Abstract
Backgrounds/Aims
It is generally accepted that non-anatomical resection (NAR) in colorectal liver metastasis (CRLM) has comparable safety and efficacy compared to anatomical resection (AR); however, there are reports that AR may have better outcomes in KRAS mutated CRLM. This study aimed to determine the effects of KRAS mutations and surgical techniques on survival outcomes in CRLM patients.
Methods
Two hundred fifty patients who underwent hepatic resection of CRLM with known KRAS mutational status between 2007 and 2018 were analyzed. A total of 94 KRAS mutated CRLM and 156 KRAS wild-type CRLM were subdivided by surgical approach and compared for short- and long-term outcomes.
Results
In both KRAS wild-type and mutated type, there was no difference in estimated blood loss, postoperative complications, and 30-day mortality. There was no difference in disease-free survival (DFS) between AR and NAR in both groups (p = 0.326, p = 0.954, respectively). Finally, there was no difference in intrahepatic DFS between AR and NAR groups in both the KRAS groups (p = 0.165, p = 0.516, respectively).
Colorectal liver metastases (CRLM) are a leading cause of cancer associated morbidity and mortality. Despite the oncological and surgical advances made, only about 25% of patients present with tumors that are amenable to surgical resection, which is regarded as the only way to achieve lasting cure [1].
To date, numerous biological markers such as RAS, BRAF, PIK3CA, TP53, SMAD4, and others have been reported to be associated with tumor aggressiveness and subsequent oncologic outcomes in patients with CRLM [2]. KRAS and NRAS mutations in particular, which are commonly screened for in clinical practice, have been associated with resistance to anti-EGFR (epidermal growth factor receptor) therapy and worse overall outcomes. Therefore, they are used as prognostic markers for predicting patterns of recurrence in patients with CRLM [3,4]. Besides these, RAS mutation has been associated with a trend toward positive surgical margins when performing resections for CRLM. Furthermore, unlike wild-type KRAS, RAS mutation has no survival benefit even with clear margins of 1 cm or more [5,6].
The potential oncological benefits of anatomical resection (AR) for CRLM have been in discussion for a long time; however, its benefits are not standardized when compared to AR for hepatocellular carcinoma [7,8]. Most reports found no difference in long-term oncologic outcomes in relation to the type of liver resection while others have stated that extensive liver resection may provide better outcomes when compared to non-anatomical resection (NAR) [7,9-13]. One report showed oncologic benefits when extensive anatomical hepatectomies were performed in KRAS mutation positive CRLM patients, and the authors suggested that such procedures may be necessary due to the generally observed highly aggressive tumors in such patients [13]. Currently, there is no standardized treatment strategy for synchronous CRLM, cases are best treated by multidisciplinary treatment teams including surgeons, medical oncologists, radiation oncologists, and radiologists. [14,15].
According to a recent RCT study, it is acceptable for patients presenting with colorectal cancer and synchronous resectable liver metastases to undergo simultaneous resection [16]. This study aimed to determine the effects of KRAS mutations and surgical techniques on long-term outcomes in synchronous CRLM patients.
Between September 2007 and December 2018, a total of 496 patients diagnosed with synchronous CRLM treated by curative resection at the Severance Hospital in Seoul, Korea, and available data on KRAS mutation status were identified. Patients undergoing only an ablative procedure without concurrent hepatic resection and those who underwent mixed AR and NAR were excluded from the final analysis. Patients with extrahepatic metastasis who underwent a staged operation, and those with an incomplete macroscopic resection (R2 resection) were also excluded from the study. This resulted in a final cohort of 250 patients meeting the criteria for analysis (Fig. 1). This study was approved by the Institutional Review Board of Yonsei University College of Medicine (registered on June 23rd, 2020, with registration number 2020-1392-001).
The extent and type of liver resection were mainly determined by the possibility of removing all tumors with a negative margin and leaving enough remnant volume for postoperative recovery. In addition, when the tumor was located in the deep liver parenchyma, AR was preferred only in case that enough remnant volume was guaranteed. AR was defined as resection of two or more complete segments as described by Couinaud, including sectionectomy, right hepatectomy, left hepatectomy, extended left hepatectomy, extended right hepatectomy or a combination of these according to previous studies (Table 1) [17,18]. When the tumor was located in the superficial liver parenchyma, NAR (wedge resection), defined as removing the tumor without regard to hepatic anatomy, was performed [13]. In addition, multiple tumors were removed by parenchymal preserving hepatectomy or multiple wedge resections in cases that not having enough remnant volume.
Detailed information including age, sex, site of primary colorectal cancer, primary tumor staging, and lymph node status were obtained. Data concerning preoperative factors were collected as well. Liver metastases were defined as synchronous when diagnosed before or at the time of colorectal resection. Tumor size was defined as the maximum diameter of the tumor in the resected specimen with the largest lesion used for reference in patients with multiple tumors. Detailed information is presented in Table 2.
The surgical approach (open, laparoscopy, and robotic) was determined by considering the case complexity in both colorectal surgery and liver surgery. This resulted in 336 patients (67.7%) undergoing conventional open surgery, 92 (18.5%) undergoing laparoscopic colorectal resection and open hepatectomy, and 67 (13.5%) undergoing minimally invasive colorectal and liver resection.
