Abstract
Objectives
The aim of this study was to compare morbidities and duration of surgery, as well as bone formation in alveolar defects reconstructed with symphysis bone combined with allograft and iliac crest bone graft in patients with cleft palate.
Patients and Methods This randomized clinical trial was performed with 22 patients with unilateral alveolar cleft with a follow-up period of 12 months. In 12 patients, alveolar defects were reconstructed with chin bone graft plus allograft (Group A), while for the other 10 patients, iliac bone crest was used as donor site (Group B). Duration of surgery as well as occurrence of morbidities and complications were recorded. In addition, cone-beam computed tomographic (CBCT) scans were performed before surgery and 12 months after surgical procedures in order to compare bone formation between the two groups.
Results
Postoperative CBCT demonstrated a mean bone fill percentage of 76.9% of the alveolar defect in Group A, compared with 77.0% in Group B. Paresthesia in the lower lip or chin did not occur in any patients of Group A. The mean duration of the surgical process was significantly shorter for Group A (40 minutes vs 76 minutes, P<0.001). In addition, patients in Group A regained normal gait faster than patients in Group B (1 day vs 9.5 days).
Orofacial clefts are the most common congenital abnormalities of the head and neck region and can involve the jaws, lips, and hard and soft palates1. Complications such as dental anomalies, malocclusion, facial deformity, malnutrition, and respiratory-auditory-vocal dysfunction can accompany orofacial clefts. Hereditary and environmental factors have pivotal roles in the etiology of facial clefts. Thus, orofacial clefts are considered to have a multi-factorial etiology2. Environmental factors such as maternal hormonal disorders, neurologic drugs, vitamins and folic acid deficiency, hypoxia and smoking, obesity of the mother, and even season in incidence of cleft patients have been reported3-8.
Patients suffering from Tessier cleft types 2 and 3 require a special sequence of treatments from childhood to adolescence. Alveolar bone graft is one of the main reconstruction techniques in this sequence9. Advantages of alveolar bone grafting for reconstruction of the alveolar cleft include maxillary arch stability, elimination of oronasal fistula, ideal bone support for tooth eruption, reconstruction of undeveloped nasal piriform aperture, providing support for soft tissue of the nasal base, and creating better bone support for future implant placement9,10. Secondary alveolar bone graft, described by Boyne and Sands in 1970 for the first time, is currently the most popular method for alveolar bone graft treatment for cleft patients. This method provides an adequate amount of bone for eruption of maxillary canines without adverse effects in the alveolar process11.
The objective of this study was to compare the success rate and morbidities of bone formation in alveolar cleft treatment using anterior iliac crest bone versus chin symphysis bone graft in combination with allograft.
This randomized controlled trial was registered at the Iranian Registry of Clinical Trials (#IRCT20210515051308n1). This study was conducted on 22 patients referred to the Cleft Palate Center of Isfahan University of Medical Sciences from 2015 to 2017. The Ethical Committee of Isfahan University of Medical Sciences approved this study (IR.MUI.REC.1397.3.131).
The inclusion criteria were unilateral alveolar cleft patients without any systemic diseases or previous reconstructive treatment for alveolar cleft. Exclusion criteria were incidence of intraoperative emergencies or changes in treatment plan, as well as patient unwillingness to participate in the study at any stage. All patients were informed about each surgical procedure and its advantages/disadvantages. Thereafter, written consent forms were signed and recorded.
Patients were divided into two groups randomly through simple randomization using random allocation software such that patients with even numbers were allocated to Group A and patients with odd numbers were put in Group B. For Group A, a combination of allogenic bone material and symphysis corticocancellous bone was used for reconstruction of alveolar defects. For patients in Group B, an autogenous anterior iliac crest graft was used as the control reconstructive treatment.
Preparation of the defect area was achieved intraorally using an advanced gingival flap technique. The mucosal membrane of the defect was divided into nasal and oral segments. For nasal floor reconstruction, nasal mucosal flaps were separated from bony walls and reconnected by sutures. Then, palatal mucosal flaps were sutured to ready the substrate receiver.
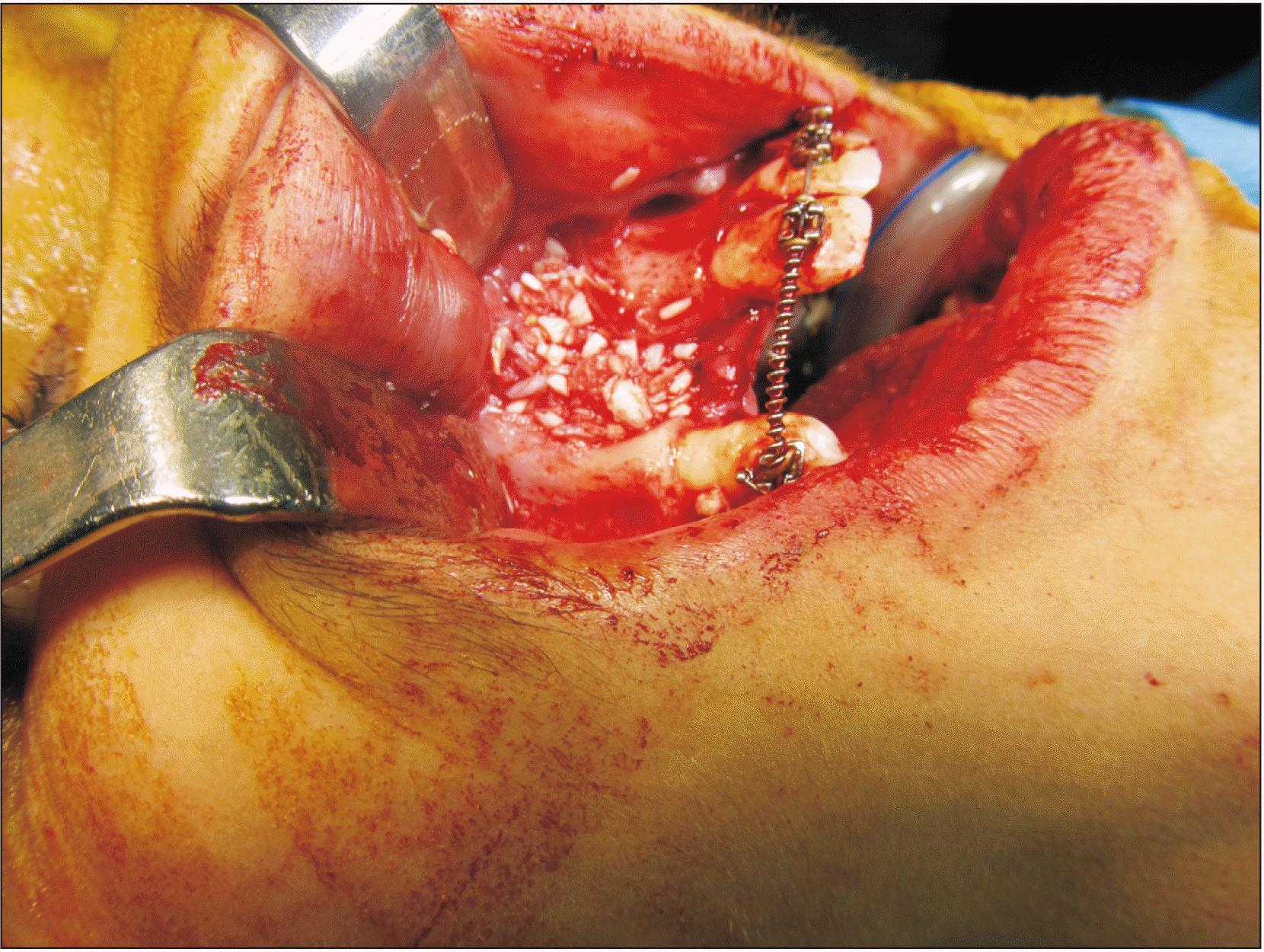
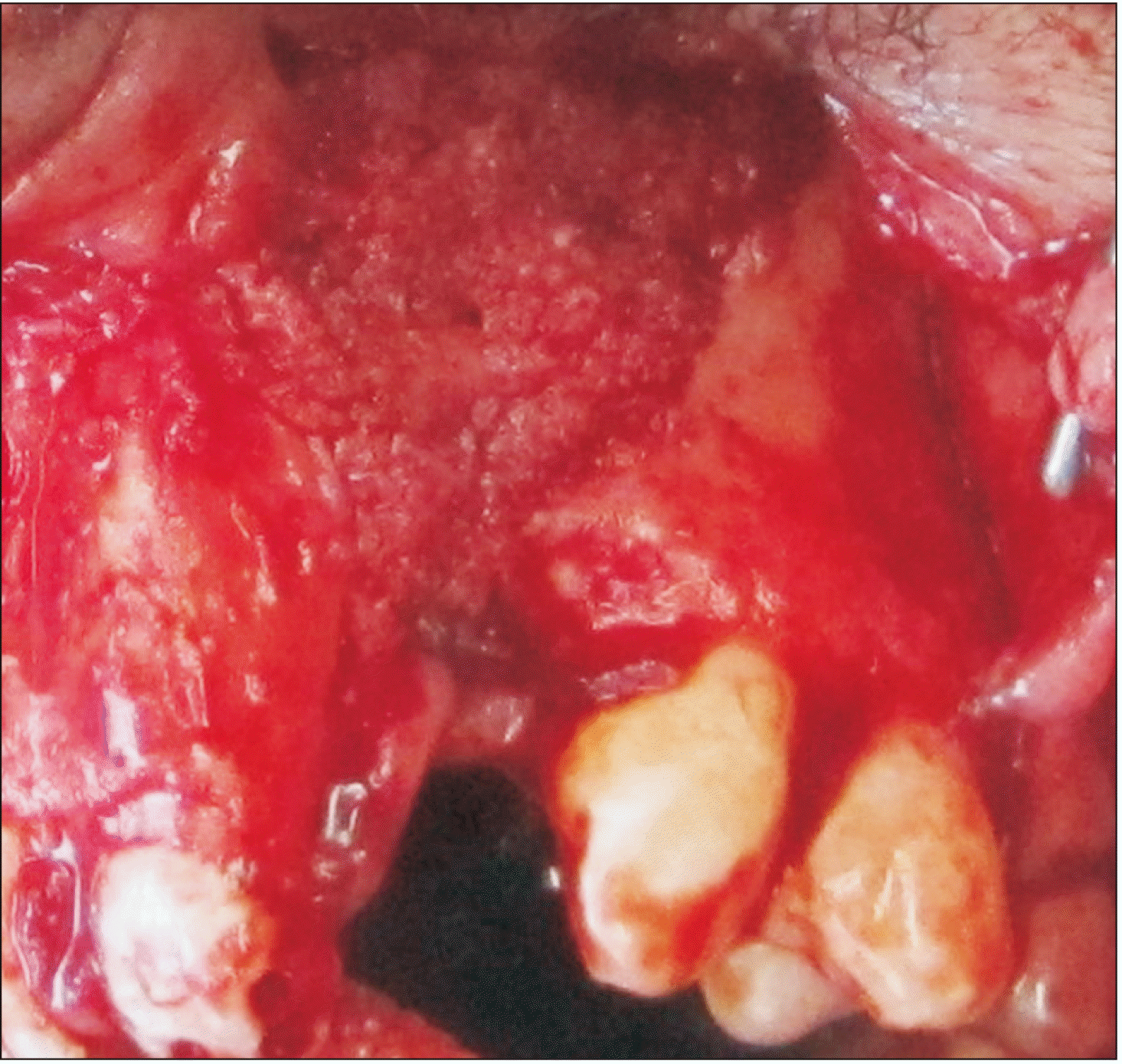
For patients in Group A, the maxillary crest mucosa was flapped using a size 15 scalpel. Then, using free mucosal tissue from the nasal bony walls and flaps suturing, the nasal floor was tightened using a watertight technique. Bony cortical pieces were extracted from the mental symphysis by embedding a sulcular incision from a canine (on one side) to a canine (on the other side), and 3-4 monocortical bone pieces with diameter of 6 milliliters were extracted using a milling trephine (Mesinger, Dusseldorf, Germany). Then, the soft tissue was sutured using polyglycolate 3.0 suture and 26 mm 3/8 reverse cutting needle (SUPA, Tehran, Iran). These pieces were divided into smaller cuts and blended with 2-5 mm allografts (Cenobone, Kish, Iran) in approximately equal amounts. Blended materials were packed in alveolar defects.(Fig. 1)
