INTRODUCTION
Chloride is the major extracellular anion in the human body, participating in water balance, osmotic equilibrium, and acid-base balance [
1-
4]. Chloride is typically measured using potentiometry. To measure a single ion with a high degree of specificity, ion-specific membranes are used to construct an ion-selective electrode (ISE) [
5].
Chloride concentration can be measured using a solid electrode such as Ag/AgCl or using chloride-selective ion exchangers embedded in a polyvinyl chloride (PVC) membrane together with a plasticizer [
6]. Quaternary ammonium salts are frequently used as chloride-selective ion exchangers. Such ion exchangers are not very specific for chloride, since all ions with an equivalent or lower ion hydration energy can bind to them; there are reports of bromide, iodine, thiocyanate (e.g., in smokers), salicylate, bicarbonate, and heparin interference [
7]. Since bicarbonate is the second most abundant anion in the human body after chloride, it most significantly interferes with chloride measurements [
1,
3,
4,
7,
8].
Schiemsky,
et al. [
1] described positive bicarbonate interference on the chloride electrode on a Cobas c501 instrument (Roche Diagnostics, Mannheim, Germany), along with under-recovery of chloride in the absence of bicarbonate. The latter is relevant when evaluating the anion gap in critically ill patients. For example, in metabolic acidosis, low bicarbonate concentrations will result in falsely decreased chloride values, leading to an erroneously increased anion gap and a possible misdiagnosis between normal and increased anion gap types of metabolic acidosis [
8].
Bicarbonate interference on chloride measurements has been described [
1-
4,
7]. However, data comparing bicarbonate interference on electrodes by multiple manufacturers are lacking. In this study, the interference of bicarbonate on chloride measurement by different electrodes and the stability of this interference were evaluated over time. The influence of varying bicarbonate calibrator concentrations was also investigated for the Roche electrodes.
DISCUSSION
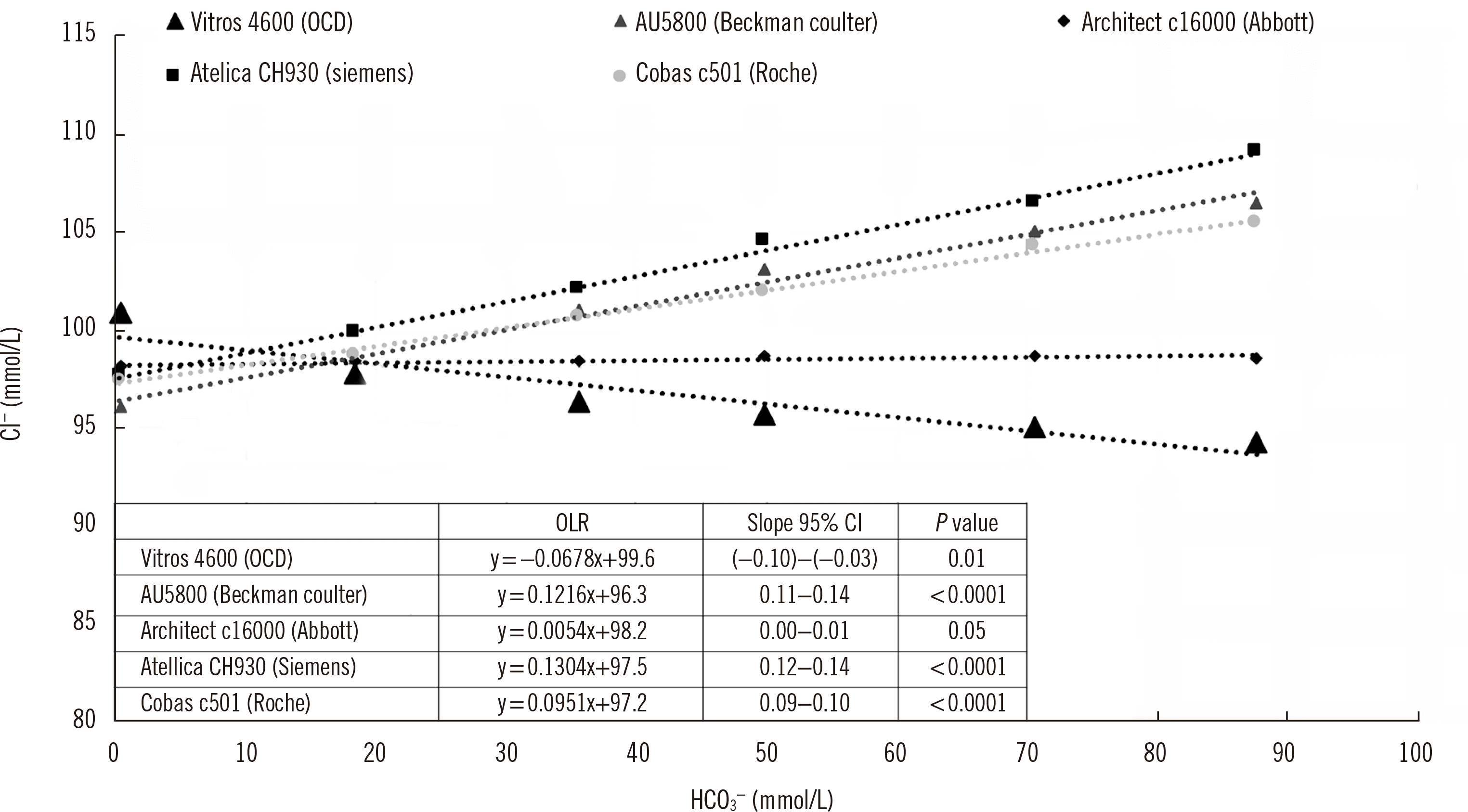
We investigated bicarbonate inte rference on chloride electrodes of five different manufacturers. The Vitros 4600 electrode from OCD is the only analyzer that applies direct potentiometry, which implies that the chloride activity is measured without pre-dilution of the sample [
5]. The activity coefficient depends on the ionic strength of the solution [
10]. The negative trend observed in the chloride measurements shown in
