Abstract
Backgrounds/Aims
Pancreaticoduodenectomy (PD) is a standard surgical procedure for patients with periampullary cancer. During the follow-up period after PD, recurrence can be observed in various places with different prognosis. The aim of this study was to clarify the pattern of recurrence and factors affecting the survival of patients with periampullary cancer.
Methods
Overall, 88 patients who received PD for distal common bile duct cancer or ampulla of Vater cancer were finally included and their clinical characteristics were analyzed. Patients were divided into three groups: recurrence-free (RF) group, an isolated locoregional recurrence (LR) group, and a distant metastasis (DM) group. Prognostic factors affecting recurrence in each group were analyzed and a survival analysis was performed.
Results
Perineural invasion (PNI), T stage, and lymphovascular invasion (LVI) were significant risk factors for LR and PNI, lymph node metastasis, LVI, and T stage were associated with DM group compared to RF group in univariate analysis, respectively. N stage and PNI were significant risk factors (p = 0.046, p = 0.041) in overall survival of the LR and the DM groups. There was no significant difference in 5-year overall survival between the LR and DM groups.
Periampullary cancer is a complex cancer that originates from adjacent structures. It can be treated with pancreaticoduodenectomy (PD) in resectable cases. However, clinical outcomes of these cancers are quite different. The recurrence rate is 28% to 42.5% in ampulla of Vater (AoV) cancers and 39% to 67% in distal common bile duct (CBD) cancers [1-6]. The 5-year survival of patients with AoV cancer is higher than that of those with distal CBD cancer [7-9].
When recurrence is found, some patients experience recurrence only within boundaries of the surgery, while distant metastasis is observed when surgeons recognize recurrence at the first time. Since recurrence has profound implications for survival of patients, it is important to predict recurrence patterns after PD. The aim of this study was to analyze recurrence patterns and prognostic factors affecting survival after PD. An optimal strategy for patients with distal CBD cancer and AoV cancer during PD is also suggested.
This study was approved by the Institutional Review Board of Yeungnam Universtiy Hospital (approval no. 2021-08-002). A total of 200 patients who received PD between 2005 and 2015 because of neoplasms of periampullary cancer at a single center were identified in the hospital database. Patients who were diagnosed with duodenal cancer or pancreatic head tumors were not included in this study. Exclusion criteria were (1) patients who underwent palliative surgery, (2) patients with combined malignancy, (3) patients who were diagnosed with benign disease at the final pathological report, (4) those with postoperative mortality (30 days), and (5) those who had a follow-up loss. A total 88 patients with pathologically confirmed adenocarcinoma for distal CBD cancer and AoV cancer were retrospectively reviewed, including 48 with distal CBD cancer and 40 with AoV cancer. Demographic characteristics, clinical presentation before surgery, tumor marker (carcinoembryonic antigen [CEA], carbohydrate antigen 19-9 [CA19-9]), preoperative biliary drainage procedures, pathological findings, recurrent patterns, and survival data were collected and analyzed for these patients.
The routine extent of lymphadenectomy was similar to that described by International Study Group of Pancreatic Surgery (ISGPS) [10]. The basic surgical strategy for PD included resection of the bile duct as proximal as possible near the hilar level. Lymph node #12 was dissected when the right and left hepatic arteries were fully skeletonized. Lymph node #8 was retrieved as much as possible. Surrounding soft tissues were dissected to the right side of the superior mesenteric artery (SMA) and the celiac artery (CA).
Postoperatively, physical examination, blood tests including tumor markers, and abdominal computed tomography were performed every 3 to 6 months for the first five years. If tumor marker increased or radiologic examination revealed doubtful recurred tumor lesion, further evaluation including positron emission tomography/computed tomography (PET/CT) was considered to detect recurrence. Patients in this study were divided into three groups: a recurrence-free group (RF group), an isolated locoregional recurrence group (LR group), and a distant metastasis group (DM group). Locoregional recurrence was defined as recurrence limited to the extent of PD, whereas distant metastasis was considered when recurrence was found beyond the regional structures of the operation site. Images were reviewed by surgeons to identify the location of recurrence in this study.
