Abstract
With the rapid spread of the coronavirus disease (COVID-19), the need for rapid testing and diagnosis and consequently, the demand for mobile laboratories have increased. Despite this need, there are no clear guidelines for the operation, maintenance, or quality control of mobile laboratories. We provide guidelines for the operation, management, and quality control of mobile laboratories, and specifically for the implementation and execution of COVID-19 molecular diagnostic testing. These practical guidelines are primarily based on expert opinions and a laboratory accreditation inspection checklist. The scope of these guidelines includes the facility, preoperative evaluation, PCR testing, internal and external quality control, sample handling, reporting, laboratory personnel, biosafety level, and laboratory safety management. These guidelines are useful for the maintenance and operation of mobile laboratories not only in normal circumstances but also during public health crises and emergencies.
Coronavirus disease (COVID-19) is a respiratory disease caused by severe acute respiratory syndrome coronavirus 2 [1]. Although large numbers of PCR tests have been conducted during the COVID-19 pandemic, the demand for faster results has increased, as conventional tests require time for sample transportation to laboratories. Specialized movable laboratories, also called mobile laboratories, rapid laboratories, on-site laboratories, or point-of-care laboratories, can be employed to meet this demand. To prepare for the implementation of mobile laboratories at COVID-19 testing sites, it is necessary to prepare detailed management requirements for mobile laboratories to ensure the quality of COVID-19 testing.
Korean Society for Laboratory Medicine (KSLM) and the Korea Disease Control and Prevention Agency (KDCA) have published guidelines for diagnosing COVID-19 in clinical laboratories in Korea [2, 3]. These guidelines were mainly for laboratories in healthcare facilities; therefore, we discuss the limitations pertaining to mobile laboratories. Board members of the COVID-19 Response Task Force within KSLM and Laboratory Medicine Foundation (LMF), who have extensive experience in conducting and reviewing the inspection and accreditation of molecular laboratories in Korea, have provided recommendations regarding the standards for operating laboratories, including management requirements for the laboratories, experts, and laboratory specialists.
We provide guidelines for implementing and conducting COVID-19 molecular diagnostic testing using real-time reverse transcription PCR in mobile laboratories. These practical guidelines are mostly based on expert opinions regarding basic facilities, pre-testing preparedness, and operating mobile laboratories, including quality control, safety control, and data handling. The operation and quality management of mobile laboratories for accurate and reliable molecular diagnostic testing of COVID-19 are reviewed.
Mobile laboratories are available in various countries, including the US, Germany, and the UK; however, only a limited number of these laboratories have the capacity to perform COVID-19 molecular diagnostic testing. Most mobile laboratories have a biosafety level (BSL) of 2/2+, and some have a BSL level between 1 and 3. The QuikLAB (TPT MedTech, Kendall, FL, USA) is designed for collecting and testing samples in a mobile setting, and the results are delivered to the test subject through a mobile application [4]. The CentoTruck (CENTOGENE, Rostock, Germany) is a trailer equipped with a mobile testing system designed for collecting samples and performing molecular diagnostic testing and antigen testing [5].
Mobile laboratories are specially permitted during public health crises such as the COVID-19 pandemic in Korea. They are operated by public health laboratories and private institutions that are capable of diagnosing infectious disease pathogens according to laws and regulations and participate in the management of laboratories and the evaluation of their testing capabilities [6]. Public institutions include the Public Health and Environment Research Institute, and private institutions include COVID-19 testing institutions with laboratories that are certified for molecular diagnostic testing by the LMF. Based on the Medical Act, mobile laboratories may be operated only when the head of the central or local government deems it necessary for the public interest, after receiving a request to run a mobile laboratory [7]. Personnel who are licensed for running the laboratories and for managing the overall tests are required. The laboratories are operated according to laws and regulations under the management and guidance of laboratory medicine specialists. In particular, specialists with practical experience in molecular diagnosis or clinical microbiology, which is required for the diagnosis of COVID-19, should be responsible for the operation of the laboratory and reporting the results. If the main laboratory is operated in a healthcare institution, each of the main and mobile laboratories must participate in external quality control evaluation. Table 1 lists the characteristics of mobile laboratories and healthcare (central) laboratories for COVID-19 molecular diagnostic testing.
