Abstract
Objectives
The purpose of this study was to evaluate risk factors and symptoms in cemento-osseous dysplasia (COD) patients.
Materials and Methods
In this study, 62 patients who were diagnosed histologically with COD were investigated from 2010 to 2020 at the author’s institution. We compared clinical and radiological characteristics of symptomatic and asymptomatic patients. The factors were sex, age, lesion size, site, radiologic stage of lesion, apical involvement, sign of infection, and history of tooth extraction. Statistical analysis was performed using Fisher’s exact test and the chi-square test.
Results
COD was more prevalent in female patients. With the exception of three cases, all were focal COD. The majority of patients presented with symptoms when the lesion was smaller than 1.5 cm in size. Symptoms were observed when the apex of the tooth was included in the lesion or there was a local infection around the lesion. The history of tooth extraction and previous endodontic treatment were evaluated, and history was not a significant predictor for the onset of symptoms.
The term “benign fibro-osseous lesion” refers to a non-neoplastic condition in which normal bone is replaced with a fibrous connective tissue matrix that contains abnormal bone or cementum1,2. The classification has been revised over time, and in 2017, the World Health Organization (WHO) announced a classification approach for benign fibro-osseous lesions into four categories based on the clinical, radiological, and pathological features: 1) ossifying fibroma, 2) cemento-osseous dysplasia (COD), 3) fibrous dysplasia3.
COD is the most common benign fibro-osseous lesion and primarily occurs in the mandible3-5. COD can be subdivided into three types with respect to the location of the lesion in the jaw: periapical, focal, and florid. Periapical COD is a dysplastic lesion that develops in the anterior site of the mandible. Focal COD describes lesions that occur in one quadrant of the posterior site of the mandible. Florid COD, in a more extensive form, features lesions in more than one quadrant of the jaw.
COD is a self-limiting and dysplastic lesion, not a neoplasm. Many patients do not present with symptoms and are diagnosed incidentally during routine dental examinations6. However, some patients experience non-specific discomfort in the affected area or show cortical bone erosion in radiographic imaging. Asymptomatic patients do not typically undergo surgery, but surgical intervention might be performed in symptomatic patients7-9. Therefore, the presence or absence of symptoms is an important factor in planning treatment. The purpose of this study was to investigate factors associated with clinical symptoms in COD patients, which will ultimately aid in treatment.
This study was approved by the Institutional Review Board (IRB) of Pusan National University Dental Hospital (IRB No. PNUDH-2021-031) and the informed consent was waived by the IRB.
Data were collected from patients who underwent surgical excision from 2010 to 2020 at the author’s institution. The assessment included cases that were considered for biopsy and with histopathological results that confirmed COD. Among them, we included symptomatic patients who underwent surgical excision and patients that were asymptomatic but wanted to confirm whether lesions were present. Data obtained from dental panoramas, computed tomography, and the chart records of each case were analyzed. Patients with metabolic diseases such as Paget’s disease, hyperparathyroidism, and osteopetrosis, i.e., patients with abnormalities in differentiation and activity of osteoblasts and osteoclasts, were excluded.
Selected cases were divided into two groups for comparison depending on the presence of symptoms. Patients were considered symptomatic if pain, swelling, discomfort, hypoesthesia, and paresthesia were reported prior to the operation. Clinical features studied were sex, age, site of the lesion, and signs of local infection.(Fig. 1) Stage of the lesion, involvement of adjacent teeth, and history of tooth extraction were examined in the radiological data.(Fig. 2) The lesion size was measured based on the largest diameter on X-rays, and suppurative exudate discharge was considered a local infection. In addition, the history of prior endodontic treatment was investigated for cases that did not undergo tooth extraction near the lesion.
Data were analyzed using IBM SPSS Statistics software (ver. 25.0; IBM, Armonk, NY, USA). The variables in the two groups were compared using Fisher’s exact test and chi-square test. Statistical analysis results with P-values less than 0.05 were considered statistically significant.
