Abstract
Objectives
This study aimed to compare the effects of systemic administration of levofloxacin or cephalexin on fracture healing in rats.
Materials and Methods
In this animal study, tibial fractures not requiring fixation were artificially induced in 30 male Wistar albino rats using a 1.1 mm surgical bur. The rats were randomly divided into 6 groups (n=5). Groups 1 and 2 received daily subcutaneous saline injections. Groups 3 and 4 received subcutaneous injections of 25 mg/kg levofloxacin twice daily. Groups 5 and 6 received daily subcutaneous injections of 20 mg/kg cephalexin. The rats in Groups 1, 3, and 5 were sacrificed after 1 week, while the rats in Groups 2, 4, and 6 were sacrificed after 4 weeks. The score of fracture healing was determined through histological assessment of sections from the fracture site according to Perry and colleagues. Data were analyzed by Kruskal–Wallis and Mann–Whitney tests.
Results
The mean score of fracture healing at 4 weeks was significantly higher than that at 1 week in the saline, levofloxacin, and cephalexin groups (P<0.001). At 1 week, no significant difference was noted among the three groups of saline, levofloxacin, and cephalexin in the mean score of fracture healing (P=0.360). However, this difference was significant at 4 weeks (P=0.018), and the mean score in the saline group was significantly higher compared to that in the levofloxacin group (P=0.015).
Successful management of maxillofacial fractures depends on correct reduction and precise fixation of the broken segments to establish normal occlusion and resume function1. Despite the advances in oral and maxillofacial surgery, delayed and incomplete bone healing remains a common concern2. Bone healing is a complex process that involves three phases of inflammation, healing, and delayed remodeling. The inflammatory phase of bone healing is completed within one week, and the remodeling process begins at the end of the third week. These biological processes are controlled by complex molecular mechanisms. Different cell types and several local and systemic factors including growth factors released from the adjacent tissues or delivered into the fracture site by blood circulation participate in the process of bone healing3,4.
The efficacy of different modalities such as injection of growth factors and medications and electrical stimulation has been evaluated for enhancement and acceleration of bone healing5-7. Evidence shows that some medications such as specific types of antibiotics can delay or impair bone healing. By identifying and avoiding such medications, complete bone healing can be expected8.
Levofloxacin is a broad-spectrum third-generation fluoroquinolone that is effective against Gram-positive and Gram-negative aerobes and some anaerobes. It exerts its bactericidal effect through inhibition of bacterial DNA replication. It is quickly absorbed when taken orally and has a half-life of 6 to 8 hours. It is mainly excreted through urine, has good bone penetration, and is among the antibiotics of choice for treatment of osteomyelitis, acute sinonasal infections, urinary tract infections, and acute bronchitis9-11.
Cephalexin is a first-generation cephalosporin that is used for treatment of several bacterial infections. It is effective against Gram-positive and Gram-negative bacteria by affecting their cell walls. It is also among the most commonly prescribed medications after maxillofacial surgical procedures8,12,13.
Recent studies have shown that the antibiotics levofloxacin, trovafloxacin, and ciprofloxacin exhibit chondrotoxic effects and decelerate bone healing14,15. Despite the availability of numerous studies regarding the effects of antibiotics on wound healing and their systemic effects, their impact on bone healing is not clearly understood. In addition, the available studies regarding the effect of antibiotics on bone healing are widely variable with respect to type of antibiotics and have reported controversial results. Therefore, this study aimed to compare the effects of systemic administration of levofloxacin or cephalexin on fracture healing in rats.
This study was performed at Dental Research Center, School of Dentistry, Isfahan University of Medical Sciences (Isfahan, Iran) taking six months period from April 2020 to October 2020. This animal study was conducted on male Wistar albino rats at 12 weeks of age and 250-300 g weights. The sample size was calculated to be 5 in each group considering α=0.05, β=0.2, and a study power of 80%. The study was conducted in accordance with the guidelines for the care and use of laboratory animals16 and was approved by the Ethics Committee of Islamic Azad University, School of Dentistry, Khorasgan Branch (IR.IAU.KHUISF.REC.1399.214). In addition, the study was carried out in accordance with the Declaration of Helsinki (2013).