As the authentic interpretation of the relevant laws and regulations by the supervisory authorities may differ among countries, it is essential to accurately interpret the laws and regulations pertaining to the installation and operation of mobile laboratories in the relevant country.
The structure of mobile laboratories and the devices included require reviewing, depending on the mobile laboratory type (container, trailer, and vehicle types). Non-combustible insulating materials are recommended for the exterior walls of mobile laboratories to reduce the temperature difference between the inside and outside of the laboratory and to prevent fire. To restrict non-authorized personnel access and maintain biosafety and security, signage must be placed on the exterior wall to indicate the COVID-19 testing room, and door locks must be installed to ensure the security of samples and personal information.
Mobile laboratories for COVID-19 testing must fulfill BSL 2 standards and be equipped with pressure control equipment. To prevent contamination, the indoor space must be sectioned and personnel must move in one direction. Additionally, there must be separate areas for sample preparation and nucleic acid extraction and amplification (Fig. 1). At least, the area for nucleic acid extraction must have negative pressure and air-conditioning equipment with high-efficiency particulate air filters installed.
The following facilities and equipment are required for mobile laboratories: air conditioners, thermo-hygrostat, external power input terminals, biological safety cabinet (BSC; Class II or higher), laboratory bench, nucleic acid extractor, thermal cycler, computer, refrigerator, freezer, centrifuge, pass box, and space to handle medical waste. These essential facilities and equipment must be installed and verified prior to the operation of mobile laboratories. Additionally, the equipment must be fixed to the ground or transported separately to prevent damage due to shock or vibration during transportation.
The following factors must be considered when selecting a location for mobile laboratories. The site must have sufficient space for transport vehicles to move and must meet the external size specification. There must be a constant main power supply, and the sample transport route must not be hindered or close to the collection site. To maintain biosafety and security, mobile laboratories must be installed in locations with as little public traffic as possible. Sufficient electric power must at all times be supplied to internal facilities and equipment, and an uninterrupted power supply (UPS) must be available in the case of main power failure or supply error.
When mobile laboratories are newly installed or reinstalled after they have moved, the facilities, equipment, and tests must be reviewed and fully evaluated prior to their operation. The pressure, temperature change, power supply, equipment location within the laboratory, and the performance of the test equipment must be evaluated. New equipment should be validated and calibrated, including proper functional evaluation and maintenance. The performance verification procedures suggested by the manufacturer must be followed.
The reliability of the tests used in mobile laboratories must be verified by running tests using sample that have been also tested in the main or another COVID-19 test laboratory. Evaluations must be performed by the same personnel who conduct laboratory tests, using the same equipment and reagents in the mobile laboratory. The comparative evaluation results must be reviewed simultaneously by the central laboratory director and the mobile laboratory director, similar to the evidence materials for preparing laboratory inspection. The results must be recorded and stored. The acceptance criteria for the results are specified; if an observed result is outside the range, appropriate corrective actions must be taken. Every time a mobile laboratory is moved, it must be reevaluated to ensure that the equipment performance and test results are not affected by the new environment or any damage caused during transportation.
A standard operating procedure (SOP) for test operation in mobile laboratories must be prepared. Administrative SOPs include guidelines regarding administrative work, including work processing, reporting, and document management. Individual SOPs include guidelines regarding test principles, clinical significance, sample conditions, reagent preparation, calibration, quality control, testing, calculation methods, reference values, result interpretation, and troubleshooting.
The general content of a mobile laboratory test SOP can be composed based on existing SOPs for the central laboratory. The SOP must be reviewed and approved by both the mobile laboratory and central laboratory directors. Guidelines must be placed near the workbench so that they are readily accessible during work. A system must be implemented to ensure that all personnel are familiar with the test manual. The SOP should be reviewed regularly by the laboratory medicine specialist and the mobile laboratory director, and records of the reviews must be stored.
Internal quality management programs and guidelines must be available in mobile laboratories. The quality management program should include evaluation criteria, quality control report form, accurate records of internal quality control results, acceptable range for quality control, and corrective actions for results outside the acceptable range. Quality control must be performed for each test batch, using both positive and negative quality control materials. The test results must fall within the acceptable range and be reported. Records must be maintained for at least 2 years.