Patients in Group B were treated using autogenic anterior iliac crest graft. In this technique, an incision was made within 1 centimeter of the upper part of the anterior-superior iliac spine. As the iliac bone was exposed, monocortical bone pieces were extracted, and the soft tissue was closed. Cartilage and connective tissues were sutured using polyglycolate 3.0 suture, and skin was closed using nylon 3.0 suture and a 26 mm 3/8 reverse cutting needle (SUPA). Following packing grafts (Fig. 2), defects were closed using mucosal flaps by polyglycolate 3.0 suture. The duration of surgery was recorded for each session.
Patients in Group A were discharged after one day, while Group B patients were discharged two days after the procedure. Sutures embedded in iliac crest were removed within 10 days after surgery in Group B patients.
Patients were examined after one week, one month, three months, six months, and 12 months for assessment of surgical procedure complications.
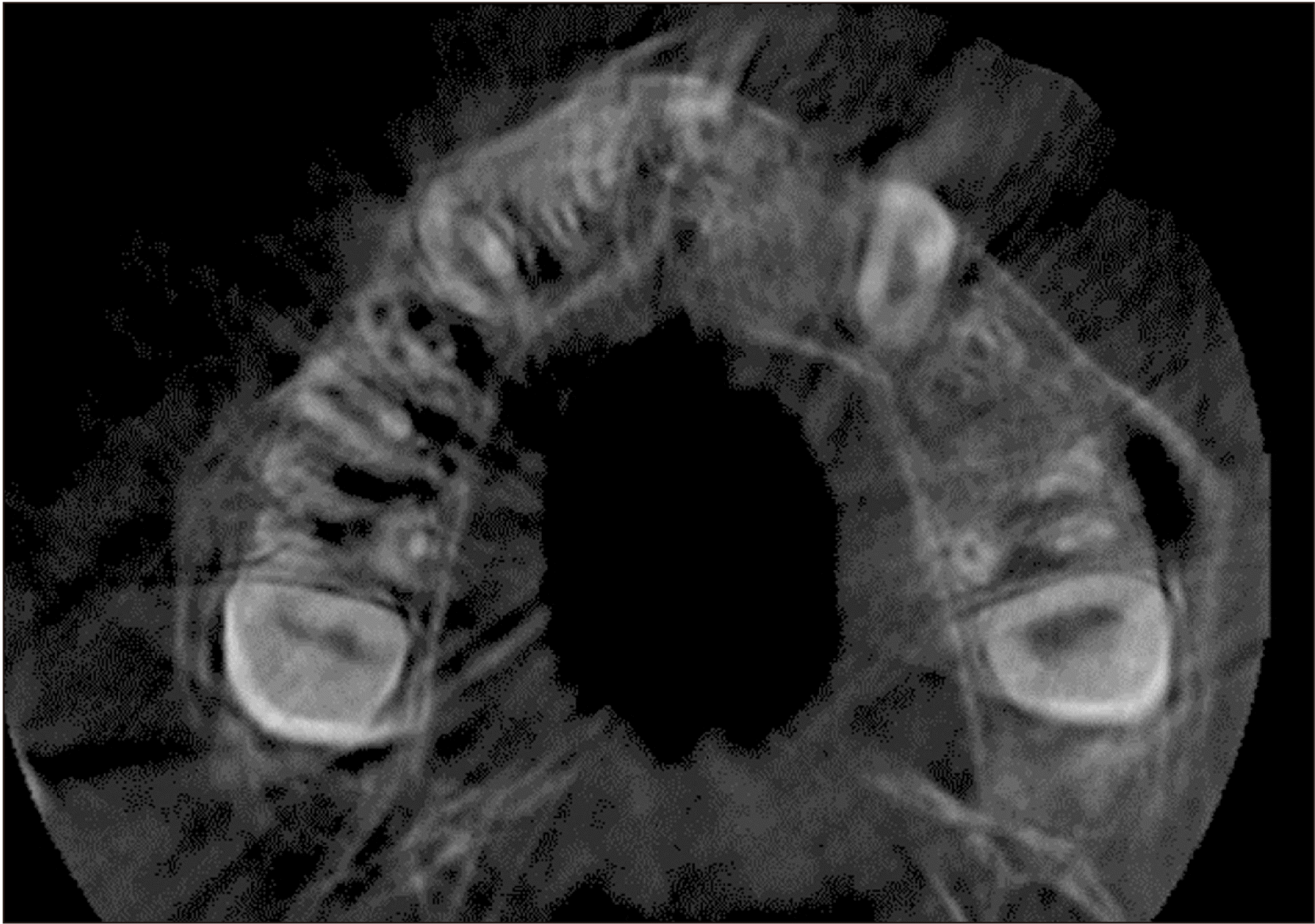
Cone-beam computed tomography (CBCT) was performed prior to and 12 months following the surgery to assess the status of the reconstructed alveolar clefts.(Fig. 3, 4) CBCT scans were obtained from the maxillary region using a Cranex 3D scanner (Soredex, Tuusula, Finland) with 90 kVp, 10 mA, voxel size of 85 µm, and field of view of 61 mm×41 mm. Images were displayed in three dimensions using OnDemand 3D software (Cybermed, Seoul, Korea). Alveolar defect diameter in the vertical plane was measured using coronal views. The lower bound of defect was considered as the cementoenamel junction adjacent to the cleft area. The superior border of the alveolar defect was considered as the base of the nasal cavity on the unaffected side. The defect volume was measured by multiplying the sum of cuts in the axial dimension by height of the defect in the coronal dimension divided by the number of assessed cuts.(Fig. 5)
A radiologist unaware of the study groups interpreted images both pre-surgically and post-surgically. CBCT images were evaluated by the radiologist again after one month to calculate intra-observer agreement.
Obtained data were statistically analyzed using IBM SPSS Statistics (ver. 23; IBM, Armonk, NY, USA) and described in mean values and percentages. The following tests were used for data analysis: intra-class correlation coefficient (ICC) to calculate intra-observer agreement, independent t-test to compare the duration of surgery and age, Fisher’s exact test to compare the sexes and sides, and multiple linear regression to compare the percentage of bone formation considering age and sex variables between the two groups. P<0.05 was considered statistically significant.
In the present study, all included patients completed the 12-month follow-up period for monitoring of sequential treatment results in the Cleft Palate Center. A total of 10 males and 12 females with a mean age of 9.7±1.7 years were recruited.(Table 1) Patient mean age (P=0.645) and sex distribution (P=0.576) were not statistically different between Group A and Group B.
Mean operation duration was 40±4.2 minutes and 76±11 minutes for Group A and Group B, respectively, indicating a significant difference (P<0.001).
No dehiscence, infection, or flap necrosis was observed in the follow-up sessions. Oronasal fistula was closed in all the patients. Paresthesia of the lips or chin was not seen in any patients of Group A or Group B. Clinical and radiographic evaluation indicated normal dental roots and teeth buds. Bridge formation was distinguishable in the CBCT scans of all patients. All patients in Group A had normal gait one day after surgery, whereas 9.5±1.2 days were required for Group B patients to walk normally.
ICC revealed high intra-observer agreement (ICC=0.980), and average volumetric measurements were included in the analysis.
The mean preoperative defect volume was 1.56 cm3 in Group A and 0.95 cm3 in Group B (P=0.001), while the mean postoperative defect was 0.35 cm3 and 0.23 cm3, respectively (P=0.102). The bone formation percentage in the groups was calculated as 76.9% in Group A and 77.0% in Group B. Multiple linear regression analysis demonstrated that, with consideration of age and sex variants, there was no significant difference in bone formation between the two groups (P=0.941).(Table 2)
According to the findings of this study, bone formation between alveolar cleft patients treated with symphysis corticocancellous bone combined with allografts and those with iliac crest bone graft is not significantly different. However, patients treated with the anterior iliac crest graft method took longer to walk normally.
Different intra- and extra-oral donor sites have been suggested for alveolar bone graft in cleft patients:
1) Anterior iliac crest is currently considered the gold standard treatment for bilateral and large alveolar defects. It provides large quantities of cancellous bone, but it has disadvantages such as donor site morbidity, operation duration, and gait disturbance12.
2) Proximal side of tibia: The tibia is gracile in childhood, and the epiphyseal cartilage of the tibia is the growth center for the bone. Therefore, it is not a suitable donor site13. Additionally, proximal tibial fractures after bone harvesting have been reported14.