Fig. 1 can be explained by the decreasing chloride activity coefficient as a function of the increasing ionic strength in the solution due to bicarbonate. To exclude the effect of the ionic strength as well as to remove the influence of the decreasing activity coefficient on the measured chloride values, the chloride concentrations were manually recalculated using a constant chloride activity coefficient. After this correction, the negative trend disappeared (results not shown). We therefore conclude that there is no effect of bicarbonate on chloride measurements with the Vitros 4600 analyzer.
By contrast, the Siemens, Beckman Coulter, Abbott, and Roche Diagnostics analyzers use indirect potentiometry that involves a pre-dilution step with a fixed ionic strength solution [
5,
11]. The Architect c16000 analyzer uses a solid-state AgCl electrode, which is not influenced by bicarbonate. The 95% confidence interval of the slope, obtained over the six solutions containing increasing bicarbonate concentrations, overlapped with zero, demonstrating a non-significant slope proving the selectivity.
Chloride values determined with the electrodes of Siemens, Beckman Coulter, and Roche Diagnostics were underestimated at low bicarbonate concentrations and were overestimated at high bicarbonate concentrations. We therefore conclude that electrodes using quaternary ammonium salts as the ion exchanger in their membrane are prone to bicarbonate interference. This ion exchanger is not specific for chloride, since bicarbonate having an equivalent ion hydration energy may also bind [
7,
11,
12]. The extent of this interference can be calculated using the Nikolsky-Eisenman equation, an extension of the Nernst equation: the smaller the selectivity coefficient, the less interference by the interfering ion [
13].
No proper selectivity study has been performed using the fixed interference or separate solution method. The selectivity coefficient has to be estimated using the slope of the curve obtained by measuring six solutions with a constant chloride concentration and an increasing bicarbonate concentration shortly after the electrode is installed. This study shows that the estimated selectivity coefficient of the chloride electrodes varies from manufacturer to manufacturer. Higher slopes indicate higher selectivity coefficients, representing greater susceptibility to interference. The largest bicarbonate interference was observed for the Siemens electrode and the smallest was observed for the Roche electrode (P<0.0001).