The internal quality control records must be comprehensively reviewed at least monthly. If the review results differ from previous cumulative results, causative analysis, countermeasures, and a record of the measures must be available and reviewed periodically by the mobile laboratory director. If multiple methods or instruments are used for the same test, the test results must be compared and evaluated at least every six months.
All tests performed in mobile laboratories must be assessed in external quality programs operated by trusted external institutions (e.g., the KDCA and Korea Clinical Laboratory Quality Control Association). In mobile laboratories, external quality control materials must be handled in the same manner as patient samples and must be tested separately from the central laboratory. The mobile laboratory director must actively review and report the test results.
Key indicators of mobile laboratory quality, including the sample recognition error rate, turnaround time, and correct result rate, must be periodically evaluated.
A system using bar codes to submit and check samples must be available in mobile laboratories to reduce human error. The sample container must have more than two types of identifiers that contain the necessary information for identification, including the test subject’s name and registration number. There must be criteria to exclude samples unsuitable for testing. It is recommended that the test request form contains the following information: the name and registration number of the test subject, name of the requested healthcare provider, name and identification information of the requesting institution (when it is not the same institution), name of the requested test, date and time of sample collection, and sample type.
To minimize nucleic acid denaturation, the sample must be processed immediately or stored at 4°C prior to processing. The criteria for sample storage conditions and duration must be available. Sample identification information must be checked at each step of the testing: sample reception, nucleic acid extraction, target gene amplification, detection, and storage.
The laboratory personnel must prepare test procedures after reviewing the SOP and follow the manufacturer’s protocol accordingly. The test subject’s information and results (e.g., raw data) must be readily available for follow-up. If the same test is conducted using multiple instruments, it must be possible to trace the instrument used.
Commercial reagents used for nucleic acid extraction and gene amplification must be used following the manufacturer’s protocol. Measures to prevent contamination during nucleic acid extraction and gene amplification must be available. Nucleic acid extraction and gene amplification should be performed in separate areas. To prevent contamination during nucleic acid extraction, equipment and laboratory benches must be frequently and thoroughly disinfected. To detect false negative results due to inhibitors or inefficient nucleic acid extraction, an internal control must be used for gene amplification.
Procedures for verifying, interpreting, and reporting test results must be included in the guidelines. For computerized laboratories, the current test status (reception, testing, interim result reporting, and report completion) must be checked in real time. If necessary, interim reports must be promptly available, and the test method, results, objective findings of the results, and interpretation must be indicated on the report in an easy-to-read manner. The address of the mobile laboratory at the time of the testing must be indicated, and the test results must be specified.
The final report (original or copy) and result record sheet must be maintained in the central and mobile laboratories for a certain period. In Korea, according to Article 15 of the Enforcement Rules of the Medical Act, result sheets are maintained for 5 years or must follow the standards specified in national laws and regulations [8].
All IVDs used for testing must be approved by the appropriate regulatory authorities. For use in Korea, IVDs must be approved by the Ministry of Food and Drug Safety. They must be supplied and stored in compliance with the manufacturer’s recommendations (e.g., temperature) and used according to the manufacturer’s instructions. All reagents must be used within the expiration date. If reagents from different companies or lots are used, a comparative evaluation must be conducted using patient samples.
Instructions for all equipment should be readily accessible to the laboratory personnel. The records regarding equipment inspection and maintenance must be carefully managed, and the equipment maintenance procedures and intervals must follow the manufacturer’s instructions.
Nucleic acid extraction and gene amplification equipment are the main equipment used in mobile laboratories. All key equipment must be inspected on a quarterly basis, and measures must be taken to prevent contamination during inspection. Automated equipment is recommended for nucleic acid extraction, and reagents suitable for the specimen type (e.g., nasopharyngeal swab, sputum) should be used.
When the equipment is reinstalled after a mobile laboratory is moved, the exterior and functionality of the nucleic acid extraction and gene amplification equipment must be inspected. Key functions, such as the heating block temperature, ultraviolet (UV) function, and reaction system alignment, must be calibrated.