Minimally invasive liver resection procedure started in 2006. Case selection was dependent on tumor size of < 5 cm and no major vascular or other organ involvement as well as a favorable tumor location (segment 2, 3, 4, 5, and 6) and achievable R0 resection. As we gained experience, indications were expanded to include even unfavorable tumor locations (segments 1, 7, and 8). For robotic surgery, only patients requiring major liver resection were selected. Patients who did not agree to the robotic approach were offered simultaneous laparoscopic colorectal and open liver resection, or open colorectal and liver resection, as deemed appropriate. Surgical technique for robotic surgery adhered to those in previously published literature [19,20]. All surgical options were explained to each patient when obtaining informed consent.
Liver resection was mostly performed after colorectal surgery. AR was performed with individual ligation and resection of inflow vessels through hilar access and glissonean approach following the ischemic demarcation line. An intraoperative ultrasound facilitated the identification of resection margins in cases resected with an NAR approach. Parenchymal transection was performed using a bipolar coagulator and a Cavitron Ultrasonic Surgical Aspirator (CUSA; Valleylab, Boulder, CO, USA). The Pringle’s maneuver was not routinely used.
All statistical analyses were performed using SPSS Statistical software (version 25.0, IBM SPSS Statistics for Windows; IBM Corp., Armonk, NY, USA). Continuous variables were expressed as means ± standard deviations or ranges, and categorical variables were expressed as frequencies or percentages. Student’s t-test was used for comparing continuous variables. Chi-squared test and Fisher’s exact test were used for comparing categorical data. Survival estimates for the study population were generated using the Kaplan-Meier curve. A Cox proportional hazards regression model was generated to assess the association of several variables with disease-free survival (DFS) rates. Statistically significance was considered when p-value was less than 0.05.
A total of 250 patients were grouped according to KRAS mutational status and further divided into AR and NAR subgroups. Among them, 156 (62.4%) had KRAS wild-type tumors, while 94 (37.6%) had KRAS mutated tumors. The rates of AR and NAR were similar between the KRAS wild-type and mutated groups (32.70% vs. 29.80% AR; p = 0.675, Supplementary Table 1). Likewise, there were no significant differences in patient demographics, primary tumor characteristics, and preoperative factors between the AR and NAR groups in both KRAS mutational status groups. In regards to CRLM characteristics, the number of CRLM in both KRAS mutational groups was not significantly different between AR and NAR groups (2.61 ± 1.87 vs. 2.23 ± 2.10; p = 0.410). However, it was noted, that the AR subgroup tended to have significantly larger tumors than the NAR subgroup in both KRAS wild and KRAS mutated groups (p < 0.01). Also, in the KRAS wild-type group, patients who underwent a NAR were more likely to present with bilateral disease compared to the KRAS mutated group (p = 0.020, p = 0.443). Pathological examination found that the frequency of R1 was similar between the AR and NAR groups regardless of the KRAS mutation status (p = 0.597, p = 0.552). Adjuvant chemotherapy was administered to both AR and NAR groups at a similar rate with the regimen of adjuvant chemotherapy also being similar between the two groups, irrespective of KRAS mutational status (p = 0.245, p = 0.680). Details are provided in Table 2.
Patients in the NAR group had significantly more extended hospital stays and showed more severe complications (Clavien-Dindo Classification ≥ IIIA) than those in the AR group (p < 0.01, p = 0.031). All other factors were not significantly different between the two groups (Table 3).
At a median follow-up, the majority of patients (n = 173, 69.20%) had developed recurrence. Median, 1-, 3-, 5-year DFS of the entire cohort were 12 months, 52.8%, 19.2%, and 10.4%, respectively. One third of patients (n = 75, 30.0%) experienced recurrence only at an intrahepatic site, whereas 75 (30.0%) patients experienced recurrence at an extrahepatic site. When comparing the DFS of patients who underwent AR or NAR, there was no significant difference (p = 0.405, Fig. 2). In multivariate analysis of DFS rates, only the presence of ≥ 3 tumors (hazard Ratio [HR]: 1.733; 95% confidence interval [CI], 1.281–2.344; p < 0.01) (Supplementary Table 2) was independently associated with lower overall DFS.
In the KRAS wild-type group, intrahepatic, extrahepatic, and combined recurrence rates were not significantly different between the AR and NAR subgroups (p = 0.106; Table 2). Patients who underwent AR had comparable median DFS with those who underwent NAR (19.00 vs. 13.00 months; p = 0.326) (Fig. 3A). Similar to the general findings, in multivariable analysis, only the presence of ≥ 3 tumors (HR, 1.843; 95% CI, 1.253–2.711; p < 0.01) (Supplementary Table 3) was significantly related to lower DFS. AR was not significantly associated with DFS changes based on a univariable analysis (HR, 1.228; 95% CI, 0.808–1.866; p = 0.337) (Supplementary Table 3). Analysis of the DFS of patients with intrahepatic recurrence showed no significant differences between the two groups (63.00 vs. 27.00 months; p = 0.165) (Fig. 4A). In multivariable analysis of DFS in patients with later development of intrahepatic recurrence, only the bilobar localization of the disease preoperatively (HR, 2.277; 95% CI, 1.454–3.567; p < 0.01) (Supplementary Table 4) was independently associated with lower DFS.
In the KRAS mutated group, intrahepatic, extrahepatic, and combined recurrence rates were not significantly different between the AR and NAR subgroups (p = 0.774, Table 2). There was no difference in the DFS associated with either surgical approach (11.00 vs. 9.00 months; p = 0.954) (Fig. 3B). DFS was not significantly associated with AR and NAR in those who later developed intrahepatic recurrence (35.00 vs. 39.00 months; p = 0.516) (Fig. 4B). Similar to the general findings and those observed in the KRAS wild-type, only the presence of ≥ 3 tumors (HR, 1.797; 95% CI, 1.091–2.959; p = 0.021; Supplementary Table 5) (HR, 2.717; 95% CI, 1.509–4.893; p < 0.01; Supplementary Table 6) in the multivariate analysis was significantly associated with lower DFS.
