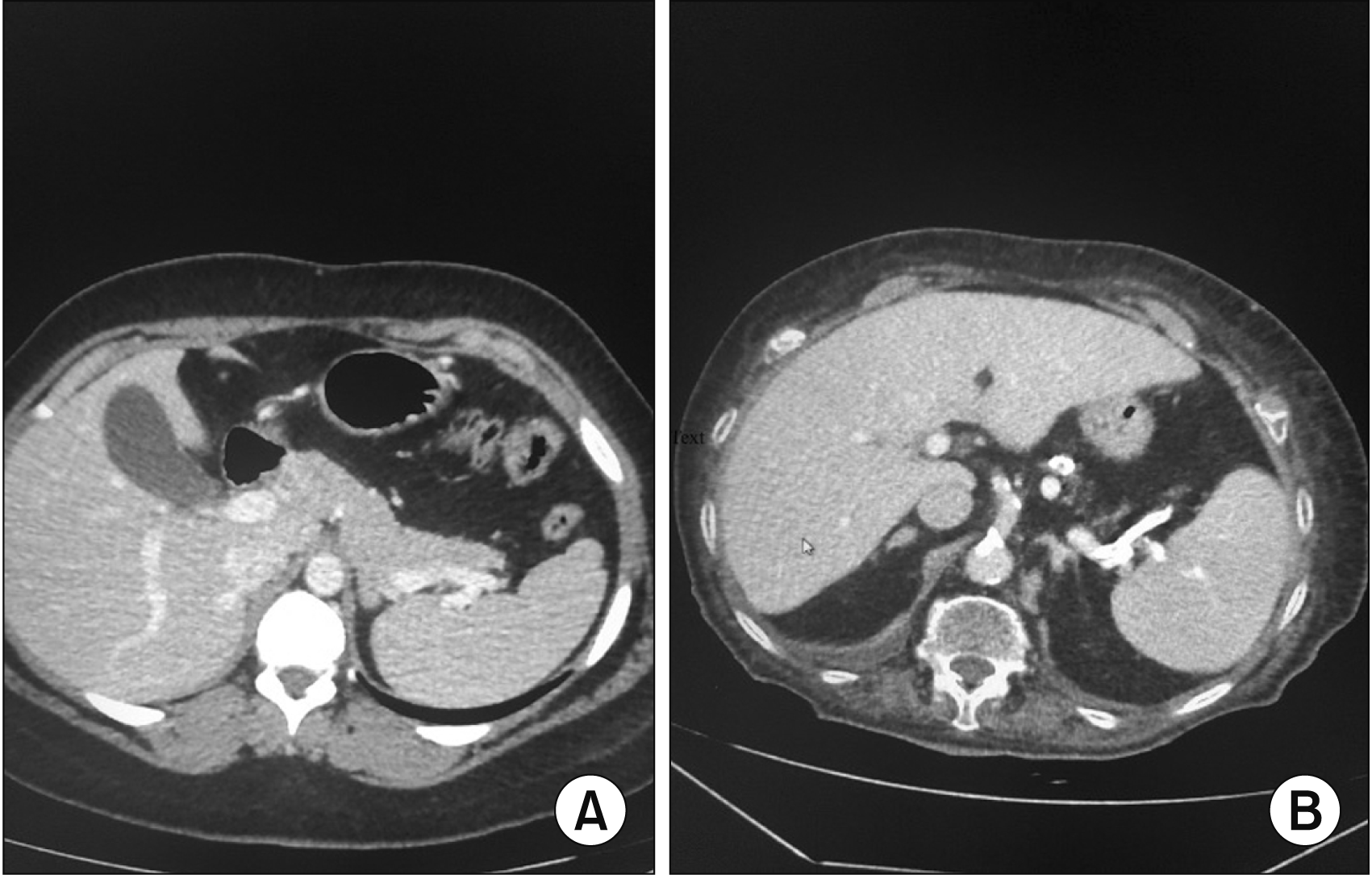
Table 1
Clinico-pathologic features and tumor stage of the study population
Table 2
A comparison of sex, tumor diameter, and appearance of a pancreatic leak in atrophic and intact pancreatic architecture
| Characteristic | Total | Pancreatic architecture | p-value | |
|---|---|---|---|---|
|
|
||||
| Intact | Atrophy | |||
| No. of cases | 154 | 103 (66.9) | 51 (33.1) | |
| Mean tumor diameter (cm) | 3.08 ± 1.5 | 3.52 ± 1.32 | 0.04a) | |
| Mean age (yr) | 61.8 | 61.65 ± 10.9 | 62.1 ± 9.2 | 0.87a) |
| Male | 89 (57.8) | 57 (64.0) | 32 (36.0) | 0.38b) |
| Female | 65 (42.2) | 46 (70.8) | 19 (29.2) | |
| Total no. (% within sex) | 103 (66.9) | 51 (33.1) | ||
| Pancreatic leak | ||||
| No leak | 120 (77.9) | 69 (67.0) | 51 (100) | ≤ 0.00001b) |
| Leak | 34 (22.1) | 34 (32.0) | 0 (0) | |
Table 3
Multivariate analysis of clinical factors associated with a pancreatic leak*
| Variable | Number | Odd ratio | 95% confidence interval | p-value |
|---|---|---|---|---|
| Sex | ||||
| Male | 112 | 1 | - | - |
| Female | 83 | 1.03 | 0.52–2.05 | 0.92 |
| Maximal diameter | 195 | 0.83 | 0.67–1.03 | 0.09 |
| Age | 195 | 1.02 | 0.99–1.06 | 0.25 |
Table 4
The relation between pancreatic architecture as shown in preoperative computed tomography and the incidence of a pancreatic leak for each sex
| Variable | No leak | Leak | p-value* |
|---|---|---|---|
| Male | |||
| Intact | 40 (70.2) | 17 (29.8) | ≤ 0.0001 |
| Atrophic | 32 (100) | 0 (0) | |
| Total | 72 (80.9) | 17 (19.1) | |
| Female | |||
| Intact | 29 (63.0) | 17 (37.0) | 0.002 |
| Atrophic | 18 (100) | 0 (0) | |
| Total | 47 (73.4) | 17 (26.6) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download