General instruments and equipment in mobile laboratories include centrifuges, automatic pipettes, temperature-related instruments, and the BSC. After the installation of mobile laboratories and at each quarter, the centrifuge speed and timers must be checked using a tachometer. The accuracy of automatic pipettes must be inspected prior to use and at least annually. Inspection results must be stored. General thermometers and built-in digital thermometers must be verified using standard thermometers before use.
For all temperature-related equipment (e.g., refrigerator and freezer), the temperature must be set within the stipulated range and must be checked every day or every test day.
BSCs (Class II or higher) that have passed performance testing and meet the relevant standards (KSJ0012, EN12469, and NSF49) must be installed, and the filter performance and flow rate must be evaluated at least once a year.
Mobile laboratories must have operating procedures and organizational charts. A job description that stipulates the laboratory staff’s responsibility, authority, and duties must be available, and the organizational chart should include information regarding the relationship between the director of the central laboratory, the director of the mobile laboratory, and the personnel in charge of each job.
All laboratory personnel must have a valid license and other necessary qualifications. Mobile laboratory directors should be full-time laboratory medicine specialists and are recommended to work exclusively in the mobile laboratory for more than 20 hours a week. Work/duties not related to mobile laboratories should not be performed during the hrs dedicated to the mobile laboratory.
The mobile laboratory director must have the appropriate education, field expertise, and experience. An appropriate number of medical technicians must be present at mobile laboratory sites for maintaining mobile laboratory quality. These medical technicians must have sufficient work experience and relevant professional education according to the standards.
The mobile laboratory space must be sufficient for work and safety management. The access of non-authorized personnel must be controlled, and animal and insect access must be prevented. The floor, walls, ceiling, and workbench in the laboratories must be clean. The laboratory should be equipped with a UPS. The recommended indoor temperature and humidity for mobile laboratories are 18–26°C and 30–70%, respectively [9]. Thermometers and hygrometers must be available in mobile laboratories, and the temperature and humidity must be recorded daily.
Mobile laboratories for molecular diagnostic testing of COVID-19 must have BSL 2 and BSCs of Class II or higher. For mobile laboratories that work with viable virus strains, BSL 3 is recommended. Procedures that can generate aerosols, such as sample aliquoting or dilution, nucleic acid extraction, and vortexing, must be performed in a BSC (Class II or higher). After processing samples in the BSC, the work area and equipment used must be disinfected with a disinfectant recommended by the KDCA. Test procedures conducted outside the BSC must be maintained at a minimum.
Regulations and guidelines regarding infection control in mobile laboratories must be prepared and laboratory personnel must be regularly trained to comply with them. Guidelines regarding personal protective equipment (gloves, gowns, masks, goggles, and shoes) must be available. Laboratory gowns must be worn by all laboratory personnel and should not be worn outside the laboratory for purposes that are not related to test procedures. Personnel that directly handle infectious samples must wear gloves and masks (KF94/N95 grade or higher), and goggles must be worn when sample containers are opened. Shoes covering the front of the feet must be worn.
Standard precautions must be taken when handling infectious samples. If possible, consumables that come in contact with infectious samples should be disposable. After the test, all protective equipment must be removed, and hand hygiene must be practiced before leaving the test area. Caution must be exercised to avoid contamination of the hands or body when removing the protective equipment. All laboratory personnel must practice hand hygiene after coming into contact with patients or handling samples.
Smoking, eating, drinking, applying makeup and lip balm, putting on and removing contact lenses, and mouth pipetting are prohibited in the laboratory work areas.
Documented plans and facilities must be available to evacuate all personnel, including those with disabilities, in case of emergency, such as fire. Emergency lights and fire extinguishers must be available. The standard for fire extinguishers in medical institutions is one unit of fire extinguisher capacity for every 50 m² of floor area.
Regulations and guidelines regarding the safe operation of electrical equipment must be available. Instructions to reduce or prevent exposure to UV light when using a UV generator must be available. Protective equipment must be used if necessary, and safety warning signage must be attached to the equipment.
Regulations and guidelines on hazardous waste collection and treatment must be available. All infectious waste materials must be handled according to national regulations. In Korea, the Special Measures for Safety Management of Waste Related to COVID-19 (3rd Edition) issued by the Ministry of Environment must be followed [10].