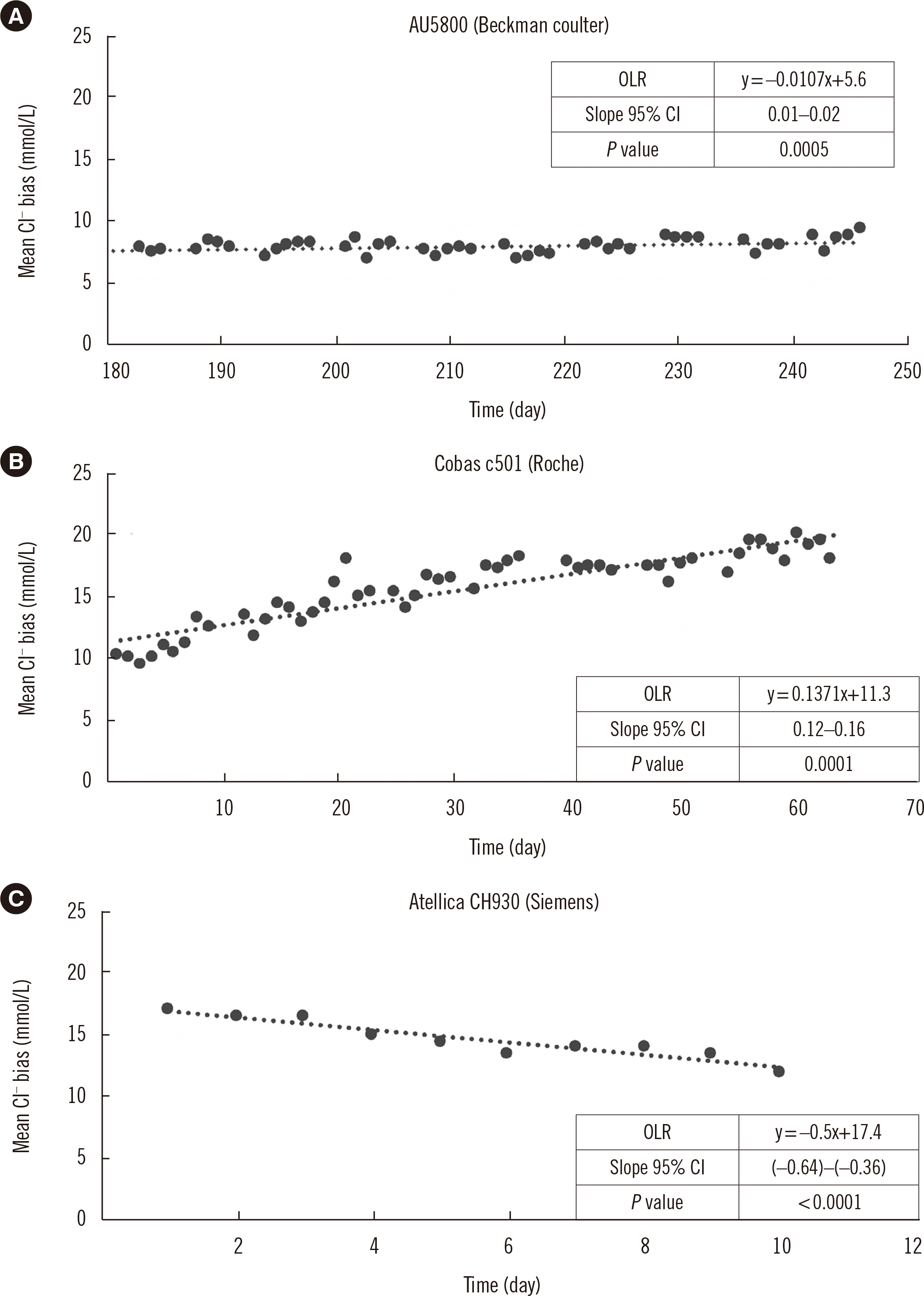
If the selectivity coefficient remains constant throughout the life of the electrode, the bicarbonate interference becomes predictable. This was the case for the Beckman Coulter electrode, since no trend was observed in the chloride values measured over time. However, the Beckman Coulter electrode has no pre-defined lifetime. According to the manufacturer, the ion-selective membrane contains approximately ten thousands macro-molecule layers of quaternary ammonium salts to assure a long life. This molecular crystallization ensures a robust structure and prevents the absorption of endogenous components from the sample, which may contribute to lower interference by other ions.