The inclusion criteria were male albino Wistar rats at 12 weeks of age and 250-300 g weights with no infections or pathological conditions. Rats that developed any infections with cardinal symptoms such as redness or pus discharge at the surgical site, fever, and those with pathological conditions were excluded.
The rats were obtained from the Torabinejad Animal Research Center. They were kept in cages at 22°C±2°C temperature and 40%-60% moisture with 12-hour dark/12-hour light cycles and ad libitum access to food and water.
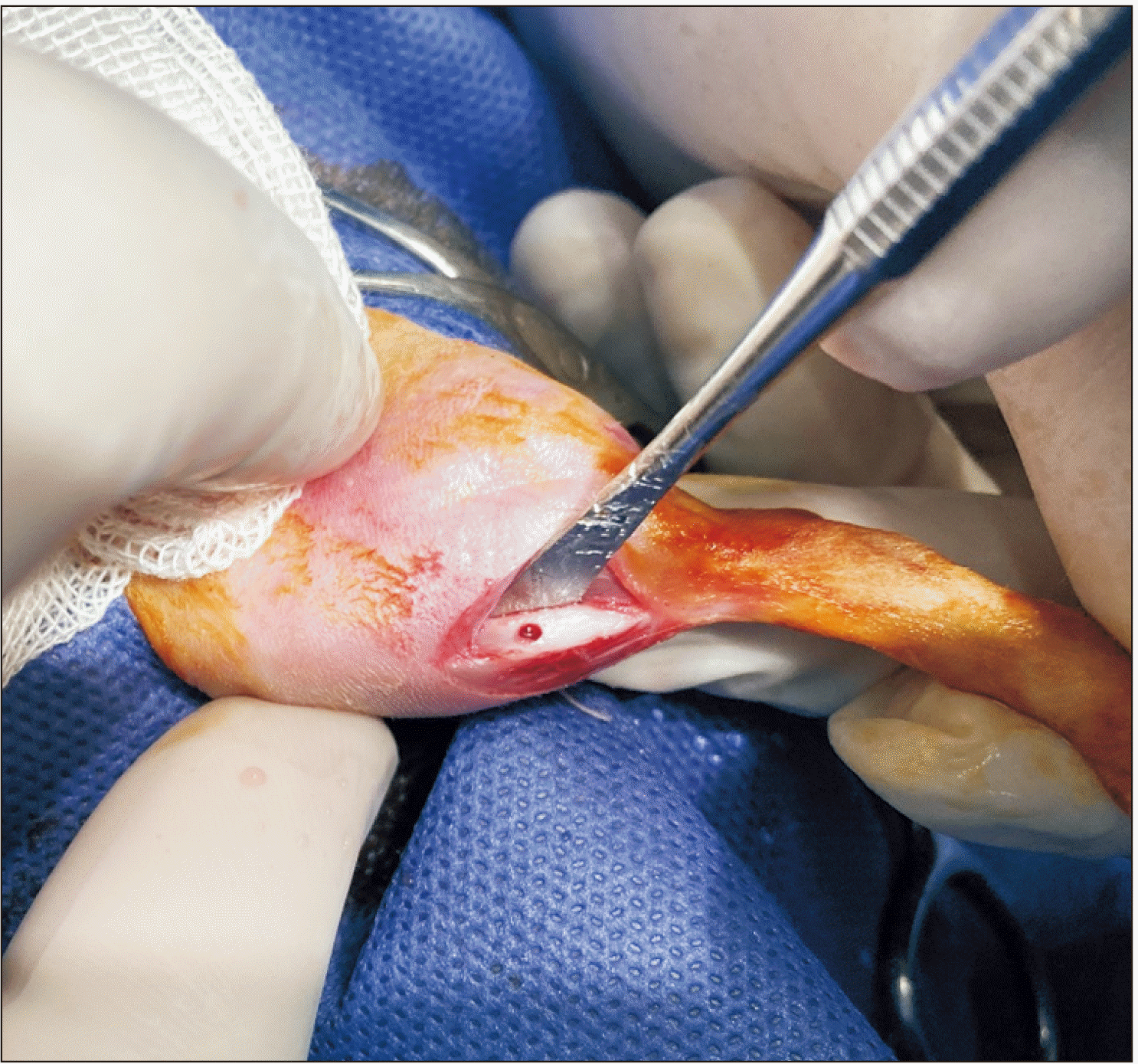
General anesthesia was induced through injection of 70 mg/kg ketamine and 0.02 mL/kg acepromazine maleate intramuscularly and 12 mg/kg xylazine intraperitoneally. Next, 0.5 mL of articaine with 1:200,000 epinephrine was injected for local anesthesia and hemostasis at the surgical site. The surgical site was shaved, and the area was prepared and disinfected with povidone iodine (Behvazan, Rasht, Iran). A tibial fracture not requiring fixation was induced (Fig. 1) using a 1.1 mm surgical bur17. All surgical procedures were performed by the same surgeon. In addition, all rats received 1 mg/kg tramadol intramuscularly for 5 days postoperatively for pain management.
After the surgical procedures, the rats were randomly divided into 6 groups (n=5). Groups 1 and 2 received daily subcutaneous saline injections. Groups 3 and 4 received subcutaneous injections of 25 mg/kg levofloxacin (Levofloxacin Solution 5 mg/mL; Normon Pharmaceutical, Madrid, Spain) twice daily18. Groups 5 and 6 received daily subcutaneous injections of 20 mg/kg cephalexin (Cefazolin Vial 1 g; Jaberebnehyyan Pharmaceutical, Tehran, Iran)19. The rats in Groups 1, 3, and 5 were sacrificed after 1 week (following completion of the inflammatory phase of bone healing), while the rats in Groups 2, 4, and 6 were sacrificed after 4 weeks (after the bone remodeling phase) through administration of high-dose sodium pentobarbital8.
The induced tibial bone fracture did not require fixation and was not extensive enough to create movement limitations. The fractured tibial bone was resected and underwent histological analysis by a pathologist blinded to the group allocation of specimens. The specimens were immediately immersed in 10% formalin for 24 hours of fixation and then decalcified in 20% nitric acid. After 2 weeks, the specimens were dehydrated and embedded in paraffin, sectioned into 4-µm-thick slices by a microtome, and stained with H&E. The stained sections were inspected under a light microscope (Nikon Eclipse E600; Nikon, Tokyo, Japan) at ×100 and ×400 magnifications, and the score of fracture healing was determined according to Perry et al.8 as follows:
Score 1. All fibrous tissue
Score 2. Mainly fibrous tissue and a small amount of cartilage tissue
Score 3. Equal amounts of fibrous and cartilage tissues
Score 4. All cartilage tissue
Score 5. Mainly cartilage tissue and a small amount of immature (woven) bone
Score 6. Equal amounts of cartilage tissue and immature bone
Score 7. Significantly immature (woven) bone and a small amount of cartilage
Score 8. All immature (woven) bone
Score 9. Immature bone and a small amount of mature bone
Score 10. All mature (lamellar) bone
Data were analyzed using IBM SPSS Statistics (ver. 22; IBM, Armonk, NY, USA) by the Kruskal–Wallis test for general comparisons and the Mann–Whitney U test for pairwise comparisons. The level of significance was set at 0.05.
None of the rats expired during the study, and they all tolerated the surgical procedures well.
Table 1 presents the frequency of fracture healing scores at 1 and 4 weeks in the rats that received saline injections. As shown, the mean score of fracture healing was 5.20±0.84 at 1 week and 9.80±0.45 at 4 weeks in the rats that received saline injections. The Mann–Whitney test showed that the mean score of fracture healing at 4 weeks was significantly higher than that at 1 week in rats that received saline injections (P=0.005).
Table 2 presents the frequency of fracture healing scores at 1 and 4 weeks in rats that received levofloxacin injections. As shown, the mean score of fracture healing was 4.60±0.55 at 1 week and 7.80±0.84 at 4 weeks in rats that received levofloxacin injections. The Mann–Whitney test showed that the mean score of fracture healing at 4 weeks was significantly higher than that at 1 week in rats that received levofloxacin injections (P=0.001).
