Abstract
Backgrounds/Aims
Splenectomy in patients with non-Hodgkin lymphoma (NHL) is performed to relieve abdominal symptoms, treat hypersplenism or confirm diagnosis. Excision of a very large spleen is technically challenging and data on outcomes of surgery in patients with NHL are scanty. The aim of study was to evaluate the impact of spleen size on the surgical outcome of splenectomy in patients with NHL.
Methods
Patients with NHL who underwent splenectomy, between 2006 and 2017, were included and divided into two groups: group 1, spleen ≤ 20 cm; group 2, spleen > 20 cm. Surgical approach, operative time, postoperative morbidity, mortality, hospital stay and re-admission rates were retrospectively compared between groups. Non-parametric data were evaluated with the Mann-Whitney U test. Differences in frequencies were analyzed with Fisher’s exact test.
Results
Sixteen patients were included (group 1, 6; group 2, 10). Laparoscopy was successful in three patients of group 1, none of group 2 (p = 0.035), the intraoperative time did not differ significantly between groups. One patient in each group developed postoperative complications. The patient in group 1 died of pneumonia. Median length of stay was 8 days (range, 3-16 days) for group 1, 5.5 days (range, 3-10 days) for group 2, showing no significant difference between the two groups. No patient was readmitted to hospital.
Splenectomy in patients with non-Hodgkin lymphoma (NHL) is indicated to relieve abdominal symptoms caused by splenomegaly, treat hypersplenism, and confirm diagnosis when other tests are inconclusive. When the disease is confined to the spleen, surgery might be considered as a curative treatment [1-3]. The procedure is deemed as high-risk due to patients’ impaired baseline hematologic reserve [4], occurrence of postoperative infections [5], and thrombotic events [6]. In the past two decades, advances in perioperative care, anaesthesia, and surgical equipment including the advent of the mini-invasive approach have led to improved postoperative outcomes [7]. Nevertheless, the excision of a very large spleen is known to be technically challenging [8]. Data on outcomes of surgery in patients with NHL are scanty.
Thus, the aim of this study was to evaluate the impact of spleen size on surgical outcome of splenectomy in patients with NHL.
The Royal Devon and Exeter NHS Foundation Trust institutional board approval was obtained for this study (approval number 19-4366). All data were fully anonymized at the time of collection. Therefore, individual informed consent was not required.
At the Royal Devon and Exeter NHS Foundation Trust, the spleen is evaluated preoperatively with computed tomography (CT) scan of the abdomen. Whenever possible, laparoscopy is attempted as the approach of choice for elective splenectomy irrespective of the spleen size. Open surgery is performed when preoperative imaging shows lymphadenopathy and/or a nodular pattern of the splenic parenchyma at the hilum that might preclude a safe access to splenic vessels. Conversion to open is undertaken when it is not possible to mobilize the spleen due to a large size and heavy weight or in case of severe intraoperative haemorrhage (uncontrollable or inaccessible pooling or spurting with visually estimated blood loss > 10 mL/minute) [9].