For the Roche and Siemens electrodes, a variable and opposite selectivity coefficient over time was demonstrated. For the Atellica CH930 electrode, the measured values slightly decreased over time. To exclude the possibility that pre-analytical errors led to the evaporation of the bicarbonate in the test solution, the bicarbonate concentrations were inspected. The bicarbonate concentration decreased by 4 mmol/L over 10 days, which could only explain a decrease in chloride of 0.6 mmol/L (data not shown). A possible explanation might be linked to the structure of the membrane itself. More specialized studies are required to clarify this reason, which is beyond the present scope.
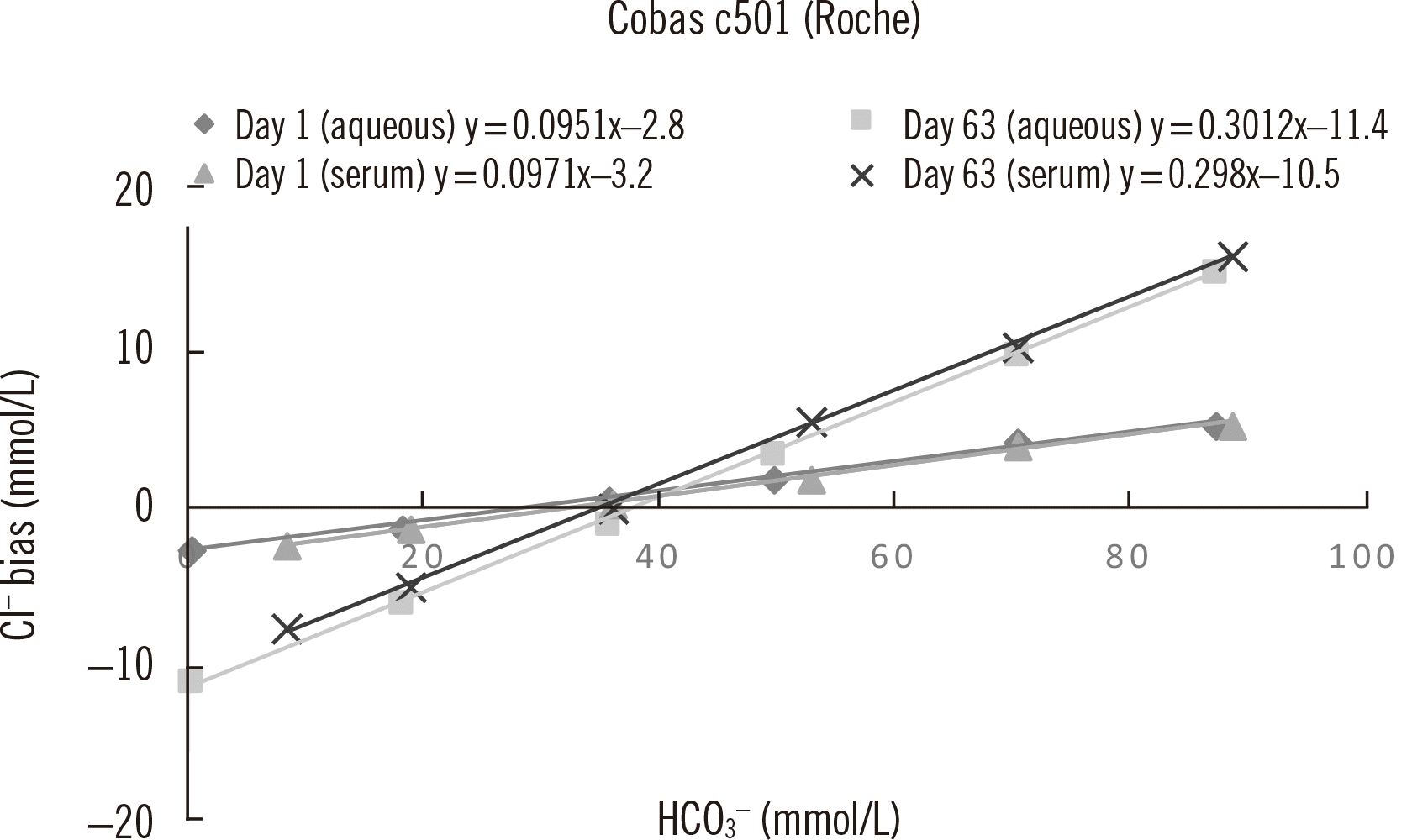
For the Roche electrode, newly installed electrodes appeared to allow a lower number of bicarbonate molecules to bind and therefore exhibited greater selectivity toward chloride. As the electrode aged, the selectivity decreased and the difference between the actual and measured chloride values increased. Co-extraction of other (positively charged) molecules from the sample might explain the higher chloride values obtained over time [
14]. This will influence the membrane selectivity, allowing more interfering ions to move into the membrane phase, which may contribute to the potential build-up. Since the bicarbonate interference is not stable over time, it is very difficult to predict the impact of bicarbonate interference on a patient’s chloride result.