Table 3 presents the frequency of fracture healing scores at 1 and 4 weeks in rats that received cephalexin injections. As shown, the mean score of fracture healing was 4.80±0.45 at 1 week and 9.00±1.00 at 4 weeks in rats that received cephalexin injections. The Mann–Whitney test showed that the mean score of fracture healing at 4 weeks was significantly higher than that at 1 week in rats that received cephalexin injections (P=0.001).
As indicated in Table 4, the Kruskal–Wallis test showed no significant difference among the saline, levofloxacin, and cephalexin groups in the mean score of fracture healing at 1 week (P=0.360). However, this difference was significant at 4 weeks (P=0.018).
As shown in Table 5, the Mann–Whitney post-hoc test with Bonferroni adjustment revealed that the mean score of fracture healing in the saline group was significantly higher than that in the levofloxacin group (P=0.015). No other significant differences were noted (P>0.05).
Fracture healing is an important topic in oral and maxillofacial surgery, and resumption of function after fracture is a major goal in oral and maxillofacial surgical procedures. The outcome of surgery is influenced by a number of patient-related factors as well as the type of bone defect and surgical approach20. In addition, due to the risk of infections such as osteomyelitis, antibiotics are commonly prescribed as a part of treatment21. The results of studies on the effects of antibiotics on bone healing have been controversial22, and several reports are available regarding their adverse effects in this respect8,14.
In the present study, tibial fractures were induced since the tibia has the same fetal origin as the mandible. The creation of defects using a 1.1 mm surgical bur was in accordance with the study by Krischak et al.17. Cephalexin was evaluated in this study since it is routinely prescribed for prevention of hospital-acquired infections. Levofloxacin was also assessed since it is a newer antibiotic with good penetration into bone. The results showed significantly greater bone healing at 4 weeks compared with 1 week in all three groups. At 4 weeks, the mean score of fracture healing in the saline group was significantly higher than that in the levofloxacin group. No other significant differences were noted.
The present study found no significant difference in fracture healing between the cephalexin and saline groups. No previous study is available regarding the effect of cephalexin on bone healing to compare our results. In the review study of Kallala et al.23 on the effects of cephalosporin antibiotics on metabolism of bone cells and fracture healing, no animal studies were found, and it was reported that the available relevant human studies concerning this topic are limited. They concluded that cephalosporin is the main antibiotic administered prophylactically in the majority of orthopedic trauma patients, and that prophylaxis with cephalosporin antibiotics most likely has no adverse effects on fracture healing. In addition, Holtom et al.24 demonstrated that ciprofloxacin (a cephalosporin antibiotic) exhibited moderate inhibitory effects on the proliferation of osteoblasts. According to Huddleston et al.14, cefazolin (a cephalosporin antibiotic) had no significant effects on bone healing in comparison with the control group. Moreover, Salzmann et al.25 reported that cefuroxime (a cephalosporin antibiotic) in low concentrations exhibited no inhibitory effects on human osteoblasts. Edin et al.26 also demonstrated that cefazolin in concentrations ≤100 µg/mL had minimal or no effect on proliferation of osteoblasts.
The present results revealed that the score of fracture healing in the levofloxacin group was significantly lower than that in the control group. This finding was in line with the results of Perry et al.8 who concluded that administration of levofloxacin would impair bone healing. Levofloxacin is a fluoroquinolone effective against a wide range of microorganisms and can penetrate into soft tissues, bone, and intracellular fluid27,28. Quinolones are extensively used for treatment of osteomyelitis. The recent generations of quinolones are highly effective against the common pathogens involved in osteomyelitis and achieve an adequate concentration in bone. In addition, they have a relatively long half-life with limited side effects and are well tolerated clinically. Quinolones inhibit bacterial topoisomerases (DNA gyrases) such as