3) Cranial bone has a mesenchymal origin, and the surgical scar will be hidden after hair growth. Morbidities involve dura exposure, subdural hemorrhage, neurologic complications (rarely), and excessive surgical duration15.
4) Intraoral donor sites: The chin is the most popular site for intraoral bone harvesting16. Bone grafting from this region provides a conservative method with lower rate of pain and complication for patients17. In addition, the surgical process can be performed in a shorter duration. Some authors reported more satisfactory results compared with iliac graft, which have been attributed to its mesenchymal origin compared to endochondral origin of the iliac crest18.
Several extraoral and intraoral sites have been proposed for autologous bone harvesting for reconstructive treatments of the alveolar cleft. The average bone mass provided from mandibular bone block is about 2.3 mL19, which is inadequate for large or bilateral alveolar clefts20-22. Therefore, a combination of these bone blocks and bone substitute material has to be used for larger alveolar defects. In 2020, Mahardawi et al.23 measured the impact of certain factors on the success of alveolar bone grafting in cleft patients. They found that bilateral clefts and bone defects greater than 10 mm in transverse and vertical dimensions (evaluation by panoramic radiograph and Bergland scale) increase the risk of failure by four to six times. In our study, CBCT was used for three-dimensional evaluation and volumetric measurements of the cleft defects. The volume of defects in the present patients ranged from less than 0.4 to 2 mL. However, the size of bone defects did not affect treatment success. Further studies to specify the effect of defect volume on bone regeneration are suggested. Additionally, it can be suggested that, in bilateral alveolar clefts or for large defects requiring large bone volume, autogenous bone can be mixed with non-autogenous bone grafting materials to reduce complications and achieve suitable results. In this study, autogenous bone and non-autogenous bone material were combined at a 1:1 ratio. Further research can be performed to obtain the best combination ratio for reconstruction of large alveolar bone defects.
In 2018, Elbokle and Elsholkamy24 performed a study on 12 patients with unilateral cleft palate. Volumetric assessment of bone defects and available bone in the chin area as the donor site was conducted using CBCT imaging. After six months, another CBCT image was obtained to evaluate bone regeneration. The average bone formation was 79%, which is close to the rate of our study (76.9% in Group A). However, in our study, the volume of the defect was measured after one year, which is longer than the follow-up period of Elbokle and Elsholkamy24. In another study performed in our institution, the average bone formation percentage in patients treated with a combination of chin symphysis bone, allograft, and platelet-rich fibrin (PRF) was comparable to that of those treated with iliac bone graft. The authors recommended the first approach to be appropriate for small and moderate alveolar clefts25. In the present study, although PRF was not used, the percentage of bone formation was very similar between the two groups. In addition to PRF, other material can be used in combination with conventional bone grafts. These alternatives have their advantages and disadvantages. For instance, rhBMP (recombinant human bone morphogenetic protein), which has been reported for reconstruction of alveolar clefts26, can eliminate bone harvesting from the donor site but is not approved by the U.S. Food and Drug Administration for application in children, can cause massive edema in the surgical site, and is an expensive treatment27-29. In a systematic review and meta-analysis conducted by Kamal et al.30 in 2018, both methods of using autologous bone graft and tissue-engineered bone material were concluded to be successful in the treatment of alveolar clefts. The average percentage of bone formation was 62% and 68.7%, respectively, and the most widely used substance in the tissue-engineered bone group was BMP-2 (bone morphogenetic protein 2). In the study by Thuaksuban et al.31, treatments with iliac bone graft alone were compared to treatments with iliac bone graft and deproteinized bovine bone in alveolar cleft patients. The combined group showed a significant advantage in reducing morbidity (e.g., shorter hospitalization period and recovery time to walking, etc.), but the density and height of the bony bridge formed in the defect area were not significantly different between the groups. Weijs et al.32 in 2010 arranged an investigation on 47 patients with alveolar clefts and compared treatment outcomes between patients treated with chin symphysis graft and those treated with chin symphysis graft combined with beta tricalcium phosphate (TCP). The results were evaluated in two-dimensional occlusal radiographs. After one year, both groups showed satisfying treatment outcomes, and there was no significant difference between the new bone bridges heights between the groups. In 2019, Miyagawa et al.33 investigated the effect of beta TCP on the quality of bone regeneration. They evaluated cleft patients with CBCT and used bone structural index and trabecular bone parameter. Their findings suggested that utilization of chin symphysis bone graft in combination with beta TCP increases the quality and density of bone regeneration compared to chin symphysis bone graft alone. Further studies can be performed surveying the combination of beta TCP with other autogenous grafts such as iliac, cranium, and tibia in order to provide an appropriate combination for more favorable graft outcomes.