Of a total of 62 patients, 57 were female and 5 were male. The mean age of the study patients was 49 years (standard deviation [SD], 13.6 years) and ranged from 16-83 years.(Table 1) For the patients who underwent surgery, no periapical COD was observed, and only two cases were diagnosed as florid COD. Only one case, in the maxilla, was determined to be florid COD. All other lesions occurred in the mandible. Regardless of the symptoms, the most common cases occurred in the posterior site of the mandible. The probability of symptoms occurring when the size of the lesion was less than 1.5 cm was approximately four times higher than for cases with a lesion size ≥1.5 cm (odds ratio, 4.038) (P=0.009). (Table 2) No statistically significant difference was identified for the association between symptom presentation and lesion stage (P=0.272). Symptomatic patients were confirmed to have apex involvement in the lesion. To detect any local infections, we compared the history of endodontic treatment of patients who did not undergo extraction. The results indicated that most of the patients did not receive any previous endodontic treatment.(Table 3)
This study was conducted to compare patients with and without symptoms to assess factors that could affect symptoms and presentation. Ideally, COD should be diagnosed based on clinical and radiological features, and biopsies should be performed only in limited cases10. Most of the patients visited the author’s institution for further evaluation after incidental recognition of the lesion. Asymptomatic patients diagnosed with COD based on clinical and radiological examination were recommended to undergo an observation period prior to surgical treatment. However, some of these patients wanted to rule out other possible pathologies and then undergo surgical excision. Therefore, surgical intervention was performed in some asymptomatic patients based on patient preferences. This approach allowed us to examine factors that impact symptom presentation by comparing asymptomatic and symptomatic patients.
Many studies have shown that COD predominantly occurs in women and is common between the ages of 40 to 50 years7-11. Consistent with the predilection identified in women in other studies, our data also showed a predominance in women (91.9%), with the highest rate of 50% at 40-69 years old. The cause for the COD prevalence in women is unknown, but one hypothesis of a hormonal imbalance that affects bone remodeling has drawn attention11. Clinically, COD can be divided into three types depending on location: periapical (anterior), focal (posterior), and florid (more than one quadrant). Olgac et al.10 reported a higher prevalence of focal COD, followed by periapical COD and florid COD. In this study, focal COD was the most frequently observed type, while florid COD was only identified in two cases. However, Cavalcanti et al.12 reported a higher prevalence of periapical COD (57.3%), followed by focal COD (28%), and florid COD (14.6%). There is no consensus on which of the three types is most prevalent13.
It is difficult to distinguish COD from many other lesions based on only radiographic properties. The differential diagnosis should take into account the stage of development of the lesion and the possibility of associated entities, including osteomyelitis and simple bone cysts14. Because the initial stage of COD presents with radiolucency on the lesion, it can be difficult to distinguish from other cysts or periapical lesions. In fact, among the cases included in this study, the following were noted: radiolucent lesions at the apex were first diagnosed as radicular cyst, and the patients underwent endodontic treatment and cyst enucleation. However, in each case, excisional biopsy revealed that the lesion was a COD. The radiological radiolucency was initially diagnosed as a radicular cyst instead of the osteolytic stage of COD. Similar to COD, an ossifying fibroma has similar clinical and radiological characteristics, which can complicate differential diagnosis. The difference between the two is that COD is a reactive lesion that does not require treatment, and an ossifying fibroma is a true neoplasm that requires intervention15. Therefore, it is important to determine a differential diagnosis from other lesions that require treatment. Further, accurate diagnosis is important because unnecessary endodontic treatment and surgery can be performed due to misdiagnosis.
COD is a self-limiting lesion with mean size of 1.5 cm16. In this study, the mean lesion size was 1.5 cm (SD, 5.01 cm). Symptoms were identified in cases with lesions smaller than 1.5 cm. However, the direct cause was not clear, and there is a limited number of studies that has assessed lesion size in COD. This is probably because asymptomatic patients are not identified until the lesion size increases, but symptomatic patients can recognize the symptoms quickly due to discomfort before the lesion size increases.