Overall, the presence or absence of KRAS mutation showed no significant association to DFS regardless of surgical approach and was not regarded as a significant prognostic factor by multivariable analysis.
The purpose of this study was to determine the relationship between surgical approach and oncological outcomes according to KRAS mutational status in patients with synchronous CRLM. To that end, we collated a large retrospective cohort of patients who underwent surgery with or without neoadjuvant chemotherapy. We found no survival benefit associated with AR in patients with both KRAS wild-type, and KRAS mutated type. Only the presence of ≥ 3 tumors was associated with a lower DFS in most patients who developed intrahepatic recurrence of the disease. Bilobar localization of disease was also related to lower DFS in KRAS wild-type group.
According to Margonis et al. [13], on the method of determining the surgical approach depending on KRAS mutational status in an era of parenchyma-sparing hepatectomies (PSH)/NAR, the performance of AR was significantly associated with a decreased risk of tumor recurrence. They also claimed that DFS was improved in the group with KRAS mutated group; however, the study excluded patients who underwent simultaneous AR and NAR. Thus, this study can help guide the decision regarding surgical approach, especially with the recent trend toward an increasing proportion of simultaneous resections in synchronous CRLM [21]. The presented analysis, in this manner, adds a new piece to the puzzle. It must be noted that as definition of the anatomical resection differs for each institution and has some ambiguity, it was important that the definition used in previous studies be followed so as to have uniformity of data [13].
Moris et al. [7] conducted a systematic review for PSH vs AR for CRLM and concluded that PSH had a comparable safety and efficacy profile compared with AR and did not compromise oncologic outcomes. PSH should be considered an appropriate surgical approach as a treatment for CRLM that facilitates preservation of hepatic parenchyma. And specific concerns have been raised by surgeons, and that safe and adequate oncologic outcome could be achieved by PSH/NAR. Torzilli et al. [22] reported that the ultrasound-guided parenchyma-sparing surgery is feasible in most patients with ill-located CRLM and they report that the parenchymal sparing policy has the higher chance of repeat surgery in case of recurrence. On the same point of view, Mise et al. [8] found that PSH does not increase recurrence in liver remnants, and more importantly, there is a significant increase in 5-year survival, if there were recurrences, so PSH/NAR in CRLM should be a standard procedure.
This study had findings which support the current mainstream PSH dominant trend in CRLM. It is important to note however, a similar study done by Margonis et al. [23], which had different findings, compared to ours. They found that the R0 resection rate in the KRAS mutated group was more than double that in the NAR group. The present study reported that the R1 resection rate of the KRAS mutated group was 0.00% in the NAR group and 4.5% in the AR group. This potentially shows more valuable results because bias caused by margin issues could be excluded. Finally, another study shows that R0 resection is a prerequisite in order to ensure long-term survival and cure [24].
For synchronous CRLM, there are two mainstays in regards to therapeutic approach: simultaneous resection and staged resection. In general, simultaneous resection has been reported to prevent the need for secondary surgery, reduce the overall length of hospital stay, and to shorten the time required for adjuvant therapy [25]. On the other hand, there is a concern that the risk of morbidity increases as the operation time increases. Staged resection can potentially minimize surgical morbidity but results in an extended total hospital stay and possible worsening of liver metastasis.
In this study, the length of hospital stay tended to be longer in the NAR group compared to that of the AR group (p < 0.01). Estimated blood loss, transfusion rate, complication of CD≥IIIA and mortality rate did not differ between the two groups. Thus, the short-term surgical outcome is not considered a factor that biases the oncologic outcome and makes the results of this study more reliable.
Previously reported prognostic factors for CRLM patients included: the absence of extrahepatic metastases, < 5 metastatic lesions, R0 resection, presence of bilobar disease, and the size of the largest metastatic lesion. Similarly, the prognostic factors associated with DFS in this study were associated with three or more metastatic lesions in both the KRAS mutated and wild types. The bilaterality of the tumor in the KRAS wild-type group was significant in regards to later development of intrahepatic recurrence.
For our study, several limitations need to be considered when interpreting the data. First, there may be inherent selection biases due to the retrospective design. Second, only patients who underwent simultaneous operation were included and those undergoing staged procedures were excluded from the analysis. Further subgroup analysis on recurrence types such as local recurrences, intrasegmental recurrences, and other intrahepatic recurrences would be merited in future similar studies.
In conclusion, the presence of KRAS mutation may not be a significant factor when deciding the surgical approach in simultaneous resection of colorectal liver metastasis. In a future study, an appropriately designed clinical trial may be warranted to address this issue definitively.
Supplementary data related to this article can be found at https://doi.org/10.14701/ahbps.21-127.
REFERENCES
1. Engstrand J, Nilsson H, Strömberg C, Jonas E, Freedman J. 2018; Colorectal cancer liver metastases - a population-based study on incidence, management and survival. BMC Cancer. 18:78. DOI: 10.1186/s12885-017-3925-x. PMID: 29334918. PMCID: PMC5769309.