Vaccination against Pneumococcus, Meningococcus, and type B Hemophilus influenzae is given two weeks before surgery. When laparoscopy is chosen, the procedure is carried out through a standard 4 trocars approach, with the patient positioned in the right lateral decubitus. The lieno-colic ligament and the short gastric vessels are divided with a harmonic scalpel and hilar vessels are divided with a stapling device. The spleen is morcellated inside the retrieving bag before being delivered out. When open surgery is considered, access is warranted via a midline laparotomy or left upper quadrant oblique incision depending on surgeon’s personal preference. Splenic vessels at the hilum are divided with a stapling device or between ligatures.
All patients are discharged on life-long oral antibiotics (penicillin G) and anti-platelet agents.
Patients who underwent elective splenectomy between 2006 and 2017 were considered for this study. Cases were identified from the hospital information center database. Exclusion criteria were: age under 18 years, surgery for splenic cysts or in the context of en-bloc resections of gastric and pancreatic tumors, and hematologic conditions other than NHL.
Subjects were divided into two groups based on the maximal longitudinal length of the spleen at preoperative abdominal CT scan: group 1, ≤ 20 cm; and group 2, > 20 cm. Demographics, American Society of Anesthesiologists (ASA) score, preoperative serum hemoglobin (Hb) and platelets (PLT) levels, surgical approach (open and laparoscopic), operative time, postoperative morbidity, mortality, hospital stay, and re-admission rates were then retrospectively compared between the two groups. Postoperative morbidity was graded according to the Clavien-Dindo Classification [10]. Postoperative complications, mortality, and re-admission rates were considered when they occurred within 30 days after surgery.
Continuous variables with a normal distribution were described using mean and range values. Data without a normal distribution were given as median, range, and 95% confidence interval (CI). Non-parametric data were evaluated with the Mann–Whitney U test. Differences in frequencies were compared with Fisher’s exact test. Results were considered statistically significant when p value was less than 0.05.
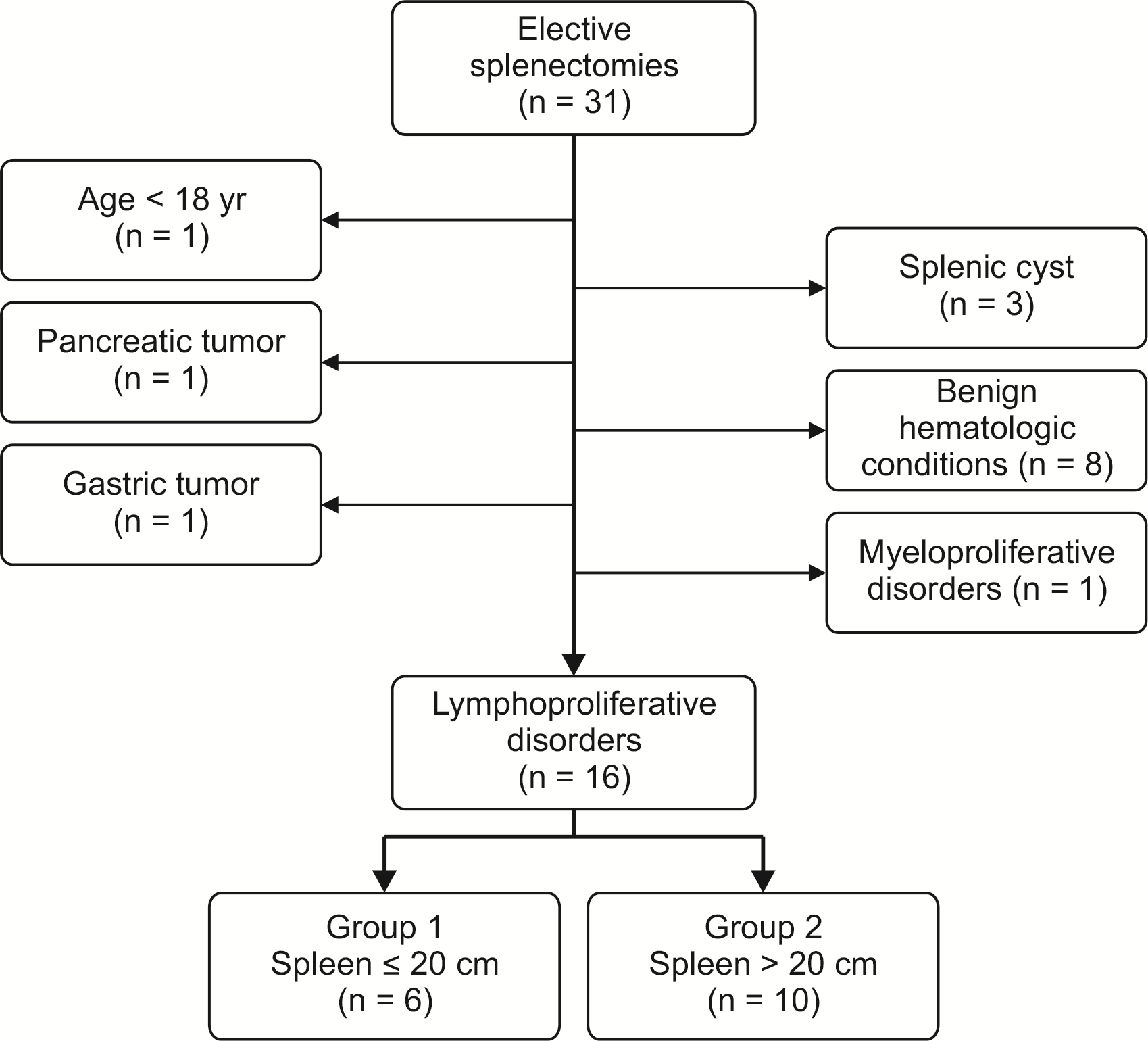
Overall, 31 patients underwent splenectomy. Fifteen were excluded because of age < 18 years (n = 1), splenic cyst (n = 3), gastro-pancreatic tumor (n = 2), or hematologic conditions other than NHL (n = 9). Eventually, 16 patients were included in this study (6 in group 1 and 10 in group 2) (Fig. 1). Subjects’ characteristics and perioperative data are described in Table 1.