No subsequent morbidity was found in the mandibular symphysis region. For each patient in the symphysis bone graft group, three to four monocortical bone pieces were harvested using a 6 mm trephine bur with the furthest distance from the mental foramens, dental roots, and tooth buds. As a result of this conservative approach and careful sulcular incision, paresthesia (even temporary) or damage to the teeth was not seen in our patients. However, paresthesia is reported in some studies after bone harvesting from the mandibular symphysis34,35. The only complication in our study after chin bone graft was regional pain that resolved after three weeks in all patients.
Surgery duration was significantly shorter for bone harvesting from the chin.(Fig. 6) The same finding was observed by Movahedian et al.25 in 2016 when applying PRF combined with chin bone graft and allograft for repairing alveolar defects in cleft patients. The average duration of each surgical session for iliac bone graft harvesting is reported to be longer in other studies36,37. In addition, Bukhari et al.38 reported longer duration of surgery for iliac crest bone graft compared with chin bone graft. The length of surgical sessions depends on various parameters, including surgical technique, available instruments, and experience of the surgeon, as well as other members of the operating room team39. Shorter surgical durations are preferred for benefits such as less need for anesthetic drugs, decreased bleeding, and lower risk of subsequent infection.
Considering postsurgical complications, no particular event occurred in patients in either group. However, morbidity of the surgical site was more significant for the iliac crest graft group, as they were unable to walk normally for an average of about nine days. Upon investigation, the patients reported pain while walking. Fortunately, all patients eventually regained normal gait. In our investigation, we discharged all Group B patients 48 hours after surgery with oral analgesic drugs. In our opinion, it is unlikely that iliac crest or chin pain would affect the quantity and quality of bone regeneration in the alveolar cleft area; thus, we did not evaluate these items.
A previous study by Swan and Goodacre40 reported that children for whom an iliac crest bone graft was performed could not walk normally for 0 to 56 days due to morbidity at the iliac crest donor sites. The duration for which the patient has difficulty in walking is largely variable among studies. However, considerable morbidity seems to be present following bone harvesting procedures. In a study of 64 patients, Rawashdeh41 examined the side effects of bone removal from the iliac crest. They reported that 91% of patients were able to walk after 24 hours but not normally. In addition, 89% of patients were able to walk as before surgery after two weeks. In our study, all patients were able to walk normally by the 11th day. It seems the cause of difficulty in walking in the first days was donor site pain for Group B patients. Due to the young age of the patients, fear could have intensified limping. Further research is recommended to investigate the role of fear in postoperative walking and limping in patients with iliac crest bone grafts.
Albuquerque et al.42 in 2011 confirmed the accuracy and efficiency of CBCT in determining the volume of bony defects after several measurements on nine human skulls. They recommended CBCT for diagnosis and treatment assessment of cleft palate patients. In the present study, CBCT scans were used to evaluate the bone formation in the alveolar cleft region after 12 months. In the process of healing and inflammation, bony resorption will occur to a degree. Therefore, most of the time, it is practically not possible for the entire cleft volume to be filled. However, bridging of the alveolar bone and continuity in the bone in the region are signs of a successful treatment.(Fig. 7, 8)
Acknowledgements
The present study was funded by Isfahan University of Medical Sciences (grant No. 397131).
Notes
Authors’ Contributions
D.D. participated in data collection and wrote the manuscript. B.M.A. and M.M. participated in the study design and performed the statistical analysis and patients managment. P.S. participated in the study design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Ethics Approval and Consent to Participate
The Ethical Committee of Isfahan University of Medical Sciences approved this study (IR.MUI.REC.1397.3.131), and the written informed consent was obtained from all patients.
References
1. Hupp JR, Tucker MR, Ellis E. 2013. Contemporary oral and maxillofacial surgery. 6th ed. Elsevier;Louis (MO): DOI: 10.25241/stomaeduj.2019.6(4).bookreview.1.
2. Vandeputte T, Bigorre M, Tramini P, Captier G. 2020; Comparison between combined cortical and cancellous bone graft and cancellous bone graft in alveolar cleft: retrospective study of complications during the first six months post-surgery. J Craniomaxillofac Surg. 48:38–42. https://doi.org/10.1016/j.jcms.2019.11.013. DOI: 10.1016/j.jcms.2019.11.013. PMID: 31874807.