Su et al.15 noted that 70% of COD is in close contact with the apex. COD typically occurs in the periodontal ligament tissue because it occurs in relation to the apical foramen of the tooth, and histopathological characteristics often indicate the proliferation of tissues such as cementum17. This study suggests that symptoms are likely to occur if the lesion contains the involved tooth apex. The apical involvement of related teeth can be communicated with the external environment through the periodontal ligament space and pulp chamber. Therefore, teeth with caries, periodontal disease, and/or pulp involvement are at increased risk of infection.
Infections that occur within the COD lesion are prone to necrosis of the affected area due to low vascularization and increased bone hardness and acellularity. The possible causes of infection include chronic inflammatory periodontal disease, tooth caries that can lead to pulp necrosis, tooth extraction, and slight irritation from dentures17,18. In this study, a relationship between symptoms and local infection was confirmed. Proximity between the oral flora and the lesion can cause infection. If infection occurs from a COD that has a sclerotic lesion with narrow blood vessel distribution, the case becomes more susceptible to necrosis18.
COD patients require periodic observation with routine panorama follow-up to assess changes, and oral hygiene should be maintained to prevent lesion infection, even while asymptomatic7-9. If symptoms present, surgical intervention is required14,17,18. COD does not destroy bone or cause root resorption but does have continuity with surrounding structures, making it difficult to separate the lesion from the surrounding tissues19. Although there is no consensus on the most appropriate treatment for COD lesions, curettage/surgical removal with or without antibiotic/analgesics is considered the most appropriate18. In other studies, surgical removal/curettage associated with antibiotics/analgesics was the most common treatment (89.4%) after which no recurrence was reported (84.8%). We first performed oral hygiene management and prescribed systemic antibiotics. Antibiotics play an adjuvant role to kill bacteria by forming a new biofilm before the bacteria attach to the necrotic bone20. We excised the lesion and curettage in cases with signs of disease progression, such as osteomyelitis. Periodontal treatment was performed if there was a periodontal problem on the affected tooth, and root canal treatment was performed if pain for percussion or with an apical lesion was reported. If the symptoms were not relieved after these steps, the related tooth was extracted.
COD is a disease without specific symptoms, and treatment methods do not vary depending on the type or intensity of symptoms. If symptoms are present, surgical intervention is required, and the approach does not differ based on the presence or absence of each symptom. Therefore, we divided the reported symptoms based on patient report and performed a simple comparative analysis. Clinical and radiological factors related to the presence of symptoms were identified, and these related factors were size, apical involvement, and local infection. Based on these results, patients with these factors tended to present with symptoms, indicating possible need for surgical invention. Therefore, we recommend that patients closely adhere to follow-up assessment scheduling.
This study was conducted in patients with COD that was confirmed histologically. However, one limitation was the number of subjects, with only 62 included in the study. Further studies with more patients are needed to support the findings. Additional studies are needed as more diverse studies reveal factors that influence the presence or absence of symptoms and provide immediate treatment options to patients and follow-up before additional symptoms appear.
COD is a benign fibrous bone lesion, and most cases are asymptomatic. However, if symptoms occur, surgical treatment is required. This retrospective study was conducted to evaluate the risk factors associated with symptoms in COD patients. Apical involvement, lesion size (less than 1.5 cm), and local infection all affected symptoms.
Notes
Authors’ Contributions
I.N. participated in data collection and wrote the manuscript. J.Y.L. participated in the study design and performed the statistical analysis. J.R., Y.D.K., and S.H.S. participated in the study design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
References
1. Alsufyani NA, Lam EW. 2011; Osseous (cemento-osseous) dysplasia of the jaws: clinical and radiographic analysis. J Can Dent Assoc. 77:b70. PMID: 21683027.
2. Mainville GN, Turgeon DP, Kauzman A. 2017; Diagnosis and management of benign fibro-osseous lesions of the jaws: a current review for the dental clinician. Oral Dis. 23:440–50. https://doi.org/10.1111/odi.12531. DOI: 10.1111/odi.12531. PMID: 27387498.