Age, sex, ASA score, preoperative Hb level, and PLT count, did not differ significantly between the two groups. None of these patients underwent bone marrow transplant. No patient was in clinical remission prior to surgery. Indications of splenectomy were hypersplenism (n = 11), need for confirmation of diagnosis on histopathological examination (n = 3), and uncontrollable abdominal symptoms—pain and bloating in the left upper quadrant and flank (n = 2).
Three patients in group 1 underwent laparoscopy. In group 2, laparoscopy was attempted in three cases who were converted to open because of inability to mobilize the spleen (n = 2) or intraoperative haemorrhage (n = 1). In the first case (inability to mobilize the spleen), both patients had a weighty, bulky spleen surrounded by dense adhesions with the spleno-renal ligament and the diaphragm.
Intraoperative bleeding occurred in three cases (1 in group 1 and 2 in group 2). The patient in group 1 underwent open surgery with marked hilar lymphadenopathy which resulted in challenging vascular dissection (estimated blood loss: 400 mL). In group 2, one patient had an open surgery with spleen measured 36 cm. The vascular access to the splenic hilum was difficult because of a narrow space (estimated blood loss: 333 mL). The second patient underwent laparoscopic access with spleen measured 28 cm. Bleeding occurred after vascular division at the splenic hilum, which prompted conversion to open (estimated blood loss: 900 mL).
In group 1, the median intraoperative time was 120 minutes (95% CI: 84.7–164 minutes) in the laparoscopic group and 115 minutes (95% CI: 36.6–201.4 minutes) in the open one (p > 0.999). Among those who underwent open splenectomy, the median duration of surgery was 115 minutes (95% CI: 36.6–201.4 minutes) in group 1 and 104 minutes (95% CI: 79.9–153.8 minutes) in group 2 (p = 0.9185).
None of the patients who underwent laparoscopic splenectomy developed postoperative complications. Among those who had an open surgery, morbidity occurred in one case in each group. In group 1, one patient died of pneumonia at 16 days after surgery (Clavien-Dindo V). In group 2, one developed pleural effusion that did not require treatment (Clavien-Dindo I).
In group 1, the median length of stay was 4 days (95% CI: -0.5–9.8 days) after a laparoscopic surgery and 8 days (95% CI: -7.3–25.3 days) after an open approach (p = 0.7414). Among those who underwent an open splenectomy, median hospital stay was longer in group 1 than in group 2. However, the difference between the two was not statistically significant (p = 0.665): 8 days (95% CI: -7.3–25.3 days) vs. 5.5 days (95% CI: 4.6–7.8 days). No patient was readmitted to the hospital.