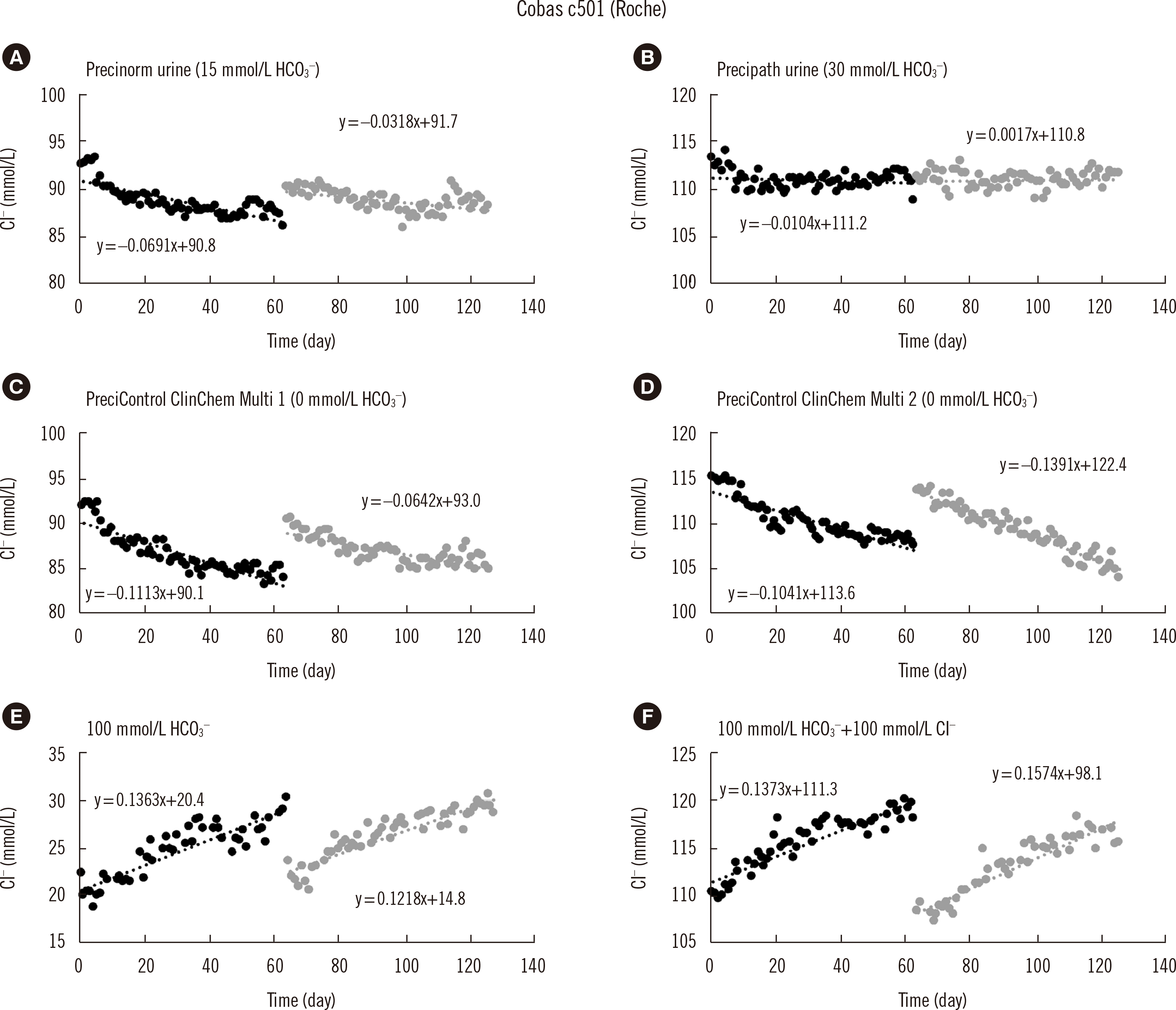
The chloride content in the sample also influences the impact of bicarbonate interference. This is demonstrated in
Table 1 for the Roche electrode. A solution consisting solely of 100 mmol/L bicarbonate induced a bias ranging from 19 to 29.1 mmol/L (depending on the age of the electrode), whereas a solution containing bicarbonate and NaCl at the same concentration (100 mmol/L) resulted in a bias ranging from 9.8 to 18.3 mmol/L. More specialized studies are required to explain this effect.