As part of the laboratory information system (LIS), it is recommended a laboratory quality control program is configured. Instructions regarding the LIS and computer use must be documented and made readily available. The mobile laboratory director must review and record the LIS instructions at least once a year.
The content and format of all prepared reports must be periodically reviewed and approved by the mobile laboratory director. It should be possible to check the personnel who performed the test and reported the results in the computerized system.
Measures for data storage and equipment in the case of fire, natural disasters, and software and hardware failures must be established. Procedures for regular data backup and information recovery from backed-up media must be specified. If the LIS program is modified or a new program is installed, all users must undergo training, and training records must be available. Data security measures, including encryption and data integrity verification, must be prepared when test information is transmitted wirelessly in mobile laboratories.
Standards for accreditation and facility safety evaluation by the LMF that satisfy the operating standards and management requirements have been implemented in existing diagnostic laboratories in medical institutions in Korea [11]. As mobile laboratories may move from one site to another, extra caution must be exercised to maintain quality. To ensure reliable test results, the necessary SOP must be provided. It is important that institutions that have previously conducted high-quality COVID-19 testing operate mobile laboratories to ensure the quality of COVID-19 testing. Institutions that operate central laboratories have experience in managing the facilities and personnel required for laboratories as well as internal and external quality control. This allows them to operate mobile laboratories rapidly and properly. Mobile laboratories require a competent director. To ensure reliability, COVID-19 tests must be evaluated before operation of a mobile laboratory.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Gye Cheol Kwon, Dr. Do-Hoon Lee, and Dr. Sang Hoon Song for help in preparing the guidelines.
Notes
REFERENCES
1. World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it. Updated on Feb 2020.
2. Hong KH, Lee SW, Kim TS, Huh HJ, Lee J, Kim SY, et al. 2020; Guidelines for laboratory diagnosis of coronavirus disease 2019 (COVID-19) in Korea. Ann Lab Med. 40:351–60. DOI: 10.3343/alm.2020.40.5.351. PMID: 32237288. PMCID: PMC7169629.

3. Hong KH, Kim GJ, Roh KH, Sung H, Lee J, Kim SY, et al. Update of Guidelines for Laboratory Diagnosis of COVID-19 in Korea. Ann Lab Med. 2022; 42:391–7. DOI: 10.3343/alm.2022.42.4.391. PMID: 35177559. PMCID: PMC8859556.

4. TPT Med Tech. QuikLAB rapid COVID-19 testing lab. https://tptmedtech.com/. Updated on Dec 2021.
5. CENTOGENE. CentoTruck, the mobile laboratory optimized for PCR tests. https://www.centogene.com/covid-19/testing/centotrucktm.html. Updated on Dec 2021.
6. Korean Law, Infectious Disease Control and Prevention Act. Article 16-2, paragraphs 1 and 2. Revised on August 11, 2020. https://elaw.klri.re.kr/kor_service/lawView.do?hseq=54658&lang=ENG. Updated on Dec 2021.
7. Korean Law, Medical Service Act. Article 33, paragraph 1, item 3, Revised on June 30, 2021. https://elaw.klri.re.kr/kor_service/lawView.do?hseq=53532&lang=ENG1. Updated on Dec 2021.
8. Korean Law, Enforcement Rules of the Medical Service Act. Article 15. Revised on December 31, 2021. https://www.law.go.kr/%EB%B2%95%EB%A0%B9/%EC%9D%98%EB%A3%8C%EB%B2%95%EC%8B%9C%ED%96%89%EA%B7%9C%EC%B9%99. Updated on Dec 2021.
9. CLSI. 2007. Laboratory design. 2nd ed. CLSI GP18-A2. Clinical and Laboratory Standards Institute;Wayne, PA: DOI: 10.3343/alm.2022.42.4.391.
10. Special Measures for Safety Management of Waste Related to COVID-19. 3rd ed. http://ncov.mohw.go.kr/shBoardView.do?brdId=2&brdGubun=28&ncvContSeq=758. Updated on Dec 2021. Ministry of Environment;2020.
11. Laboratory accreditation inspection checklist-laboratory management and molecular diagnostic test. http://lmf.or.kr/english/sub/catalog.php?CatNo=65. Updated on Dec 2021. Seoul: Korean Society for Laboratory Medicine/Laboratory Medicine Foundation;2021.