topoisomerase IV in Gram-positive bacteria and exert their antibacterial effects as such. Topoisomerases are imperative for induction of negative supercoiling in the tertiary structure of DNA. Attachment of quinolone antibiotics to DNA gyrase is fatal for division of prokaryotic cells24. Nonetheless, several studies have shown that quinolones cause irreversible arthropathy in many animal species29-33. Egerbacher et al.34,35 demonstrated that quinolones caused cytoskeletal changes and resulted in separation of human and rat cartilage under in vitro conditions. Huddleston et al.14 indicated that administration of ciprofloxacin (which is a fluoroquinolone) at the time of bone fracture would decelerate the process of bone healing particularly in the initial phases. Quinolones have been shown to be cytotoxic for eukaryotic cells. Preclinical toxicological assessments have shown nephrotoxicity and cardiac toxicity of fluoroquinolones in laboratory animals24,36. Chondrotoxicity is the most important type of cytotoxicity related to fluoroquinolones. Several in vitro and in vivo studies have confirmed the chondrotoxicity of quinolones8,14,34,37,38. Fracture healing begins with endochondral osteogenesis, which is then followed by intramembranous and endochondral ossification39. Chondrotoxicity can affect the cartilage and impair the next steps of endochondral ossification. Although the exact mechanism of the chondrotoxicity of quinolones has not been well-elucidated, it appears that their ability to chelate two-valence and three-valence cations is involved24. Perry et al.8 and Huddleston et al.14 showed that levofloxacin and ciprofloxacin impaired the proliferation of chondrocytes, significantly decreased callus formation at the fracture site, and prevented complete fracture healing. However, in the present study, levofloxacin (which is also a fluoroquinolone) exhibited no adverse effects on bone healing at 1 week. This difference might be due to the cancellous bone and optimal blood supply of the tibia, as well as the greater safety of levofloxacin than ciprofloxacin. However, Huddleston et al.14 showed that administration of cefazolin for 3 weeks had no adverse effects on osteogenesis, which was similar to the present findings. Förster et al.37 indicated that the in vitro chondro-pathogenic effects of quinolones were greater in tissues exposed to higher concentrations of quinolones and were detected sooner. Nonetheless, other mechanisms have also been suggested for the chondrotoxicity of quinolones in eukaryotic cells. Holtom et al.24 indicated that quinolones inhibited extracellular matrix mineralization, and treatment with levofloxacin slightly inhibited calcium deposition in injured bone tissue. This statement can explain the adverse effects of levofloxacin noted in the present study at 4 weeks.
This study had some limitations. The bone response to higher and lower doses of the drugs remains unknown and should be evaluated in further studies. In addition, bone healing was evaluated at 1 and 4 weeks, and the effects of these antibiotics over longer periods of time must be investigated in future studies. Moreover, similar studies on larger animals such as rabbits and dogs should be carried out to obtain results more generalizable to humans. Furthermore, radiographic, biomechanical, and genetic assessments (expression of genes involved in bone healing) can provide valuable information and further elucidate this topic.
The present study was the first to show the possible adverse effects of long-term administration of levofloxacin versus cephalexin on fracture healing. Therefore, it is recommended not to prescribe levofloxacin for more than 1 week after surgical management of bone fractures due to its possible adverse effects on fracture healing.
Notes
Authors’ Contributions
S.G. conceived of the presented idea and planned the experiments and participated in data collection wrote the manuscript with support from A.G. and A.A.G. A.G. supervised and designed the project. A.A.G. participated in histological species preparation and verified the analytical methods.
References
1. Shokier H, Khalifa G, Fawzy A, Sallam MM. 2010; Experimental use of autogenous bone grafts as an alternative method for bone plates in treatment of mandibular fracture. Aust J Basic Appl Sci. 4:1466–72.
2. Uçan MC, Koparal M, Ağaçayak S, Gunay A, Ozgoz M, Atilgan S, et al. 2013; Influence of caffeic acid phenethyl ester on bone healing in a rat model. J Int Med Res. 41:1648–54. https://doi.org/10.1177/0300060513490613. DOI: 10.1177/0300060513490613. PMID: 24065455.