3. Itikala PR, Watkins ML, Mulinare J, Moore CA, Liu Y. 2001; Maternal multivitamin use and orofacial clefts in offspring. Teratology. 63:79–86. https://doi.org/10.1002/1096-9926(200102)63:2<79::AID-TERA1013>3.0.CO;2-3. DOI: 10.1002/1096-9926(200102)63:2<79::AID-TERA1013>3.0.CO;2-3. PMID: 11241430.

4. Laron Z, Taube E, Kaplan I. 1969; Pituitary growth hormone insufficiency associated with cleft lip and palate. An embryonal developmental defect. Helv Paediatr Acta. 24:576–81. PMID: 5370797.
5. Loffredo LC, Souza JM, Freitas JA, Mossey PA. 2001; Oral clefts and vitamin supplementation. Cleft Palate Craniofac J. 38:76–83. https://doi.org/10.1597/1545-1569_2001_038_0076_ocavs_2.0.co_2. DOI: 10.1597/1545-1569_2001_038_0076_ocavs_2.0.co_2. PMID: 11204686.

6. Little J, Cardy A, Munger RG. 2004; Tobacco smoking and oral clefts: a meta-analysis. Bull World Health Organ. 82:213–8. PMID: 15112010. PMCID: PMC2585921.
7. Stothard KJ, Tennant PW, Bell R, Rankin J. 2009; Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA. 301:636–50. https://doi.org/10.1001/jama.2009.113. DOI: 10.1001/jama.2009.113. PMID: 19211471.