Choosing an appropriate bicarbonate concentration in a calibrator is important to control the impact of the interference in routine practice. During calibration, the offset due to the interference of bicarbonate in the calibrators is set to zero. Samples containing less bicarbonate compared with that in the calibrators will be overcompensated, yielding falsely decreased chloride values, whereas samples with a higher bicarbonate concentration will be undercompensated, resulting in falsely elevated chloride values. Only samples within a limited bicarbonate range, close to the concentration in the calibrator, will be measured with minimal bias.
When monitoring time-dependent selectivity, the control materials should contain bicarbonate concentrations that differ from those present in the calibrators (
Table 1). With the Precipath urine QC material, which contains approximately 30 mmol/L of bicarbonate, only a very limited time effect was observed (−0.3 mmol/L during the electrode’s lifetime). By contrast, Precipath ClinChem Multi controls, without any bicarbonate, exhibited a much more pronounced time effect (−5.8 to −7.4 mmol/L during the electrode’s lifetime). Each laboratory should define the clinically relevant bicarbonate range to choose appropriate control materials. A control material with a bicarbonate concentration close to that of the calibrator can be used to monitor inherent analytical variation, without issues of interference. A second control material, at the border of the clinically relevant bicarbonate range, should be included to specifically monitor the selectivity of the electrode.
Recently, Roche reduced the concentration of bicarbonate in their calibrator. The bicarbonate concentration in the calibrator lots used in this study changed from 30 mmol/L for both calibrator concentrations to 20 mmol/L in the high ISE standard (120 mmol/L chloride) and 24 mmol/L in the low standard (80 mmol/L chloride). The lower bicarbonate concentrations caused the complete calibration curve to shift toward less negative electromotive force (EMF) values, and the slope of the calibration curve became less steep in the ISE high standard compared with that of the low standard. The chloride results became more accurate in the normal and lower bicarbonate ranges, which, from a clinical point of view (e.g., anion gap calculation in the case of metabolic acidosis), is more important than the higher bicarbonate range.
The bicarbonate concentration of a calibrator depends on the calibration lot owing to factors in the production process. The bicarbonate concentrations in the tested calibrator lot numbers of Roche changed from 24 to 25.1 mmol/L for the low standard and from 20 to 22.4 mmol/L for the high standard. For Beckman Coulter, the low standard changed from 21 to 23.8 mmol/L and the high standard changed from 20.7 to 23.4 mmol/L. The extent of over/underestimation of chloride values is defined by the difference in the bicarbonate concentration between the sample and the calibrator. This induces yet another source of variation on the chloride measurement, making it even more complex to predict the magnitude of the induced error and emphasizing the importance of carefully selected internal QC materials.
A limitation of this study is that data for the stability of the Beckman Coulter electrode were obtained over 65 days, starting at day 183 after installation of a new electrode, and the stability was not investigated until defect. This makes it impossible to infer the stability of the selectivity of the electrode over its entire lifetime. Furthermore, a supraclinical solution with a bicarbonate concentration up to 100 mmol/L was used to enlarge the effect occurring in the clinically relevant range.
In conclusion, bicarbonate interference is inherent to electrodes that use quaternary ammonium salts as the ion exchanger in the membrane. The selectivity coefficient varies among manufacturers. Relevant instability over time was observed for the Roche and Siemens electrodes. The bicarbonate-induced error on the measured chloride value depends on the difference in the bicarbonate concentration between the calibrator and test sample. The bicarbonate-induced error varies according to the type, manufacturer, and wear of the electrode; the bicarbonate concentration in the calibrators and the tested sample; and the chloride concentration. Clinical laboratories should be aware of the impact of bicarbonate interference on chloride measurements to assess the reliability of the obtained chloride results and to establish appropriate QC procedures to monitor the effect over time.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download