Surgical excision of an enlarged spleen in the context of hematologic diseases represents a challenge. This is because the organ is congested and fragile. It often adheres to surrounding tissues, making it difficult to mobilize [11]. Moreover, the presence of lymphadenopathy and/or parenchymal nodularity or hypertrophy at the hilum might result in a narrow access to the splenic artery and vein, thus increasing the risk of inadvertent iatrogenic vascular injury [12,13]. Such conditions become even more relevant if a laparoscopic approach is chosen as spleen mobilization and vascular control can be limited by the small room inside the peritoneal cavity. In most published series, the spleen is described as “giant” or “massive” based on its weight [11,14-16], while a few reports make such definitions based on its size [13,17]. Moreover, cut-off measurements to make such definitions vary greatly among authors. In the present study, spleen weight was not considered since specimens removed laparoscopically were morcellated, making it impossible to accurately weigh them. Data on how to best approach an enlarged spleen are scanty. In the review by Klingler et al. [18], laparoscopic splenectomy was not recommended for patients with organs > 20 cm. Guidelines published by the European Association of Endoscopic Surgery also suggest that open or hand assisted surgery should be considered if spleen size > 20 cm [19]. Nevertheless, successful laparoscopic excision of spleens > 20 cm has been reported by other authors [17].
In our series, laparoscopic splenectomy was feasible in patients with spleen ≤ 20 cm. In group 2, the mini-invasive approach was not possible because of the lack of space and adhesions within the peritoneal cavity, making it difficult to mobilize the organ and access the splenic hilum. Published studies have reported the correlation between enlarged spleen and conversion to open [20-23]. Our results revealed that three patients had intraoperative bleeding. In all three cases, the intraoperative bleeding was secondary to difficult access to the splenic hilum and inadequate vascular control. The correlation between large spleen size and increased risk of intraoperative bleeding during splenectomy has been reported in the published literature [24-25].
In this series, comparison of patients who underwent open splenectomy depending on spleen size did not show significant difference in intraoperative time, rate of postoperative complications, or length of stay. One patient in group 1 died of hospital-acquired pneumonia. Results from the study of Lemaire et al. [11] seem to confirm good outcomes after excision of massive spleen. However, their series included patients with a more heterogeneous spectrum of underlying hematological conditions.
This study is biased due to its small sample size and the retrospective nature. Therefore, definitive conclusions could not be drawn. Within these limitations, the authors conclude that spleen size does not affect the outcome of splenectomy in patients with NHL. If a mini-invasive approach is chosen, laparoscopic splenectomy might not be feasible when the spleen size is > 20 cm.
REFERENCES
1. Xiros N, Economopoulos T, Christodoulidis C, Dervenoulas J, Papageorgiou E, Mellou S, et al. 2000; Splenectomy in patients with malignant non-Hodgkin's lymphoma. Eur J Haematol. 64:145–150. DOI: 10.1034/j.1600-0609.2000.90079.x. PMID: 10997879.