The overall survival (OS) was defined as the interval between the date of surgery and the date of death. Survival status was determined based on data from the Ministry of Public Administration and Security, Korea.
All statistical analyses were performed using IBM SPSS version 22.0 (IBM Corp., Armonk, NY, USA). Nominal data were compared using Pearson’s chi-square test and linear by linear association, while continuous data were analyzed using one-way ANOVA. Logistic regression analysis was used for multivariate analysis using factors that were found to be significant in the univariate analysis. Survival outcomes were estimated using the Kaplan–Meier method and compared using the log-rank test. p-values of < 0.05 were considered statistically significant.
Demographic and clinicopathologic factors of patients were collected and analyzed (Table 1). Among 88 patients, 48 patients underwent PD due to distal CBD cancer, while 40 patients underwent PD due to AoV cancer. The median age of patients at the time of surgery was 61 years. Seventy patients received preoperative biliary drainage for biliary decompression. With regard to T classification, 28, 16, 31, and 13 patients were classified as having T1, T2, T3, and T4 disease, respectively. Intraoperative lymph node positivity was noted in 38 patients. Well-, moderately-, and poorly-differentiated cells based on histological differentiation were noted in 14, 55, and 19 patients, respectively. Microscopic examination revealed perineural invasion in 43 patients and lymphovascular invasion in 58 patients.
During the follow-up period, 14 patients with locoregional recurrence, 32 patients with distant metastases, 42 patients without recurrence were found. When isolated LR and DM groups were compared, significantly higher rates of T stage, N stage, lymphovascular invasion, and perineural invasion were observed in the DM group. The medial value of preoperative CA19-9 was also higher in the DM group. Distal CBD cancer tended to metastasize to distant organs as compared to AoV cancer (p = 0.046). In addition, poorly differentiated cancers tended to be found in the DM group.
Locations of recurrence confirmed by imaging studies during the follow-up period were summarized. All recurrence sites were included in cases involving multiple sites at the time of the first recognition of recurrence (Table 2). The region around the SMA in AoV cancer and portocaval region in distal CBD cancer were the most common sites of recurrence in patients with isolated locoregional recurrence at the time surgeons found recurrence. The liver was the most common site of distant metastasis.
In analysis between RF and LR groups, there were significant differences in T stage, lymphovascular invasion, and perineural invasion in univariate analysis, while only T stage was a significant factor in multivariate analysis (Table 3). When the DM group was compared to the RF group, cancer type (distal CBD cancer > AoV cancer), T stage, lymph node metastasis, lymphovascular invasion, and perineural invasion were significant factors in univariate analysis, while perineural invasion remained a significant factor in multivariate analysis (Table 4).
Among patients with recurrence, 21 patients had palliative chemotherapy, including gemcitabine (19 patients) and 5-fluorouracil (2 patients). Three patients had radiotherapy. Two patients had operations to remove recurred lesions. Others received conservative treatment which alleviated patients’ symptoms or billiary drainage.
There was no significant difference in 5-year overall survival according to the recurrence pattern: 89.8% in the RF group, 21.4% in the LR group, and 15.6% in the DM group (Fig. 1). With regard to cancer type, patients with AoV cancer showed better survival than those with distal CBD cancer (Fig. 2).
Several studies have investigated recurrence and prognostic factors of AoV cancer and distal CBD cancer. A number of studies have reported that patients with positive lymph node, lymphovascular invasion, perineural invasion, and higher medial value of CA19-9 have higher recurrence rates [1,2,4,7,11-14]. In this study, perineural invasion and lymphovasuclar invasion were significant risk factors of both LR and DM group. Positive lymph node was a significant risk factor of recurrence in the DM group in the present study.