2. Tsilimigras DI, Ntanasis-Stathopoulos I, Bagante F, Moris D, Cloyd J, Spartalis E, et al. 2018; Clinical significance and prognostic relevance of KRAS, BRAF, PI3K and TP53 genetic mutation analysis for resectable and unresectable colorectal liver metastases: a systematic review of the current evidence. Surg Oncol. 27:280–288. DOI: 10.1016/j.suronc.2018.05.012. PMID: 29937183.

3. Shindoh J, Nishioka Y, Yoshioka R, Sugawara T, Sakamoto Y, Hasegawa K, et al. 2016; KRAS mutation status predicts site-specific recurrence and survival after resection of colorectal liver metastases irrespective of location of the primary lesion. Ann Surg Oncol. 23:1890–1896. DOI: 10.1245/s10434-016-5087-5. PMID: 26786089.

4. Vauthey JN, Zimmitti G, Kopetz SE, Shindoh J, Chen SS, Andreou A, et al. 2013; RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Ann Surg. 258:619–626. discussion 626–627. DOI: 10.1097/SLA.0b013e3182a5025a. PMID: 24018645. PMCID: PMC3856211.

5. Margonis GA, Sasaki K, Andreatos N, Kim Y, Merath K, Wagner D, et al. 2017; KRAS mutation status dictates optimal surgical margin width in patients undergoing resection of colorectal liver metastases. Ann Surg Oncol. 24:264–271. DOI: 10.1245/s10434-016-5609-1. PMID: 27696170.

6. Brudvik KW, Mise Y, Chung MH, Chun YS, Kopetz SE, Passot G, et al. 2016; RAS mutation predicts positive resection margins and narrower resection margins in patients undergoing resection of colorectal liver metastases. Ann Surg Oncol. 23:2635–2643. DOI: 10.1245/s10434-016-5187-2. PMID: 27016292. PMCID: PMC5527830.

7. Moris D, Ronnekleiv-Kelly S, Rahnemai-Azar AA, Felekouras E, Dillhoff M, Schmidt C, et al. 2017; Parenchymal-sparing versus anatomic liver resection for colorectal liver metastases: a systematic review. J Gastrointest Surg. 21:1076–1085. DOI: 10.1007/s11605-017-3397-y. PMID: 28364212.

8. Mise Y, Aloia TA, Brudvik KW, Schwarz L, Vauthey JN, Conrad C. 2016; Parenchymal-sparing hepatectomy in colorectal liver metastasis improves salvageability and survival. Ann Surg. 263:146–152. DOI: 10.1097/SLA.0000000000001194. PMID: 25775068.

9. Kokudo N, Tada K, Seki M, Ohta H, Azekura K, Ueno M, et al. 2001; Anatomical major resection versus nonanatomical limited resection for liver metastases from colorectal carcinoma. Am J Surg. 181:153–159. DOI: 10.1016/S0002-9610(00)00560-2. PMID: 11425058.