3. MacDonald DS. 2021; Classification and nomenclature of fibro-osseous lesions. Oral Surg Oral Med Oral Pathol Oral Radiol. 131:385–9. https://doi.org/10.1016/j.oooo.2020.12.004. DOI: 10.1016/j.oooo.2020.12.004. PMID: 33518490.

4. de Noronha Santos Netto J, Machado Cerri J, Miranda AM, Pires FR. 2013; Benign fibro-osseous lesions: clinicopathologic features from 143 cases diagnosed in an oral diagnosis setting. Oral Surg Oral Med Oral Pathol Oral Radiol. 115:e56–65. https://doi.org/10.1016/j.oooo.2012.05.022. DOI: 10.1016/j.oooo.2012.05.022. PMID: 22981804.

5. Kim NK, Kim HS, Kim J, Nam W, Cha IH, Kim HJ. 2011; Florid cemento-osseous dysplasia: a report of two cases. J Korean Assoc Oral Maxillofac Surg. 37:515–9. https://doi.org/10.5125/jkaoms.2011.37.6.515. DOI: 10.5125/jkaoms.2011.37.6.515. PMID: 34210933.

6. Nelson BL, Phillips BJ. 2019; Benign fibro-osseous lesions of the head and neck. Head Neck Pathol. 13:466–75. https://doi.org/10.1007/s12105-018-0992-5. DOI: 10.1007/s12105-018-0992-5. PMID: 30887390. PMCID: PMC6684826.

7. Brody A, Zalatnai A, Csomo K, Belik A, Dobo-Nagy C. 2019; Difficulties in the diagnosis of periapical translucencies and in the classification of cemento-osseous dysplasia. BMC Oral Health. 19:139. https://doi.org/10.1186/s12903-019-0843-0. DOI: 10.1186/s12903-019-0843-0. PMID: 31291935. PMCID: PMC6617922.

8. Kato CN, Barra SG, Pereira MJ, Gomes LT, Amaral TM, Abreu LG, et al. 2020; Mandibular radiomorphometric parameters of women with cemento-osseous dysplasia. Dentomaxillofac Radiol. 49:20190359. https://doi.org/10.1259/dmfr.20190359. DOI: 10.1259/dmfr.20190359. PMID: 31846355. PMCID: PMC7213529.

9. Mufeed A, Mangalath U, George A, Hafiz A. 2015; Infected florid osseous dysplasia: clinical and imaging follow-up. BMJ Case Rep. 2015:bcr2014209099. https://doi.org/10.1136/bcr-2014-209099. DOI: 10.1136/bcr-2014-209099. PMID: 25754168. PMCID: PMC4368933.

10. Olgac V, Sinanoglu A, Selvi F, Soluk-Tekkesin M. 2021; A clinicopathologic analysis of 135 cases of cemento-osseous dysplasia: to operate or not to operate? J Stomatol Oral Maxillofac Surg. 122:278–82. https://doi.org/10.1016/j.jormas.2020.06.002. DOI: 10.1016/j.jormas.2020.06.002. PMID: 32565263.

11. Kawai T, Hiranuma H, Kishino M, Jikko A, Sakuda M. 1999; Cemento-osseous dysplasia of the jaws in 54 Japanese patients: a radiographic study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 87:107–14. https://doi.org/10.1016/s1079-2104(99)70303-3. DOI: 10.1016/S1079-2104(99)70303-3. PMID: 9927089.