8. Jahanbin A, Mokhber N, Sahafian AA. 2008; Seasonal and yearly fluctuations in birth date of cleft lip and palate children in northern east of Iran, 1992-2007. Iran J Otorhinolaryngol. 20:45–50.
9. Eppley BL, Sadove AM. 2000; Management of alveolar cleft bone grafting--state of the art. Cleft Palate Craniofac J. 37:229–33. https://doi.org/10.1597/1545-1569_2000_037_0229_moacbg_2.3.co_2. DOI: 10.1597/1545-1569_2000_037_0229_moacbg_2.3.co_2. PMID: 10830800.

10. Morselli PG, Giuliani R, Pinto V, Oranges CM, Negosanti L, Tavaniello B, et al. 2009; Treatment of alveolar cleft performing a pyramidal pocket and an autologous bone grafting. J Craniofac Surg. 20:1566–70. https://doi.org/10.1097/SCS.0b013e3181b0dacd. DOI: 10.1097/SCS.0b013e3181b0dacd. PMID: 19816297.

11. Boyne PJ. 1970; Autogenous cancellous bone and marrow transplants. Clin Orthop Relat Res. 73:199–209. DOI: 10.1097/00003086-197011000-00022. PMID: 4920990.

12. Wang LX, Zhou ZG, Wang PL. 2008; [Clinical study of cancellous iliac bone graft in cleft alveolar repair]. Shanghai Kou Qiang Yi Xue. 17:555–7. Chinese. PMID: 18989604.
13. Besly W, Ward Booth P. 1999; Technique for harvesting tibial cancellous bone modified for use in children. Br J Oral Maxillofac Surg. 37:129–33. https://doi.org/10.1054/bjom.1998.0433. DOI: 10.1054/bjom.1998.0433. PMID: 10371319.

14. Walker TW, Modayil PC, Cascarini L, Williams L, Duncan SM, Ward-Booth P. 2009; Retrospective review of donor site complications after harvest of cancellous bone from the anteriomedial tibia. Br J Oral Maxillofac Surg. 47:20–2. https://doi.org/10.1016/j.bjoms.2008.05.014. DOI: 10.1016/j.bjoms.2008.05.014. PMID: 18676069.

15. Kline RM Jr, Wolfe SA. 1995; Complications associated with the harvesting of cranial bone grafts. Plast Reconstr Surg. 95:5–13. discussion 14–20. DOI: 10.1097/00006534-199501000-00003. PMID: 7809267.

16. van der Meij AJW, Baart JA, Prahl-Andersen B, Kostense PJ, van der Sijp JR, Tuinzing DB. 2003; Outcome of bone grafting in relation to cleft width in unilateral cleft lip and palate patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 96:19–25. https://doi.org/10.1016/s1079-2104(03)00266-x. DOI: 10.1016/S1079-2104(03)00266-X. PMID: 12847439.

17. Freihofer HP, Borstlap WA, Kuijpers-Jagtman AM, Voorsmit RA, van Damme PA, Heidbüchel KL, et al. 1993; Timing and transplant materials for closure of alveolar clefts. A clinical comparison of 296 cases. J Craniomaxillofac Surg. 21:143–8. https://doi.org/10.1016/s1010-5182(05)80102-7. DOI: 10.1016/S1010-5182(05)80102-7. PMID: 8335724.

18. Sindet-Pedersen S, Enemark H. 1990; Reconstruction of alveolar clefts with mandibular or iliac crest bone grafts: a comparative study. J Oral Maxillofac Surg. 48:554–8. discussion 559–60. https://doi.org/10.1016/s0278-2391(10)80466-5. DOI: 10.1016/S0278-2391(10)80466-5. PMID: 2341935.

19. Rahpeyma A, Khajehahmadi S. 2014; Chin bone graft for maxillary alveolar cleft: indications and limitations. J Craniofac Surg. 25:1650–2. https://doi.org/10.1097/SCS.0000000000001132. DOI: 10.1097/SCS.0000000000001132. PMID: 25162555.

20. Nwoku AL, Al Atel A, Al Shlash S, Oluyadi BA, Ismail S. 2005; Retrospective analysis of secondary alveolar cleft grafts using iliac of chin bone. J Craniofac Surg. 16:864–8. https://doi.org/10.1097/01.scs.0000179742.45424.0a. DOI: 10.1097/01.scs.0000179742.45424.0a. PMID: 16192872.

21. Booij A, Raghoebar GM, Jansma J, Kalk WW, Vissink A. 2005; Morbidity of chin bone transplants used for reconstructing alveolar defects in cleft patients. Cleft Palate Craniofac J. 42:533–8. https://doi.org/10.1597/03-158.1. DOI: 10.1597/03-158.1. PMID: 16149836.

22. Verdugo F, Simonian K, Smith McDonald R, Nowzari H. 2010; Quantitation of mandibular symphysis volume as a source of bone grafting. Clin Implant Dent Relat Res. 12:99–104. https://doi.org/10.1111/j.1708-8208.2008.00140.x. DOI: 10.1111/j.1708-8208.2008.00140.x. PMID: 19220848.

23. Mahardawi B, Boonsiriseth K, Pairuchvej V, Wongsirichat N. 2020; Alveolar cleft bone grafting: factors affecting case prognosis. J Korean Assoc Oral Maxillofac Surg. 46:409–16. https://doi.org/10.5125/jkaoms.2020.46.6.409. DOI: 10.5125/jkaoms.2020.46.6.409. PMID: 33377466. PMCID: PMC7783176.