3. Hausman MR, Schaffler MB, Majeska RJ. 2001; Prevention of fracture healing in rats by an inhibitor of angiogenesis. Bone. 29:560–4. https://doi.org/10.1016/s8756-3282(01)00608-1. DOI: 10.1016/S8756-3282(01)00608-1. PMID: 11728927.

4. Keramaris NC, Calori GM, Nikolaou VS, Schemitsch EH, Giannoudis PV. 2008; Fracture vascularity and bone healing: a systematic review of the role of VEGF. Injury. 39 Suppl 2:S45–57. https://doi.org/10.1016/S0020-1383(08)70015-9. DOI: 10.1016/S0020-1383(08)70015-9. PMID: 18804573.

5. Cook JJ, Summers NJ, Cook EA. 2015; Healing in the new millennium: bone stimulators: an overview of where we've been and where we may be heading. Clin Podiatr Med Surg. 32:45–59. https://doi.org/10.1016/j.cpm.2014.09.003. DOI: 10.1016/j.cpm.2014.09.003. PMID: 25440417.

6. MalekiGorji M, Golestaneh A, Razavi SM. 2020; The effect of two phosphodiesterase inhibitors on bone healing in mandibular fractures (animal study in rats). J Korean Assoc Oral Maxillofac Surg. 46:258–65. https://doi.org/10.5125/jkaoms.2020.46.4.258. DOI: 10.5125/jkaoms.2020.46.4.258. PMID: 32855373. PMCID: PMC7469969.

7. Massari L, Caruso G, Sollazzo V, Setti S. 2009; Pulsed electromagnetic fields and low intensity pulsed ultrasound in bone tissue. Clin Cases Miner Bone Metab. 6:149–54. PMID: 22461165. PMCID: PMC2781224.
8. Perry AC, Prpa B, Rouse MS, Piper KE, Hanssen AD, Steckelberg JM, et al. 2003; Levofloxacin and trovafloxacin inhibition of experimental fracture-healing. Clin Orthop Relat Res. 414:95–100. https://doi.org/10.1097/01.blo.0000087322.60612.14. DOI: 10.1097/01.blo.0000087322.60612.14. PMID: 12966282.

9. Maxwell A. 1992; The molecular basis of quinolone action. J Antimicrob Chemother. 30:409–14. https://doi.org/10.1093/jac/30.4.409. DOI: 10.1093/jac/30.4.409. PMID: 1337067.

10. Rissing JP. 1997; Antimicrobial therapy for chronic osteomyelitis in adults: role of the quinolones. Clin Infect Dis. 25:1327–33. https://doi.org/10.1086/516150. DOI: 10.1086/516150. PMID: 9431371.

11. Davis R, Bryson HM. 1994; Levofloxacin. A review of its antibacterial activity, pharmacokinetics and therapeutic efficacy. Drugs. 47:677–700. https://doi.org/10.2165/00003495-199447040-00008. DOI: 10.2165/00003495-199447040-00008. PMID: 7516863.

12. Shahabadi N, Hashempour S. 2019; DNA binding studies of antibiotic drug cephalexin using spectroscopic and molecular docking techniques. Nucleosides Nucleotides Nucleic Acids. 38:428–47. https://doi.org/10.1080/15257770.2018.1562071. DOI: 10.1080/15257770.2018.1562071. PMID: 30931791.

13. Papich MG, Lindeman C. 2018; Cephalexin susceptibility breakpoint for veterinary isolates: Clinical Laboratory Standards Institute revision. J Vet Diagn Invest. 30:113–20. https://doi.org/10.1177/1040638717742434. DOI: 10.1177/1040638717742434. PMID: 29145786. PMCID: PMC6504153.

14. Huddleston PM, Steckelberg JM, Hanssen AD, Rouse MS, Bolander ME, Patel R. 2000; Ciprofloxacin inhibition of experimental fracture healing. J Bone Joint Surg Am. 82:161–73. https://doi.org/10.2106/00004623-200002000-00002. DOI: 10.2106/00004623-200002000-00002. PMID: 10682725.