In this study, no significant survival benefit was found for the LR group compared with the DM group. This finding was different from that of Bhandare’s study [15] showing that isolated locoregional recurrence was associated with better prognosis than distant recurrence. The prominent difference between our study and Bhandare’s study was the most common site of locoregional recurrence site. In Bhandare’s study, the region around the SMA was the most common site of isolated locoregional recurrence. However, we found that both SMA nodal regions in AoV cancer and the region near the hepatic hilum in distal CBD cancer were common sites of isolated locoregional recurrence. In both centers, soft tissues at the right site of the SMA were dissected as a routine lymphadenectomy. Therefore, there was no link between the extent of lymphadenectomy and results. Such difference might be due to the fact that R1 resection was often found in the final pathological report in some distal CBD cancer cases, although no tumor cells at the resection margin were found in intraoperative frozen biopsy. Efforts to secure a negative resection margin on the bile duct and to have wide excision around hepatoduodenal ligament might be needed to improve survival of patients with distal CBD cancer and locoregional recurrence.
Several studies have reported which node around the SMA (#14) as a common site of nodal metastasis [5,15-17]. Among five nodal recurrence cases of AoV cancer, two cases (40.0%) showed recurrence on the left side of the SMA. In the study of Kim et al. [5] 10 of 17 nodal (58.8%) recurrence cases showed recurrence located in the left side of the SMA. This outcome suggests that radical lymph node dissection that encircles the SMA should be performed in carefully selected patients with a high-risk of AoV cancer recurrence [5].
This study revealed that distal CBD cancer was more associated with distal metastasis than AoV cancer. Survival analysis showed that distal CBD cancer had a poor survival rate. Although multivariable analysis failed to prove inferior prognosis of distal CBD cancer, a univariable study verified that more aggressive adjuvant therapy and closer follow-up examination after surgery should be given to patients diagnosed with distal CBD cancer.
This study has some limitations. First, the present study was a single institutional study that included less than 100 cases. Therefore, further multi-institutional studies with larger sample sizes are required in the future. In the 8th edition of AJCC, primary tumor (T) staging was changed from the extent of tumor to the depth of invasion for staging of distal bile duct cancer [18]. As the pathological report in our study did not describe the depth of invasion in our center, we could not analyze the relation between T stage and other prognostic factors based on the AJCC 8th edition. As updated descriptions of pathological reports are available these days, further studies will be needed to support new staging systems. In addition, the standard regimens of chemotherapy for these cancers have not been established at that time. Since many patients in this study were not able to receive chemotherapy due to poor performance status, we could not investigate the relationship between the regimen of adjuvant chemotherapy and oncologic prognosis. Therefore, further studies of adjuvant therapies to reduce recurrence in high-risk patients are needed.
In conclusion, T stage was a significant risk factor of locoregional recurrence, while perineural invasion was a significant risk factor of distant metastasis. There was no significant difference in overall survival depending on the site of recurrence. While both distal CBD cancer and AoV cancer were treated by PD, AoV cancer had a tendency to recur near the SMA nodal lesion in this study. Therefore, more aggressive strategy to dissect near the SMA will be needed to reduce locoregional recurrence in patients with advanced AoV cancer.
REFERENCES
1. Park JS, Yoon DS, Kim KS, Choi JS, Lee WJ, Chi HS, et al. 2007; Factors influencing recurrence after curative resection for ampulla of Vater carcinoma. J Surg Oncol. 95:286–290. DOI: 10.1002/jso.20665. PMID: 17326125.