Fig. 1
Layout of a mobile laboratory for COVID-19 molecular diagnostic testing.
Abbreviation: BSC, biological safety cabinet.
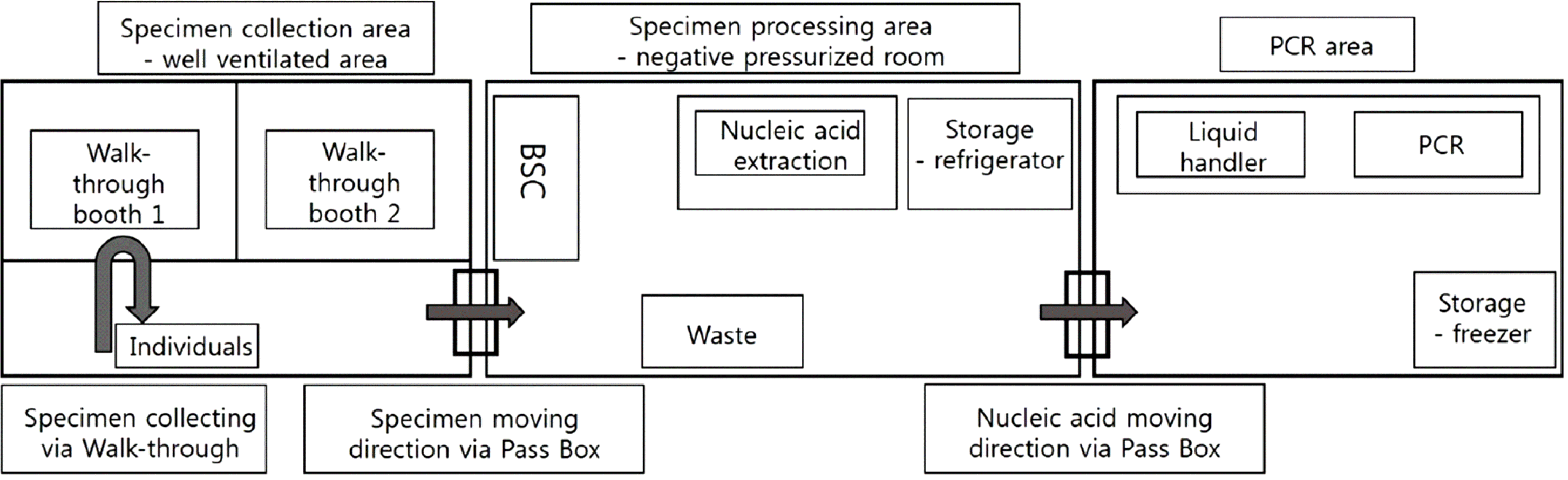
Table 1
Characteristics of healthcare (central) laboratories and mobile laboratories for COVID-19 molecular diagnostic testing
| Comparison item | Healthcare or central laboratories | Mobile laboratories |
|---|---|---|
| Laboratory location | Metropolitan area or major cities | Airport, national park, or sites where people gather |
| Maximum throughput | 10,000–100,000 tests/day | 400–1,000 tests/8-hr shift |
| Turnaround time |
3–6 hr (healthcare laboratory) 18–24 hr (independent laboratory) |
3–6 hr |
| Essential facility | Negative pressure facility | Negative pressure facility |
| Key instruments |
BSC Automated nucleic acid extraction instrument Liquid handler or reagent preparation room Refrigerator and freezer Storage for waste |
BSC Automated nucleic acid extraction instrument Liquid handler (optional) Refrigerator and freezer Storage for waste Self-power generator |
| Accreditation | Certified by accreditation agency (e.g., KSLM, KDCA in Korea) | Certified by accreditation agency (e.g., KSLM, KDCA in Korea) |
| Proficiency test | Participate with appropriate proficiency test (e.g., KAEQAS in Korea) | Participate with appropriate proficiency test (e.g., KAEQAS in Korea) |
| Director of laboratory | Laboratory Medicine Specialist* | Laboratory Medicine Specialist |
| Duty of work | Full-time work (40 hr/week) | 20 hr/week per mobile laboratory site minimally recommended |
| Report | Report to healthcare institutes | Report to individuals and healthcare authorities |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download