15. Atalay Y, Gunes N, Guner MD, Akpolat V, Celik MS, Guner R. 2015; Pentoxifylline and electromagnetic field improved bone fracture healing in rats. Drug Des Devel Ther. 9:5195–201. https://doi.org/10.2147/DDDT.S89669. DOI: 10.2147/DDDT.S89669. PMID: 26388687. PMCID: PMC4571933.

16. Clark JD, Gebhart GF, Gonder JC, Keeling ME, Kohn DF. 1997; Special report: the 1996 guide for the care and use of laboratory animals. ILAR J. 38:41–8. https://doi.org/10.1093/ilar.38.1.41. DOI: 10.1093/ilar.38.1.41. PMID: 11528046.

17. Krischak GD, Augat P, Blakytny R, Claes L, Kinzl L, Beck A. 2007; The non-steroidal anti-inflammatory drug diclofenac reduces appearance of osteoblasts in bone defect healing in rats. Arch Orthop Trauma Surg. 127:453–8. https://doi.org/10.1007/s00402-007-0288-9. DOI: 10.1007/s00402-007-0288-9. PMID: 17245601.

18. Sitovs A, Voiko L, Kustovs D, Kovalcuka L, Bandere D, Purvina S, et al. 2020; Pharmacokinetic profiles of levofloxacin after intravenous, intramuscular and subcutaneous administration to rabbits (Oryctolagus cuniculus). J Vet Sci. 21:e32. https://doi.org/10.4142/jvs.2020.21.e32. DOI: 10.4142/jvs.2020.21.e32. PMID: 32233138. PMCID: PMC7113567.

19. Williams BH. 2000; Therapeutics in ferrets. Vet Clin North Am Exot Anim Pract. 3:131–53. vihttps://doi.org/10.1016/s1094-9194(17)30098-1. DOI: 10.1016/S1094-9194(17)30098-1. PMID: 11228824. PMCID: PMC7110454.

20. Hernandez RK, Do TP, Critchlow CW, Dent RE, Jick SS. 2012; Patient-related risk factors for fracture-healing complications in the United Kingdom General Practice Research Database. Acta Orthop. 83:653–60. https://doi.org/10.3109/17453674.2012.747054. DOI: 10.3109/17453674.2012.747054. PMID: 23140093. PMCID: PMC3555441.

21. Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MK, et al. 2013; ; American Society of Health-System Pharmacists (ASHP); Infectious Diseases Society of America (IDSA); Surgical Infection Society (SIS); Society for Healthcare Epidemiology of America (SHEA). Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect (Larchmt). 14:73–156. https://doi.org/10.1089/sur.2013.9999. DOI: 10.1089/sur.2013.9999. PMID: 23461695.

22. Pountos I, Georgouli T, Bird H, Kontakis G, Giannoudis PV. 2011; The effect of antibiotics on bone healing: current evidence. Expert Opin Drug Saf. 10:935–45. https://doi.org/10.1517/14740338.2011.589833. DOI: 10.1517/14740338.2011.589833. PMID: 21824037.

23. Kallala R, Graham SM, Nikkhah D, Kyrkos M, Heliotis M, Mantalaris A, et al. 2012; In vitro and in vivo effects of antibiotics on bone cell metabolism and fracture healing. Expert Opin Drug Saf. 11:15–32. https://doi.org/10.1517/14740338.2012.643867. DOI: 10.1517/14740338.2012.643867. PMID: 22149454.

24. Holtom PD, Pavkovic SA, Bravos PD, Patzakis MJ, Shepherd LE, Frenkel B. 2000; Inhibitory effects of the quinolone antibiotics trovafloxacin, ciprofloxacin, and levofloxacin on osteoblastic cells in vitro. J Orthop Res. 18:721–7. https://doi.org/10.1002/jor.1100180507. DOI: 10.1002/jor.1100180507. PMID: 11117292.

25. Salzmann GM, Naal FD, von Knoch F, Tuebel J, Gradinger R, Imhoff AB, et al. 2007; Effects of cefuroxime on human osteoblasts in vitro. J Biomed Mater Res A. 82:462–8. https://doi.org/10.1002/jbm.a.31158. DOI: 10.1002/jbm.a.31158. PMID: 17295250.

26. Edin ML, Miclau T, Lester GE, Lindsey RW, Dahners LE. 1996; Effect of cefazolin and vancomycin on osteoblasts in vitro. Clin Orthop Relat Res. 333:245–51. DOI: 10.1097/00003086-199612000-00027. PMID: 8981903.

27. Brown SA. 1996; Fluoroquinolones in animal health. J Vet Pharmacol Ther. 19:1–14. https://doi.org/10.1111/j.1365-2885.1996.tb00001.x. DOI: 10.1111/j.1365-2885.1996.tb00001.x. PMID: 8992019.

28. Martinez M, McDermott P, Walker R. 2006; Pharmacology of the fluoroquinolones: a perspective for the use in domestic animals. Vet J. 172:10–28. https://doi.org/10.1016/j.tvjl.2005.07.010. DOI: 10.1016/j.tvjl.2005.07.010. PMID: 16154368.