2. Komaya K, Ebata T, Shirai K, Ohira S, Morofuji N, Akutagawa A, et al. 2017; Recurrence after resection with curative intent for distal cholangiocarcinoma. Br J Surg. 104:426–433. DOI: 10.1002/bjs.10452. PMID: 28138968.

3. Choi SB, Park SW, Kim KS, Choi JS, Lee WJ. 2009; The survival outcome and prognostic factors for middle and distal bile duct cancer following surgical resection. J Surg Oncol. 99:335–342. DOI: 10.1002/jso.21238. PMID: 19226533.

4. Woo SM, Ryu JK, Lee SH, Yoo JW, Park JK, Kim YT, et al. 2007; Recurrence and prognostic factors of ampullary carcinoma after radical resection: comparison with distal extrahepatic cholangiocarcinoma. Ann Surg Oncol. 14:3195–3201. DOI: 10.1245/s10434-007-9537-y. PMID: 17710498.

5. Kim H, Kwon W, Kim JR, Byun Y, Jang JY, Kim SW. 2019; Recurrence patterns after pancreaticoduodenectomy for ampullary cancer. J Hepatobiliary Pancreat Sci. 26:179–186. DOI: 10.1002/jhbp.618. PMID: 30849209.

6. Courtin-Tanguy L, Rayar M, Bergeat D, Merdrignac A, Harnoy Y, Boudjema K, et al. 2016; The true prognosis of resected distal cholangiocarcinoma. J Surg Oncol. 113:575–580. DOI: 10.1002/jso.24165. PMID: 26776934.

7. El Nakeeb A, El Sorogy M, Ezzat H, Said R, El Dosoky M, Abd El Gawad M, et al. 2018; Predictors of long-term survival after pancreaticoduodenectomy for peri-ampullary adenocarcinoma: a retrospective study of 5-year survivors. Hepatobiliary Pancreat Dis Int. 17:443–449. DOI: 10.1016/j.hbpd.2018.08.004. PMID: 30126828.

8. Yeo CJ, Sohn TA, Cameron JL, Hruban RH, Lillemoe KD, Pitt HA. 1998; Periampullary adenocarcinoma: analysis of 5-year survivors. Ann Surg. 227:821–831. DOI: 10.1097/00000658-199806000-00005. PMID: 9637545. PMCID: PMC1191384.
9. Howe JR, Klimstra DS, Moccia RD, Conlon KC, Brennan MF. 1998; Factors predictive of survival in ampullary carcinoma. Ann Surg. 228:87–94. DOI: 10.1097/00000658-199807000-00013. PMID: 9671071. PMCID: PMC1191432.

10. Tol JA, Gouma DJ, Bassi C, Dervenis C, Montorsi M, Adham M, et al. 2014; Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery. 156:591–600. DOI: 10.1016/j.surg.2014.06.016. PMID: 25061003.

11. Lee JH, Whittington R, Williams NN, Berry MF, Vaughn DJ, Haller DG, et al. 2000; Outcome of pancreaticoduodenectomy and impact of adjuvant therapy for ampullary carcinomas. Int J Radiat Oncol Biol Phys. 47:945–953. DOI: 10.1016/S0360-3016(00)00537-X. PMID: 10863064.

12. Kim RD, Kundhal PS, McGilvray ID, Cattral MS, Taylor B, Langer B, et al. 2006; Predictors of failure after pancreaticoduodenectomy for ampullary carcinoma. J Am Coll Surg. 202:112–119. DOI: 10.1016/j.jamcollsurg.2005.08.002. PMID: 16377504.

13. de Castro SM, Kuhlmann KF, van Heek NT, Busch OR, Offerhaus GJ, van Gulik TM, et al. 2004; Recurrent disease after microscopically radical (R0) resection of periampullary adenocarcinoma in patients without adjuvant therapy. J Gastrointest Surg. 8:775–784. discussion 784DOI: 10.1016/j.gassur.2004.08.006. PMID: 15531230.

14. van der Gaag NA, ten Kate FJ, Lagarde SM, Busch OR, van Gulik TM, Gouma DJ. 2008; Prognostic significance of extracapsular lymph node involvement in patients with adenocarcinoma of the ampulla of Vater. Br J Surg. 95:735–743. DOI: 10.1002/bjs.6076. PMID: 18300268.

15. Bhandare MS, Mondal A, Chaudhari V, Bal M, Yadav S, Ramaswamy A, et al. 2021; Factors influencing local and distant recurrence following resection of periampullary cancer. Br J Surg. 108:427–434. DOI: 10.1093/bjs/znaa143. PMID: 33723577.