24. Elbokle N, ElSholkamy MA. 2018; Volumetric assessment of mandibular symphyseal bone graft for secondary reconstruction of unilateral alveolar clefts. Egypt Dent J. 64:2081–8. https://doi.org/10.21608/edj.2018.76756. DOI: 10.21608/edj.2018.76756.

25. Movahedian Attar B, Naghdi N, Etemadi Sh M, Mehdizadeh M. 2017; Chin symphysis bone, allograft, and platelet-rich fibrin: is the combination effective in repair of alveolar cleft? J Oral Maxillofac Surg. 75:1026–35. https://doi.org/10.1016/j.joms.2016.12.026. DOI: 10.1016/j.joms.2016.12.026. PMID: 28093204.

26. Liang F, Yen S, Florendo E, Urata M, Hammoudeh J. 2015; 3D cone beam computed tomography volumetric outcomes of rhBMP-2/demineralized bone matrix vs. Iliac crest bone graft for alveolar cleft reconstruction. Plast Reconstr Surg. 136(4S):3–4. https://doi.org/10.1097/01.prs.0000472273.84427.35. DOI: 10.1097/01.prs.0000472273.84427.35.

27. Neovius E, Lemberger M, Docherty Skogh AC, Hilborn J, Engstrand T. 2013; Alveolar bone healing accompanied by severe swelling in cleft children treated with bone morphogenetic protein-2 delivered by hydrogel. J Plast Reconstr Aesthet Surg. 66:37–42. https://doi.org/10.1016/j.bjps.2012.08.015. DOI: 10.1016/j.bjps.2012.08.015. PMID: 22980542.

28. Myeroff C, Archdeacon M. 2011; Autogenous bone graft: donor sites and techniques. J Bone Joint Surg Am. 93:2227–36. https://doi.org/10.2106/JBJS.J.01513. DOI: 10.2106/JBJS.J.01513. PMID: 22159859.

29. Francis CS, Mobin SSN, Lypka MA, Rommer E, Yen S, Urata MM, et al. 2013; rhBMP-2 with a demineralized bone matrix scaffold versus autologous iliac crest bone graft for alveolar cleft reconstruction. Plast Reconstr Surg. 131:1107–15. https://doi.org/10.1097/PRS.0b013e3182865dfb. DOI: 10.1097/PRS.0b013e3182865dfb. PMID: 23385986.

30. Kamal M, Ziyab AH, Bartella A, Mitchell D, Al-Asfour A, Hölzle F, et al. 2018; Volumetric comparison of autogenous bone and tissue-engineered bone replacement materials in alveolar cleft repair: a systematic review and meta-analysis. Br J Oral Maxillofac Surg. 56:453–62. https://doi.org/10.1016/j.bjoms.2018.05.007. DOI: 10.1016/j.bjoms.2018.05.007. PMID: 29859781.

31. Thuaksuban N, Nuntanaranont T, Pripatnanont P. 2010; A comparison of autogenous bone graft combined with deproteinized bovine bone and autogenous bone graft alone for treatment of alveolar cleft. Int J Oral Maxillofac Surg. 39:1175–80. https://doi.org/10.1016/j.ijom.2010.07.008. DOI: 10.1016/j.ijom.2010.07.008. PMID: 20813500.

32. Weijs WL, Siebers TJ, Kuijpers-Jagtman AM, Bergé SJ, Meijer GJ, Borstlap WA. 2010; Early secondary closure of alveolar clefts with mandibular symphyseal bone grafts and beta-tri calcium phosphate (beta-TCP). Int J Oral Maxillofac Surg. 39:424–9. https://doi.org/10.1016/j.ijom.2010.02.004. DOI: 10.1016/j.ijom.2010.02.004. PMID: 20303237.

33. Miyagawa K, Tanaka S, Hiroishi S, Matsushita Y, Murakami S, Kogo M. 2020; Comparative evaluation of bone microstructure in alveolar cleft repair by cone beam CT: influence of different autologous donor sites and additional application of β-tricalcium phosphate. Clin Oral Investig. 24:2789–97. https://doi.org/10.1007/s00784-019-03142-1. DOI: 10.1007/s00784-019-03142-1. PMID: 31707628.

34. Shirzadeh A, Rahpeyma A, Khajehahmadi S. 2018; A prospective study of chin bone graft harvesting for unilateral maxillary alveolar cleft during mixed dentition. J Oral Maxillofac Surg. 76:180–8. https://doi.org/10.1016/j.joms.2017.07.143. DOI: 10.1016/j.joms.2017.07.143. PMID: 28774851.

35. Hunt DR, Jovanovic SA. 1999; Autogenous bone harvesting: a chin graft technique for particulate and monocortical bone blocks. Int J Periodontics Restorative Dent. 19:165–73. PMID: 10635182.
36. MacIsaac ZM, Rottgers SA, Davit AJ 3rd, Ford M, Losee JE, Kumar AR. 2012; Alveolar reconstruction in cleft patients: decreased morbidity and improved outcomes with supplemental demineralized bone matrix and cancellous allograft. Plast Reconstr Surg. 130:625–32. https://doi.org/10.1097/PRS.0b013e31825dcb75. DOI: 10.1097/PRS.0b013e31825dcb75. PMID: 22929248.

37. Abramowicz S, Katsnelson A, Forbes PW, Padwa BL. 2012; Anterior versus posterior approach to iliac crest for alveolar cleft bone grafting. J Oral Maxillofac Surg. 70:211–5. https://doi.org/10.1016/j.joms.2011.02.044. DOI: 10.1016/j.joms.2011.02.044. PMID: 21683495.

38. Bukhari SGA, Pasha B, Ahmed W, Fazal M, Jan H. 2009; Alveolar bone grafting with mandibular symphysis as donor material. Pak Oral Dent J. 29:3–8.
39. Laskin DM, Abubaker AO, Strauss RA. 2013; Accuracy of predicting the duration of a surgical operation. J Oral Maxillofac Surg. 71:446–7. https://doi.org/10.1016/j.joms.2012.10.009. DOI: 10.1016/j.joms.2012.10.009. PMID: 23351763.

40. Swan MC, Goodacre TE. 2006; Morbidity at the iliac crest donor site following bone grafting of the cleft alveolus. Br J Oral Maxillofac Surg. 44:129–33. https://doi.org/10.1016/j.bjoms.2005.04.015. DOI: 10.1016/j.bjoms.2005.04.015. PMID: 15961201.

41. Rawashdeh MA. 2008; Morbidity of iliac crest donor site following open bone harvesting in cleft lip and palate patients. Int J Oral Maxillofac Surg. 37:223–7. https://doi.org/10.1016/j.ijom.2007.11.009. DOI: 10.1016/j.ijom.2007.11.009. PMID: 18272337.

42. Albuquerque MA, Gaia BF, Cavalcanti MG. 2011; Comparison between multislice and cone-beam computerized tomography in the volumetric assessment of cleft palate. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 112:249–57. https://doi.org/10.1016/j.tripleo.2011.03.006. DOI: 10.1016/j.tripleo.2011.03.006. PMID: 21664153.