10. Lalmahomed ZS, Ayez N, van der Pool AE, Verheij J, IJzermans JN, Verhoef C. 2011; Anatomical versus nonanatomical resection of colorectal liver metastases: is there a difference in surgical and oncological outcome? World J Surg. 35:656–661. DOI: 10.1007/s00268-010-0890-9. PMID: 21161655. PMCID: PMC3032901.

11. Sarpel U, Bonavia AS, Grucela A, Roayaie S, Schwartz ME, Labow DM. 2009; Does anatomic versus nonanatomic resection affect recurrence and survival in patients undergoing surgery for colorectal liver metastasis? Ann Surg Oncol. 16:379–384. DOI: 10.1245/s10434-008-0218-2. PMID: 19020941.

12. DeMatteo RP, Palese C, Jarnagin WR, Sun RL, Blumgart LH, Fong Y. 2000; Anatomic segmental hepatic resection is superior to wedge resection as an oncologic operation for colorectal liver metastases. J Gastrointest Surg. 4:178–184. DOI: 10.1016/S1091-255X(00)80054-2. PMID: 10675241.

13. Margonis GA, Buettner S, Andreatos N, Sasaki K, Ijzermans JNM, van Vugt JLA, et al. 2017; Anatomical resections improve disease-free survival in patients with KRAS-mutated colorectal liver metastases. Ann Surg. 266:641–649. DOI: 10.1097/SLA.0000000000002367. PMID: 28657938.

14. Kye BH, Lee SH, Jeong WK, Yu CS, Park IJ, Kim HR, et al. 2019; Which strategy is better for resectable synchronous liver metastasis from colorectal cancer, simultaneous surgery, or staged surgery? Multicenter retrospective analysis. Ann Surg Treat Res. 97:184–193. DOI: 10.4174/astr.2019.97.4.184. PMID: 31620392. PMCID: PMC6779956.

15. Abelson JS, Michelassi F, Sun T, Mao J, Milsom J, Samstein B, et al. 2017; Simultaneous resection for synchronous colorectal liver metastasis: the new standard of care? J Gastrointest Surg. 21:975–982. DOI: 10.1007/s11605-017-3422-1. PMID: 28411351.

16. Boudjema K, Locher C, Sabbagh C, Ortega-Deballon P, Heyd B, Bachellier P, et al. 2021; Simultaneous versus delayed resection for initially resectable synchronous colorectal cancer liver metastases: a prospective, open-label, randomized, controlled trial. Ann Surg. 273:49–56. DOI: 10.1097/SLA.0000000000003848. PMID: 32209911.

17. Majno P, Mentha G, Toso C, Morel P, Peitgen HO, Fasel JH. 2014; Anatomy of the liver: an outline with three levels of complexity--a further step towards tailored territorial liver resections. J Hepatol. 60:654–662. DOI: 10.1016/j.jhep.2013.10.026. PMID: 24211738.

18. Kaibori M, Kon M, Kitawaki T, Kawaura T, Hasegawa K, Kokudo N, et al. 2017; Comparison of anatomic and non-anatomic hepatic resection for hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 24:616–626. DOI: 10.1002/jhbp.502. PMID: 28887834.

19. Navarro J, Rho SY, Kang I, Choi GH, Min BS. 2019; Robotic simultaneous resection for colorectal liver metastasis: feasibility for all types of liver resection. Langenbecks Arch Surg. 404:895–908. DOI: 10.1007/s00423-019-01833-7. PMID: 31797029.

20. Choi SH, Choi GH, Han DH, Choi JS. 2015; Laparoscopic liver resection using a rubber band retraction technique: usefulness and perioperative outcome in 100 consecutive cases. Surg Endosc. 29:387–397. DOI: 10.1007/s00464-014-3680-x. PMID: 24986021.

21. Gavriilidis P, Sutcliffe RP, Hodson J, Marudanayagam R, Isaac J, Azoulay D, et al. 2018; Simultaneous versus delayed hepatectomy for synchronous colorectal liver metastases: a systematic review and meta-analysis. HPB (Oxford). 20:11–19. DOI: 10.1016/j.hpb.2017.08.008. PMID: 28888775.

22. Torzilli G, Viganò L, Gatti A, Costa G, Cimino M, Procopio F, et al. 2017; Twelve-year experience of "radical but conservative" liver surgery for colorectal metastases: impact on surgical practice and oncologic efficacy. HPB (Oxford). 19:775–784. DOI: 10.1016/j.hpb.2017.05.006. PMID: 28625391.

23. Margonis GA, Buettner S, Andreatos N, Wagner D, Sasaki K, Barbon C, et al. 2019; Prognostic factors change over time after hepatectomy for colorectal liver metastases: a multi-institutional, international analysis of 1099 patients. Ann Surg. 269:1129–1137. DOI: 10.1097/SLA.0000000000002664. PMID: 31082912.

24. Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, et al. 2007; Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 25:4575–4580. DOI: 10.1200/JCO.2007.11.0833. PMID: 17925551.

25. Yin Z, Liu C, Chen Y, Bai Y, Shang C, Yin R, et al. 2013; Timing of hepatectomy in resectable synchronous colorectal liver metastases (SCRLM): simultaneous or delayed? Hepatology. 57:2346–2357. DOI: 10.1002/hep.26283. PMID: 23359206.

Fig. 1
Study flow diagram. CRC, colorectal cancer; AR, anatomical resection; NAR, non-anatomical resection.
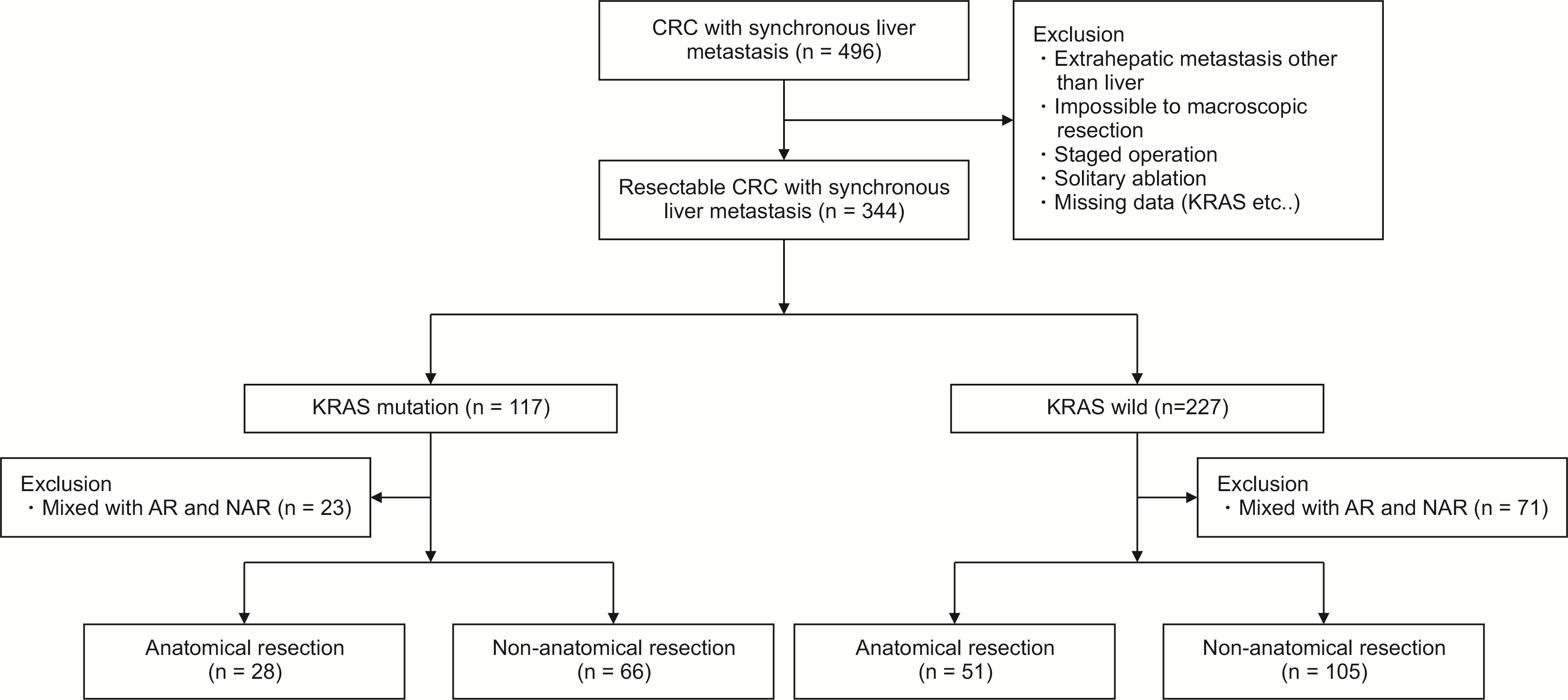
Fig. 2