29. Förster C, Kociok K, Shakibaei M, Merker HJ, Stahlmann R. 1996; Quinolone-induced cartilage lesions are not reversible in rats. Arch Toxicol. 70:474–81. https://doi.org/10.1007/s002040050301. DOI: 10.1007/s002040050301. PMID: 8783810.

30. Gough A, Johnson R, Campbell E, Hall L, Tylor J, Carpenter A, et al. 1996; Quinolone arthropathy in immature rabbits treated with the fluoroquinolone, PD 117596. Exp Toxicol Pathol. 48:225–32. https://doi.org/10.1016/S0940-2993(96)80003-0. DOI: 10.1016/S0940-2993(96)80003-0. PMID: 8811288.

31. Kato M, Takada S, Ogawara S, Takayama S. 1995; Effect of levofloxacin on glycosaminoglycan and DNA synthesis of cultured rabbit chondrocytes at concentrations inducing cartilage lesions in vivo. Antimicrob Agents Chemother. 39:1979–83. https://doi.org/10.1128/AAC.39.9.1979. DOI: 10.1128/AAC.39.9.1979. PMID: 8540702. PMCID: PMC162867.

32. Linseman DA, Hampton LA, Branstetter DG. 1995; Quinolone-induced arthropathy in the neonatal mouse. Morphological analysis of articular lesions produced by pipemidic acid and ciprofloxacin. Fundam Appl Toxicol. 28:59–64. https://doi.org/10.1006/faat.1995.1146. DOI: 10.1006/faat.1995.1146. PMID: 8566484.

33. Shakibaei M, Kociok K, Förster C, Vormann J, Günther T, Stahlmann R, et al. 1996; Comparative evaluation of ultrastructural changes in articular cartilage of ofloxacin-treated and magnesium-deficient immature rats. Toxicol Pathol. 24:580–7. https://doi.org/10.1177/019262339602400507. DOI: 10.1177/019262339602400507. PMID: 8923679.

34. Egerbacher M, Edinger J, Tschulenk W. 2001; Effects of enrofloxacin and ciprofloxacin hydrochloride on canine and equine chondrocytes in culture. Am J Vet Res. 62:704–8. https://doi.org/10.2460/ajvr.2001.62.704. DOI: 10.2460/ajvr.2001.62.704. PMID: 11341389.

35. Egerbacher M, Seiberl G, Wolfesberger B, Walter I. 2000; Ciprofloxacin causes cytoskeletal changes and detachment of human and rat chondrocytes in vitro. Arch Toxicol. 73:557–63. https://doi.org/10.1007/s002040050008. DOI: 10.1007/s002040050008. PMID: 10663387.

36. Stahlmann R, Schwabe R. 1997; Safety profile of grepafloxacin compared with other fluoroquinolones. J Antimicrob Chemother. 40 Suppl A:83–92. https://doi.org/10.1093/jac/40.suppl_1.83. DOI: 10.1093/jac/40.suppl_1.83. PMID: 9484877.

37. Förster C, Rücker M, Shakibaei M, Baumann-Wilschke I, Vormann J, Stahlmann R. 1998; Effects of fluoroquinolones and magnesium deficiency in murine limb bud cultures. Arch Toxicol. 72:411–9. https://doi.org/10.1007/s002040050521. DOI: 10.1007/s002040050521. PMID: 9708880.

38. Menschik M, Neumüller J, Steiner CW, Erlacher L, Köller M, Ullrich R, et al. 1997; Effects of ciprofloxacin and ofloxacin on adult human cartilage in vitro. Antimicrob Agents Chemother. 41:2562–5. https://doi.org/10.1128/AAC.41.11.2562. DOI: 10.1128/AAC.41.11.2562. PMID: 9371369. PMCID: PMC164164.

39. Long F, Ornitz DM. 2013; Development of the endochondral skeleton. Cold Spring Harb Perspect Biol. 5:a008334. https://doi.org/10.1101/cshperspect.a008334. DOI: 10.1101/cshperspect.a008334. PMID: 23284041. PMCID: PMC3579395.