2. Brodsky J, Abcar A, Styler M. 1996; Splenectomy for non-Hodgkin's lymphoma. Am J Clin Oncol. 19:558–561. DOI: 10.1097/00000421-199612000-00004. PMID: 8931670.

3. Walsh RM, Heniford BT. 1999; Laparoscopic splenectomy for non-Hodgkin lymphoma. J Surg Oncol. 70:116–121. DOI: 10.1002/(SICI)1096-9098(199902)70:2<116::AID-JSO10>3.0.CO;2-Y. PMID: 10084655.

4. Horowitz J, Smith JL, Weber TK, Rodriguez-Bigas MA, Petrelli NJ. 1996; Postoperative complications after splenectomy for hematologic malignancies. Ann Surg. 223:290–296. DOI: 10.1097/00000658-199603000-00010. PMID: 8604910. PMCID: PMC1235118.

5. Edgren G, Almqvist R, Hartman M, Utter GH. 2014; Splenectomy and the risk of sepsis: a population-based cohort study. Ann Surg. 260:1081–1087. DOI: 10.1097/SLA.0000000000000439. PMID: 24374533.
6. Bickenbach KA, Gonen M, Labow DM, Strong V, Heaney ML, Zelenetz AD, et al. 2013; Indications for and efficacy of splenectomy for haematological disorders. Br J Surg. 100:794–800. DOI: 10.1002/bjs.9067. PMID: 23436638.

7. Balagué C, Targarona EM, Cerdán G, Novell J, Montero O, Bendahan G, et al. 2004; Long-term outcome after laparoscopic splenectomy related to hematologic diagnosis. Surg Endosc. 18:1283–1287. DOI: 10.1007/s00464-003-9092-y. PMID: 15457387.

8. Uranues S, Alimoglu O. 2005; Laparoscopic surgery of the spleen. Surg Clin North Am. 85:75–90. ixDOI: 10.1016/j.suc.2004.09.003. PMID: 15619530.

9. Lewis KM, Li Q, Jones DS, Corrales JD, Du H, Spiess PE, et al. 2017; Development and validation of an intraoperative bleeding severity scale for use in clinical studies of hemostatic agents. Surgery. 161:771–781. DOI: 10.1016/j.surg.2016.09.022. PMID: 27839931.

10. Dindo D, Demartines N, Clavien PA. 2004; Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 240:205–213. DOI: 10.1097/01.sla.0000133083.54934.ae. PMID: 15273542. PMCID: PMC1360123.
11. Lemaire J, Rosière A, Bertrand C, Bihin B, Donckier JE, Michel LA. 2017; Surgery for massive splenomegaly. BJS Open. 1:11–17. DOI: 10.1002/bjs5.1. PMID: 29951600. PMCID: PMC5989945.

12. Saboo SS, Krajewski KM, O'Regan KN, Giardino A, Brown JR, Ramaiya N, et al. 2012; Spleen in haematological malignancies: spectrum of imaging findings. Br J Radiol. 85:81–92. DOI: 10.1259/bjr/31542964. PMID: 22096219. PMCID: PMC3473934.

13. Grahn SW, Alvarez J 3rd, Kirkwood K. 2006; Trends in laparoscopic splenectomy for massive splenomegaly. Arch Surg. 141:755–761. DOI: 10.1001/archsurg.141.8.755. PMID: 16924082.

14. Targarona EM, Balagué C, Trías M. 2002; Laparoscopic splenectomy for splenomegaly. Probl Gen Surg. 19:58–64. DOI: 10.1097/00013452-200209000-00010. PMID: 30720697.

15. Delpero JR, Houvenaeghel G, Gastaut JA, Orsoni P, Blache JL, Guerinel G, et al. 1990; Splenectomy for hypersplenism in chronic lymphocytic leukaemia and malignant non-Hodgkin's lymphoma. Br J Surg. 77:443–449. DOI: 10.1002/bjs.1800770427. PMID: 2340397.

16. Walsh RM, Brody F, Brown N. 2004; Laparoscopic splenectomy for lymphoproliferative disease. Surg Endosc. 18:272–275. DOI: 10.1007/s00464-003-8916-0. PMID: 14691699.

17. Tsamalaidze L, Stauffer JA, Permenter SL, Asbun HJ. 2017; Laparoscopic splenectomy for massive splenomegaly: does size matter? J Laparoendosc Adv Surg Tech A. 27:1009–1014. DOI: 10.1089/lap.2017.0384. PMID: 28799827.

18. Klingler PJ, Tsiotos GG, Glaser KS, Hinder RA. 1999; Laparoscopic splenectomy: evolution and current status. Surg Laparosc Endosc. 9:1–8. DOI: 10.1097/00019509-199901000-00001. PMID: 9950119.
19. Habermalz B, Sauerland S, Decker G, Delaitre B, Gigot JF, Leandros E, et al. 2008; Laparoscopic splenectomy: the clinical practice guidelines of the European Association for Endoscopic Surgery (EAES). Surg Endosc. 22:821–848. DOI: 10.1007/s00464-007-9735-5. PMID: 18293036.