Disease-free survival in entire cohort grouped according to type of resection. AR, anatomical resection; NAR, non-anatomical resection.
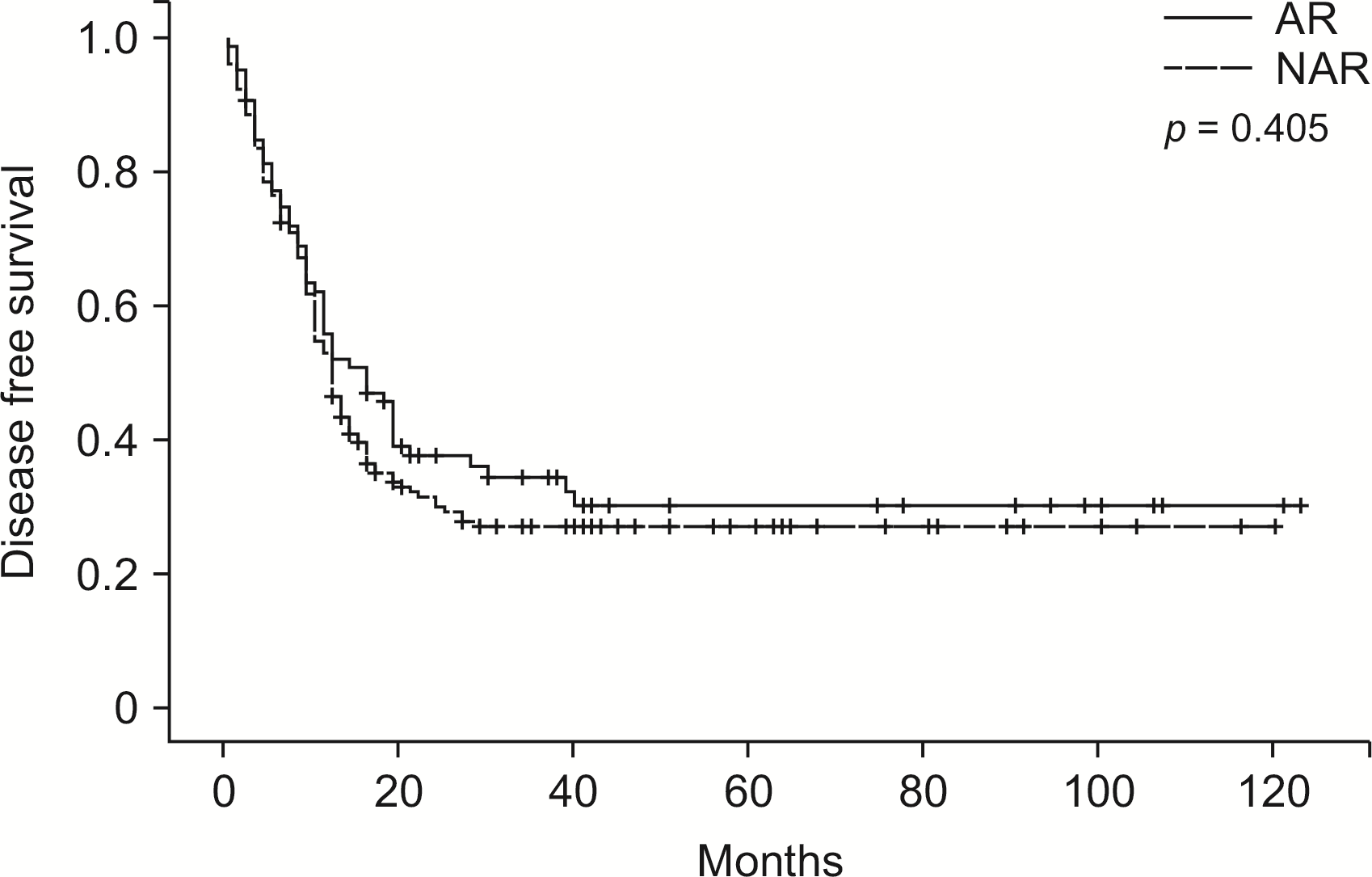
Fig. 3
(A) Disease-free survival in patients with KRAS wild-type tumors grouped according to type of resection. (B) Disease-free survival in patients with KRAS mutated tumors grouped according to type of resection. AR, anatomical resection; NAR, non-anatomical resection.
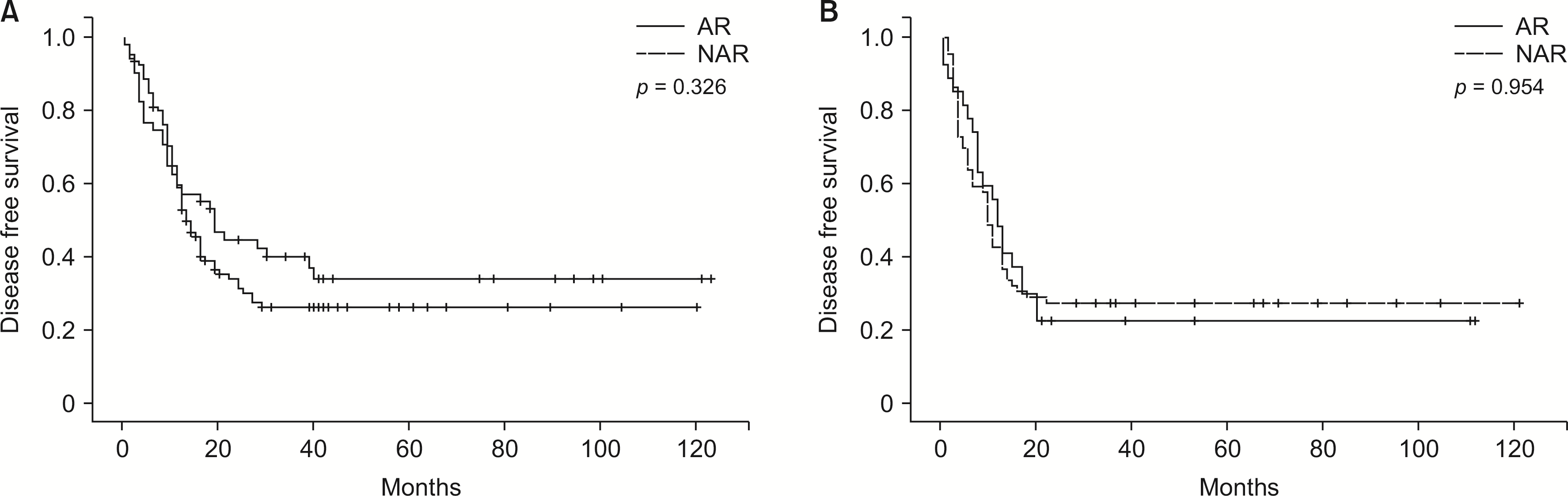
Fig. 4
(A) Intrahepatic disease-free survival in patients with KRAS wild-type tumors grouped according to type of resection. (B) Intrahepatic disease-free survival in patients with KRAS