Fig. 2
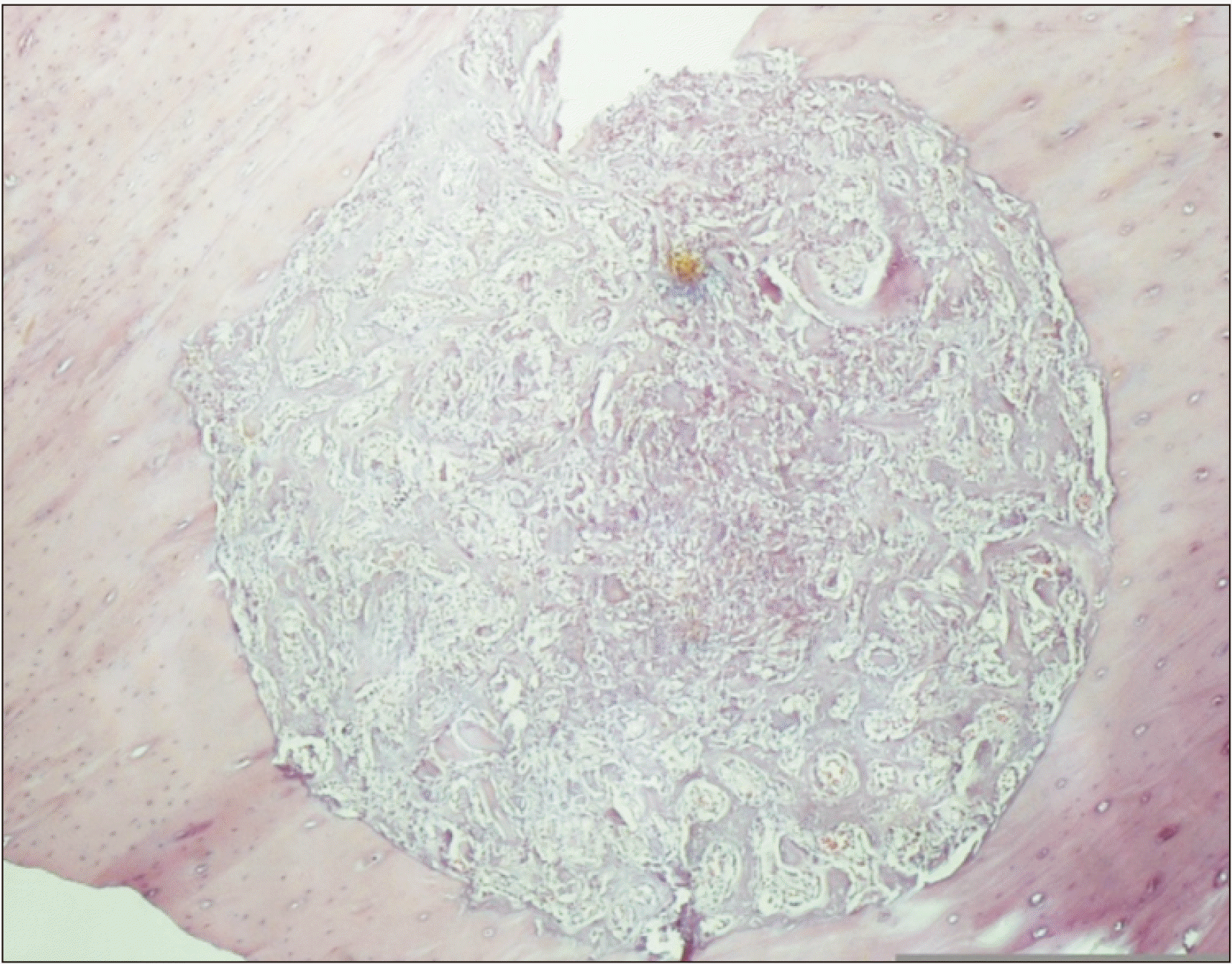
A-F. Representative histological micrographs at ×400 (H&E staining). A. Fracture healing score 5 in a specimen from the saline group at 1 week (mainly cartilage tissue and a small amount of immature woven bone). B. Score 4 in a specimen from the levofloxacin group at 1 week (all cartilage tissue). C. Score 5 in a specimen from the cephalexin group at 1 week (mainly cartilage tissue and a small amount of immature woven bone). D. Score 9 in a specimen from the saline group at 4 weeks (immature bone and a small amount of mature bone). E. Score 7 in a specimen from the levofloxacin group at 4 weeks (significantly immature woven bone and a small amount of cartilage). F. Score 9 in a specimen from the cephalexin group at 4 weeks (immature bone and a small amount of mature bone).

Table 1
Frequency of fracture healing scores at 1 and 4 weeks in rats that received saline injections
Table 2
Frequency of fracture healing scores at 1 and 4 weeks in rats that received levofloxacin injections
Table 3
Frequency of fracture healing scores at 1 and 4 weeks in rats that received cephalexin injections
Table 4
Comparison of the mean score of fracture healing among the saline, levofloxacin, and cephalexin groups at 1 and 4 weeks using the Kruskal–Wallis test (n=5)
| Time point | Group | Mean rank | χ2 | Degree of freedom | P-value |
|---|---|---|---|---|---|
| 1 week | Saline | 9.90 | 2.043 | 2 | 0.360 |
| Levofloxacin | 6.40 | ||||
| Cephalexin | 7.70 | ||||
| 4 weeks | Saline | 11.60 | 7.994 | 2 | 0.018 |
| Levofloxacin | 4.00 | ||||
| Cephalexin | 8.40 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download