16. Kayahara M, Nagakawa T, Ohta T, Kitagawa H, Miyazaki I. 1997; Surgical strategy for carcinoma of the papilla of Vater on the basis of lymphatic spread and mode of recurrence. Surgery. 121:611–617. DOI: 10.1016/S0039-6060(97)90048-9. PMID: 9186460.

17. Lee JH, Lee KG, Ha TK, Jun YJ, Paik SS, Park HK, et al. 2011; Pattern analysis of lymph node metastasis and the prognostic importance of number of metastatic nodes in ampullary adenocarcinoma. Am Surg. 77:322–329. DOI: 10.1177/000313481107700322. PMID: 21375845.

18. Edge SB, Byrd DR, Carducci MA, Compton CC, Fritz A, Greene F. 2010. AJCC cancer staging manual. 7th ed. Springer;New York: DOI: 10.1177/000313481107700322.
Fig. 2
Overall survival according to cancer type. dCBD, distal common bile duct; AoV, ampulla of Vater.
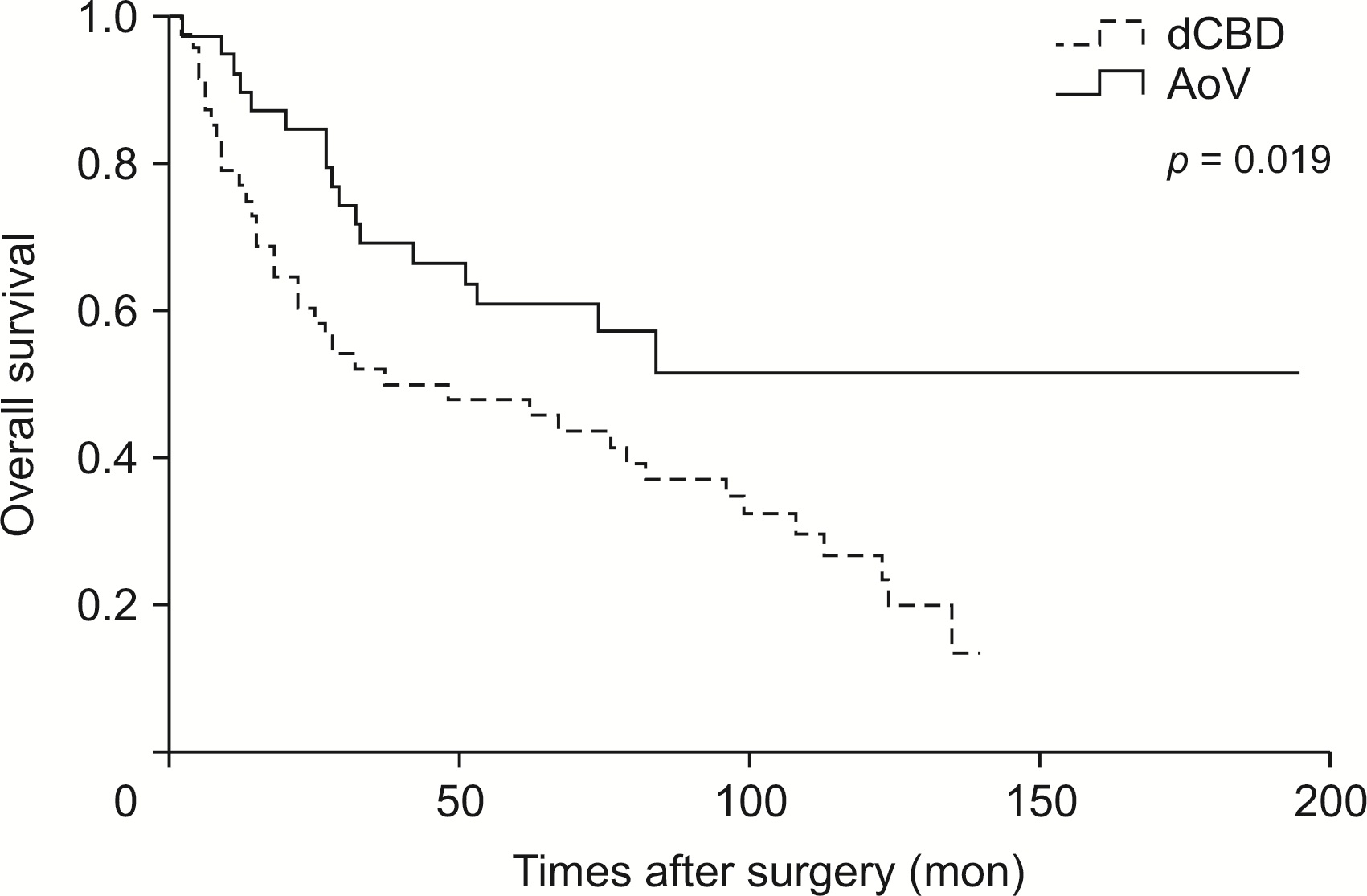
Table 1
Demographic and clinicopathologic factors of patients who underwent pancreaticoduodenectomy as primary treatment for periampullary cancer
| Characteristic | No recurrence (n = 42) | Isolated LR (n = 14) | DM (n = 32 ) | p-value |
|---|---|---|---|---|
| Mean age (yr) | 60.5 ± 8.6 | 62.2 ± 7.7 | 61.8 ± 10.1 | 0.162 |
| Sex | 0.642 | |||
| Male | 26 (61.9) | 10 (71.4) | 18 (56.3) | |
| Female | 16 (38.1) | 4 (28.6) | 14 (43.8) | |
| Preoperative CEA (ng/mL) | 2.68 (0.01–17) | 1.7 (0.01–5) | 2.7 (0.01–26) | 0.474 |