12. Cavalcanti PHP, Nascimento EHL, Pontual MLDA, Pontual ADA, Marcelos PGCL, Perez DEDC, et al. 2018; Cemento-osseous dysplasias: imaging features based on cone beam computed tomography scans. Braz Dent J. 29:99–104. https://doi.org/10.1590/0103-6440201801621. DOI: 10.1590/0103-6440201801621. PMID: 29267533.

13. Gumru B, Akkitap MP, Deveci S, Idman E. 2021; A retrospective cone beam computed tomography analysis of cemento-osseous dysplasia. J Dent Sci. 16:1154–61. https://doi.org/10.1016/j.jds.2021.03.009. DOI: 10.1016/j.jds.2021.03.009. PMID: 34484583. PMCID: PMC8403794.

14. Shibata N, Inamoto K, Naitoh M, Ariji E. 2021; Clinical assessment of cemento-osseous dysplasia based on three-dimensional diagnostic imaging: a case report. Aust Endod J. 47:105–12. https://doi.org/10.1111/aej.12488. DOI: 10.1111/aej.12488. PMID: 33523556.

15. Su L, Weathers DR, Waldron CA. 1997; Distinguishing features of focal cemento-osseous dysplasia and cemento-ossifying fibromas. II. A clinical and radiologic spectrum of 316 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 84:540–9. https://doi.org/10.1016/s1079-2104(97)90271-7. DOI: 10.1016/S1079-2104(97)90271-7. PMID: 9394387.

16. Ravikumar SS, Vasupradha G, Menaka TR, Sankar SP. 2020; Focal cemento-osseous dysplasia. J Oral Maxillofac Pathol. 24(Suppl 1):S19–22. https://doi.org/10.4103/jomfp.JOMFP_209_19. DOI: 10.4103/jomfp.JOMFP_209_19. PMID: 32189898. PMCID: PMC7069129.

17. Min CK, Koh KJ, Kim KA. 2018; Recurrent symptomatic cemento-osseous dysplasia: a case report. Imaging Sci Dent. 48:131–7. https://doi.org/10.5624/isd.2018.48.2.131. DOI: 10.5624/isd.2018.48.2.131. PMID: 29963485. PMCID: PMC6015922.

18. Kato CNAO, de Arruda JAA, Mendes PA, Neiva IM, Abreu LG, Moreno A, et al. 2020; Infected cemento-osseous dysplasia: analysis of 66 cases and literature review. Head Neck Pathol. 14:173–82. https://doi.org/10.1007/s12105-019-01037-x. DOI: 10.1007/s12105-019-01037-x. PMID: 31011984. PMCID: PMC7021850.

19. Günhan Ö, Kahraman D, Yalçın ÜK. 2021; The possible pathogenesis of cemento-osseous dysplasia: a case series and discussion. Adv Oral Maxillofac Surg. 3:100105. https://doi.org/10.1016/j.adoms.2021.100105. DOI: 10.1016/j.adoms.2021.100105.

20. Ray JM, Triplett RG. 2011; What is the role of biofilms in severe head and neck infections? Oral Maxillofac Surg Clin North Am. 23:497–505. https://doi.org/10.1016/j.coms.2011.07.002. DOI: 10.1016/j.coms.2011.07.002. PMID: 21982601.