Fig. 3
Axial view of cone-beam computed tomography before bone graft (Group A patient). Group A: patients treated with chin symphysis bone+allograft.
Fig. 5
Axial view of cone-beam computed tomography after bone graft (Group A patient). Group A: patients treated with chin symphysis bone+allograft.
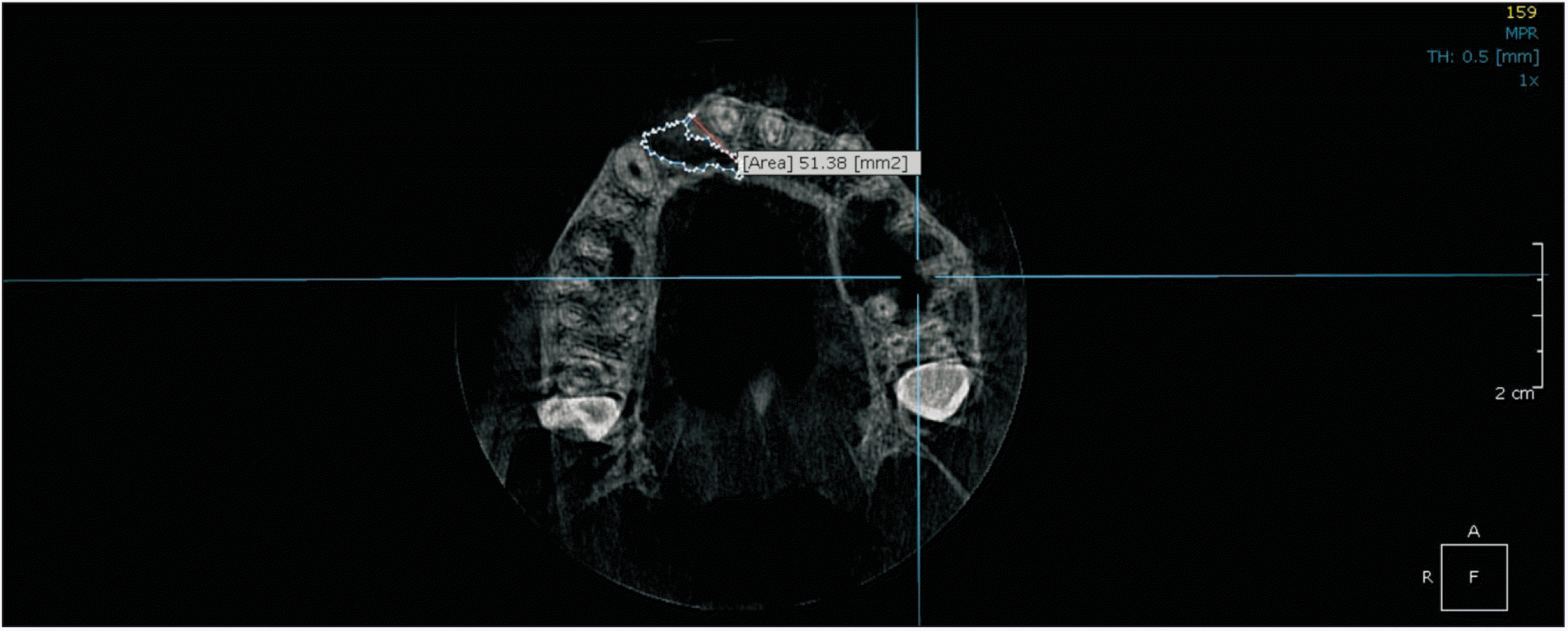
Fig. 6
Box plot of operation duration. Group A: patients treated with chin symphysis bone+allograft, Group B: patients treated with iliac bone graft.
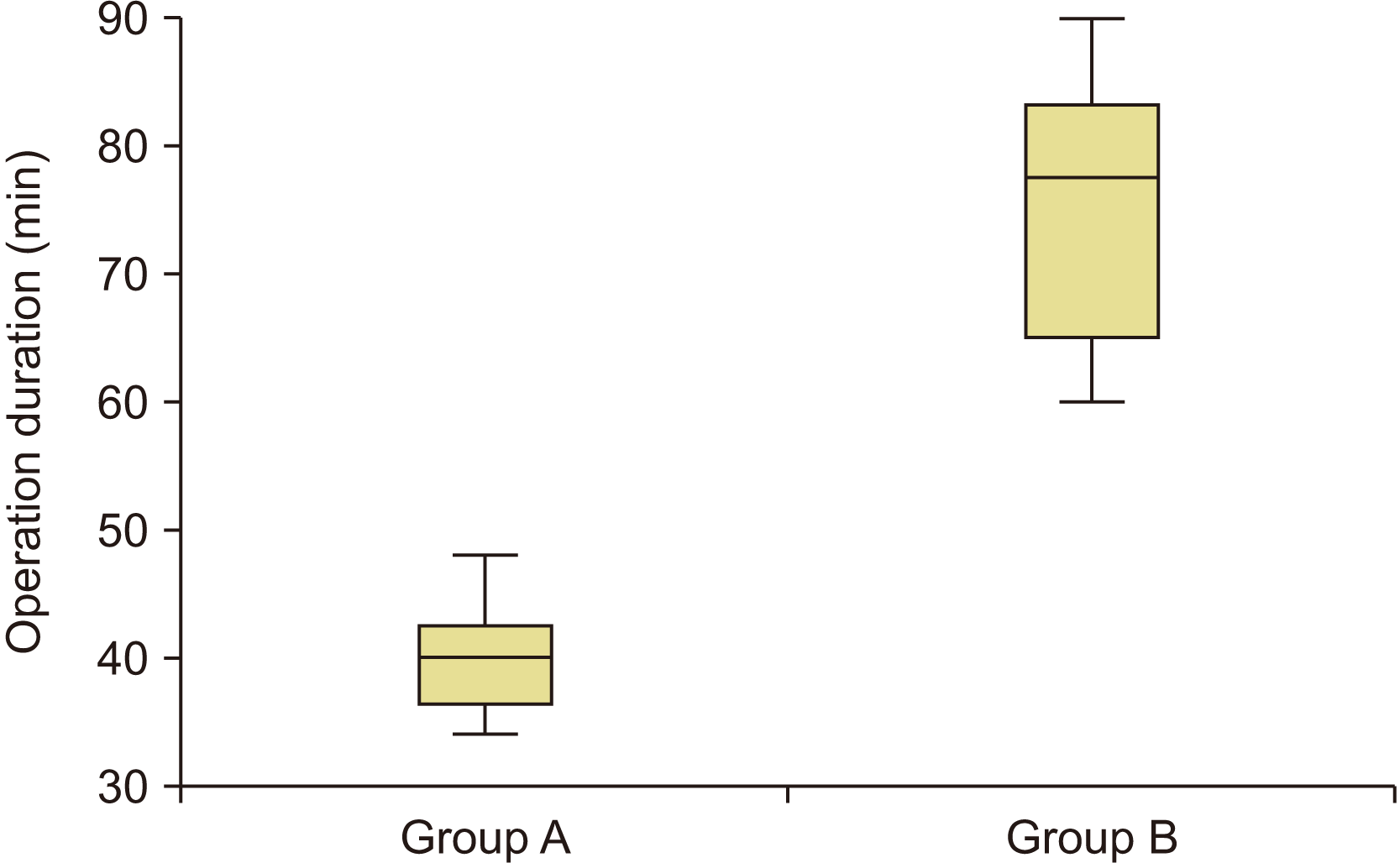
Fig. 7
Reformatted panoramic view before bone graft (Group B patient) (arrow). Group B: patients treated with iliac bone graft.
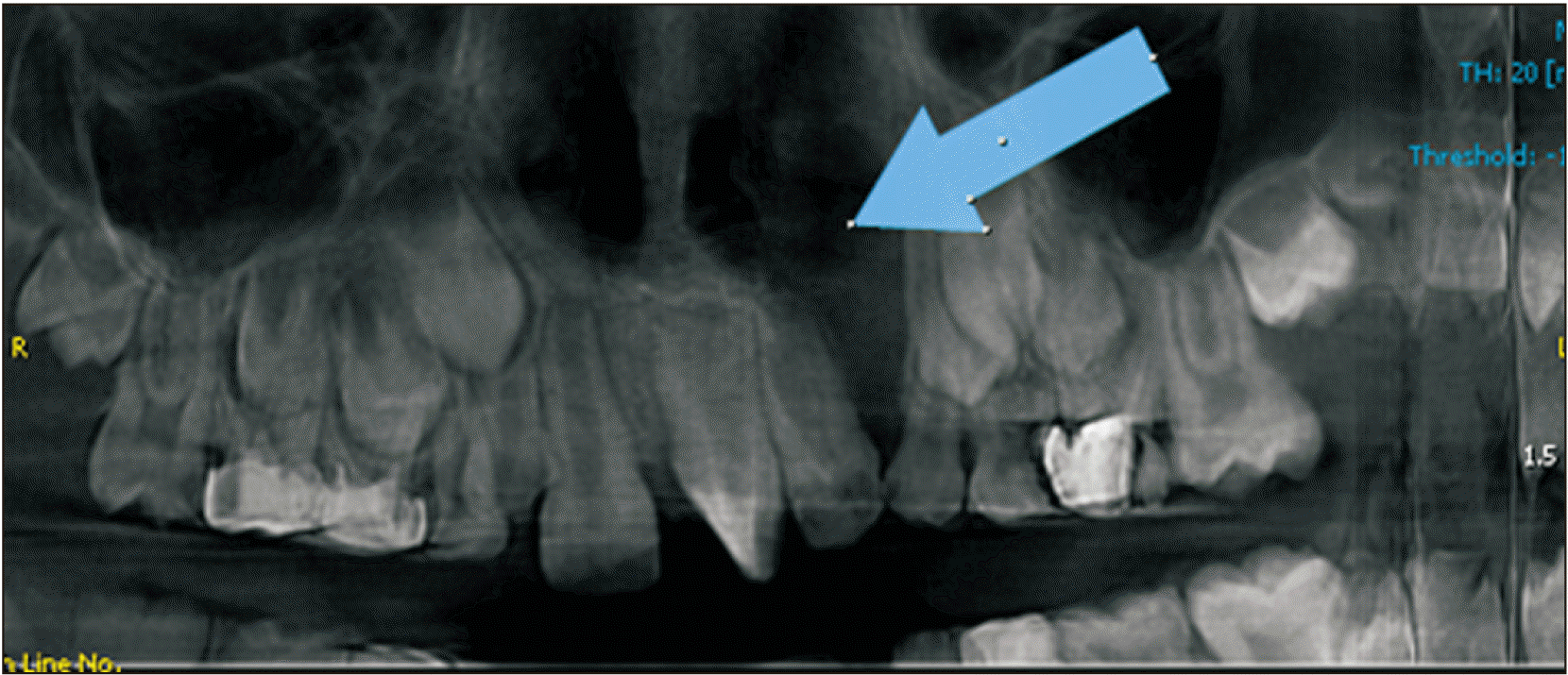
Fig. 8
Reformatted panoramic view after bone graft (Group B patient). Group B: patients treated with iliac bone graft.
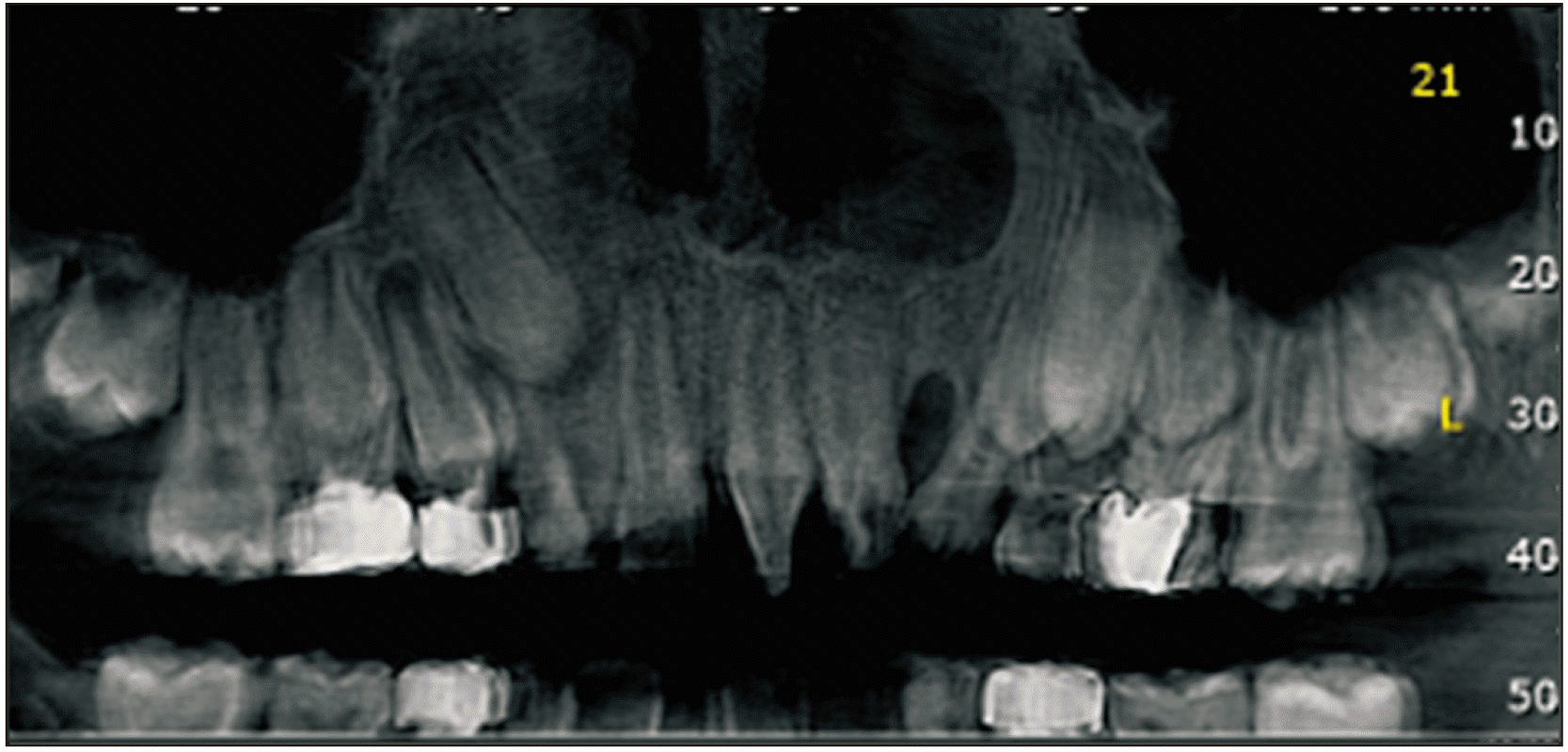
Table 1
Characteristics of study patients
Table 2
Preoperative and postoperative defect volumes in study patients




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download