| Preoperative CA19-9 (U/mL) | 26.4 (1.92–100,000) | 25.6 (0–217) | 62.1 (0–1,000) | 0.023* |
| Preoperative biliary drainage | 31 (73.8) | 13 (92.9) | 26 (81.3) | 0.472 |
| Cancer type | 0.046* | |||
| Distal CBD cancer | 18 (42.9) | 7 (50.0) | 23 (71.9) | |
| AoV cancer | 24 (57.1) | 7 (50.0) | 9 (28.1) | |
| Adjuvant chemotherapy | 15 (35.7) | 7 (50.0) | 21 (65.7) | 0.02* |
| Differentiation | 0.045* | |||
| Well | 10 (23.8) | 2 (14.3) | 2 (6.3) | |
| Moderated | 25 (59.5) | 9 (64.3) | 21 (65.6) | |
| Poor | 7 (16.7) | 3 (21.4) | 9 (28.1) | |
| T stagea) | 0.016* | |||
| 1 | 18 (42.9) | 3 (21.4) | 7 (21.9) | |
| 2 | 11 (26.2) | 1 (7.1) | 4 (12.5) | |
| 3 | 8 (19.0) | 6 (42.9) | 17(53.1) | |
| 4 | 5 (11.9) | 4 (28.6) | 4 (12.5) | |
| N stagea) | 0.001*,b) | |||
| 0 | 31 (73.8) | 9 (64.3) | 10 (31.3) | |
| 1 | 11 (26.2) | 5 (35.7) | 22 (68.8) | |
| Lymphovascular invasion | 21 (50.0) | 10 (71.4) | 27 (84.4) | 0.008* |
| Perineural invasion | 10 (23.8) | 7 (50.0) | 26 (81.3) | < 0.001* |
Table 2
Site of recurrence at the first time of recognition
Table 3
Univariate and multivariate analyses of risk factors associated with isolated local recurrence
Table 4
Univariate and multivariate analyses of risk factors associated with distant metastasis




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download