Fig. 1
Types of radiographic findings. A. Osteolytic stage (Stage I). The entire lesion is radiolucent. B. Cementoblastic stage (Stage II). The entire lesion is radiolucent, with nodular radiopaque deposits. C. Mature stage (Stage III). The entire lesion is radiopaque.
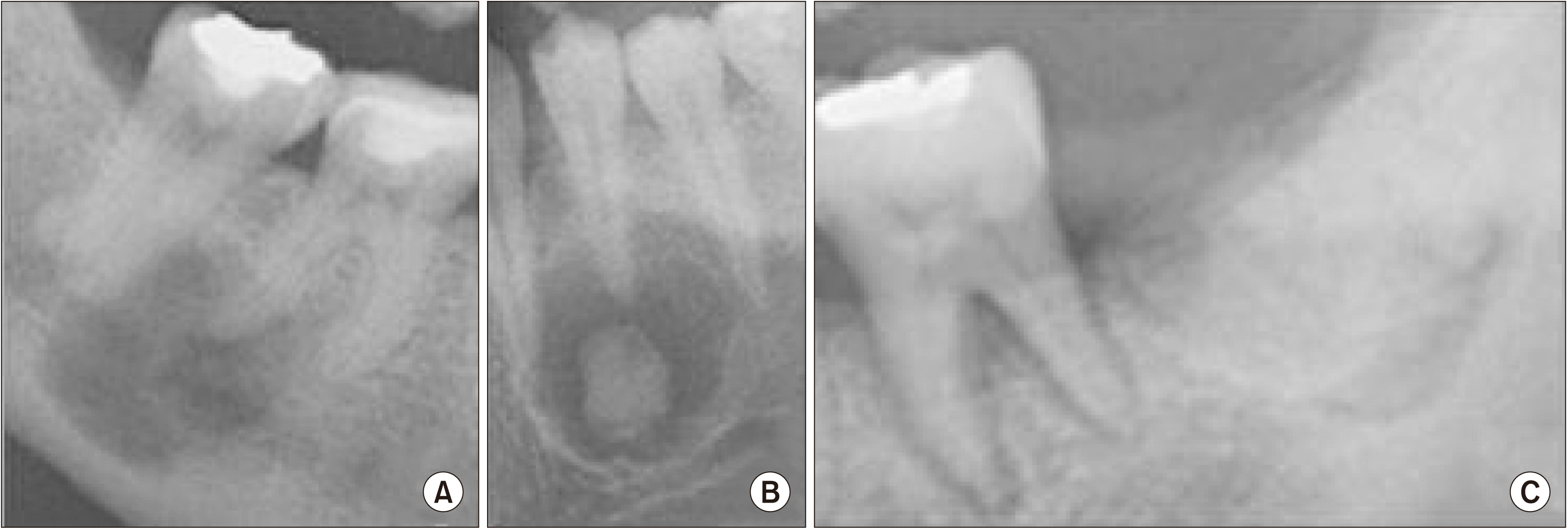
Table 1
Sex and age distributions (n=62)
Table 2
Analysis by study variable based on symptom presentation (n=62)
| Variable | Symptomatic | P-value | OR (95% CI) | |
|---|---|---|---|---|
|
|
||||
| Yes (n=28) | No (n=34) | |||
| Size | 0.009* | 4.038 (1.381-11.805) | ||
| <1.5 cm | 20 (71.4) | 13 (38.2) | ||
| ≥1.5 cm | 8 (28.6) | 21 (61.8) | ||
| Site | 0.494 | - | ||
| Premolar | 5 (17.9) | 9 (26.5) | ||
| Molar | 20 (71.4) | 19 (55.9) | ||
| Wisdom teeth | 3 (10.7) | 6 (17.6) | ||
| Stage | 0.272 | - | ||
| 1 (osteolytic) | 7 (25.0) | 10 (29.4) | ||
| 2 (cementoblastic) | 14 (50.0) | 10 (29.4) | ||
| 3 (mature) | 7 (25.0) | 14 (41.2) | ||
| Apical involvement | 0.006* | - | ||
| Yes | 28 (100) | 26 (76.5) | ||
| No | 0 (0) | 8 (23.5) | ||
| Local infection | 0.039* | 9.000 (1.013-79.988) | ||
| Yes | 6 (21.4) | 1 (2.9) | ||
| No | 22 (78.6) | 33 (97.1) | ||
| Extraction | 0.844 | 1.128 (0.339-3.75) | ||
| Yes | 6 (21.4) | 8 (23.5) | ||
| No | 22 (78.6) | 26 (76.5) | ||




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download