mutated tumors grouped according to type of resection. AR, anatomical resection; NAR, non-anatomical resection.
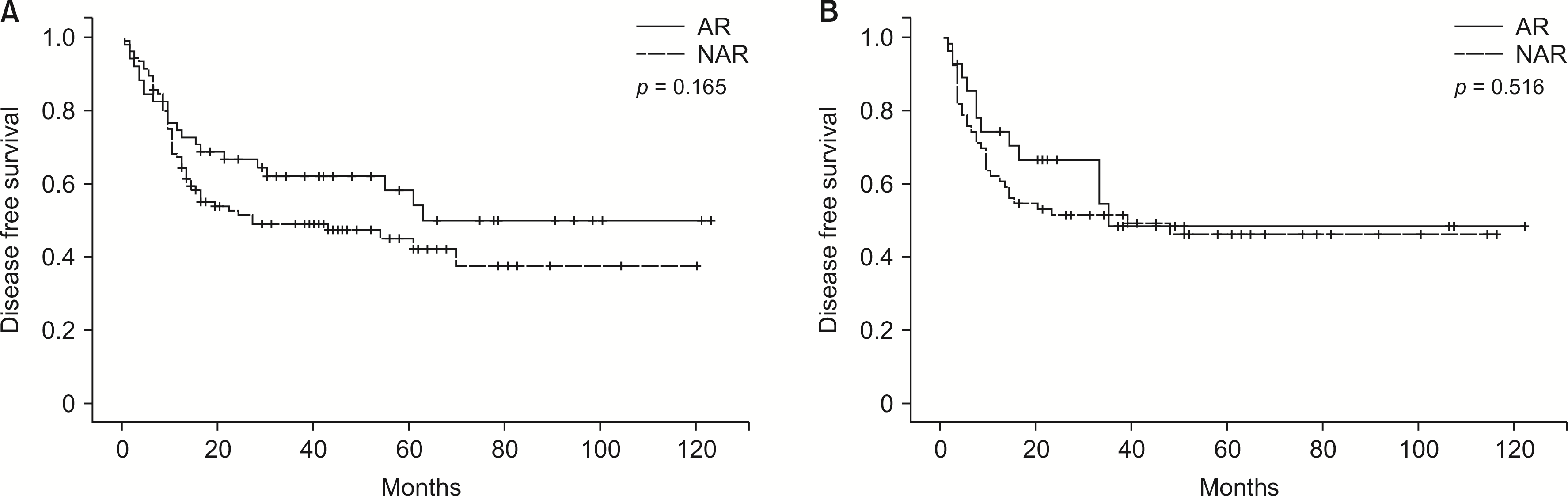
Table 1
The extent and type of liver resection
| Anatomical resection | Non-anatomical resection |
|---|---|
| Sectionectomy (bisegmentectomy) | Segmentectomy |
| Right hepatectomy | Wedge resection |
| Left heptectomy | |
| Extended right hepatectomy | |
| Extended left hepatectomy |
Table 2
Clinical and pathological characteristics according to KRAS mutational status
Table 3
Short-term surgical outcomes




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download