20. Knauer EM, Ailawadi G, Yahanda A, Obermeyer RJ, Millie MP, Ojeda H, et al. 2003; 101 laparoscopic splenectomies for the treatment of benign and malignant hematologic disorders. Am J Surg. 186:500–504. DOI: 10.1016/j.amjsurg.2003.07.026. PMID: 14599614.

21. Bagrodia N, Button AM, Spanheimer PM, Belding-Schmitt ME, Rosenstein LJ, Mezhir JJ. 2014; Morbidity and mortality following elective splenectomy for benign and malignant hematologic conditions: analysis of the American College of Surgeons National Surgical Quality Improvement Program data. JAMA Surg. 149:1022–1029. DOI: 10.1001/jamasurg.2014.285. PMID: 25143027.

22. Kavic SM, Segan RD, Park AE. 2005; Laparoscopic splenectomy in the elderly: a morbid procedure? Surg Endosc. 19:1561–1564. DOI: 10.1007/s00464-005-0125-6. PMID: 16189722.

23. Targarona EM, Espert JJ, Cerdán G, Balagué C, Piulachs J, Sugrañes G, et al. 1999; Effect of spleen size on splenectomy outcome. A comparison of open and laparoscopic surgery. Surg Endosc. 13:559–562. DOI: 10.1007/s004649901040. PMID: 10347290.
24. Ohta M, Nishizaki T, Matsumoto T, Shimabukuro R, Sasaki A, Shibata K, et al. 2005; Analysis of risk factors for massive intraoperative bleeding during laparoscopic splenectomy. J Hepatobiliary Pancreat Surg. 12:433–437. DOI: 10.1007/s00534-005-1027-7. PMID: 16365814.

25. Wysocki M, Radkowiak D, Zychowicz A, Rubinkiewicz M, Kulawik J, Major P, et al. 2018; Prediction of technical difficulties in laparoscopic splenectomy and analysis of risk factors for postoperative complications in 468 cases. J Clin Med. 7:547. DOI: 10.3390/jcm7120547. PMID: 30558132. PMCID: PMC6306709.

Table 1
Patients’ characteristics
| Characteristic | Group 1 (n = 6) | Group 2 (n = 10) | p-value |
|---|---|---|---|
| Male : female (n) | 2 : 4 | 4 : 6 | > 0.999 |
| Mean age (range), yr | 66.2 (52–82) | 64.5 (51–75) | 0.7394 |
| Median ASA (range) | 1.5 (1–2) | 1 (1–2) | 0.3575 |
| Indication for surgery (n) | |||
| Hypersplenism | 4 | 7 | - |
| Abdominal symptoms | 1 | 1 | |
| To clarify histologic type | 1 | 2 | |
| Preoperative serum Hb (range), g/dL | 11.8 (8–13) | 12 (7.1–14.7) | 0.9578 |
| Preoperative PLT count (range), ×103/mL | 112.5 (51–184) | 116.5 (37–326) | 0.6642 |
| Median spleen size (range), cm | 18.7 (16–20) | 25.3 (21–45) | - |
| Access (n) | |||
| Laparoscopy | 3 | - | 0.035* |
| Conversion | - | 3 | |
| Open | 3 | 7 | |
| Median intraoperative time (range), min | 117.5 (88–154) | 116.5 (77–191) | |
| Laparoscopy | 120 | - | |
| Open | 115 | 104 | 0.9185 |
| Converted | - | 137 | |
| Intraoperative haemorrhage (n) | 1 | 2 | - |
| Estimated blood loss, mL | 400 | 333/900 | |
| In-hospital mortality (n) | 1 | 0 | - |
| Postoperative complications (n) | 1 | 1 | - |
| Type | Pneumonia | Pleural effusion | |
| Clavien-Dindo | V | I | |
| Median length of stay (range), day | |||
| Laparoscopy | 4 (3–7) | - | |
| Open | 8 (3–16) | 5.5 (3–10) | 0.665 |
| Readmission (n) | 0 | 0 | - |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download