Abstract
Technologies in laboratory diagnostics are changing fast with progress in understanding and therapy of diseases. Unfortunately, new analyzers are often needed to be installed in a clinical laboratory to implement such techniques. The demand for new hardware is a bottleneck in improving the diagnostic services for many facilities with limited resources. In this regard, hemostasis laboratories take a slightly different position. Because many in vitro diagnostic tests target the functional aspects of hemostasis, further meaningful information can be obtained from the same analyzers as in current use. Automated coagulometers are good candidates for such further utilization. Clot waveform analysis is a leading example. Behind the simple values reported as clotting time, clotting curves exist that represent the process of fibrin clot formation. Clot waveform analysis examines the clotting curves and derives new parameters other than clotting times. The clot waveform parameters are now in active use in assessing the hemostatic potential of hemorrhagic patients. Clinical application of coagulometers can also be widened by modifying the reagent formulation. For example, the chromogenic factor VIII assay with bovine source reagent compositions has recently been introduced for hemophilia A patients on emicizumab prophylaxis. Also, new immunoturbidimetric functional assays for von Willebrand factor have been developed recently. Thus, new clinically relevant information can be mined from the automated coagulometers that are based on old technology.
The routine laboratory hemostasis tests are based on several common assay principles. The methods have long been developed and established and are now employed in most hemostasis analyzers. They form the backbone of laboratory evaluation of hemostatic function and thrombotic diseases. Most high-throughput coagulometers are equipped with three modules based on three assay principles that measure and examine different aspects of hemostasis [1, 2]. The first one measures the clotting time and probably accounts for most of the test size in a clinical hemostasis laboratory. Various stimulators of various sources are used to initiate coagulation factor activation and induce blood clot formation. The best-known clotting time tests are prothrombin time (PT) and activated partial thromboplastin time (APTT). Tissue factor and substances with negative-charge surfaces are used to initiate coagulation cascade along the extrinsic and intrinsic pathways. Other substances can be used to serve specific diagnostic purposes triggering the coagulation cascade at different points of the coagulation system. For example, we use activated thrombin to measure thrombin time. Because thrombin directly acts on fibrinogen to form a fibrin clot, a prolonged thrombin time means abnormal fibrinogen status (i.e., hypofibrinogenemia and dysfibrinogenemia).
Spectrophotometry and turbidimetry (or nephelometry) are also important modalities of modern coagulometers. Spectrophotometry, as the second method, measures color developing from a chromogenic reaction, where a chromogenic substrate is cleaved by a proteolytic enzyme to release a chromophore, usually p-nitroaniline [3-7]. Because coagulation factors are proteolytic enzymes or cofactors thereof, the intensity of a chromogenic reaction correlates with the coagulation factor activity selective for the chromogenic substrate. In addition to chromogenic reaction, a fluorogenic substrate for thrombin can be used to track the time course of global coagulating activity. The method is used for the popular thrombin generation assay. Turbidimetry or nephelometry can detect clot formation and be utilized to measure clotting time in optical coagulometers. They are also applied to perform immunoassays, that is, immunoturbidimetric assays for quantifying markers related to hemostasis and thrombosis [8]. The immunoassay, the third method coagulometers are capable of, is commonly implemented in every clinical and research field. The results are easy to understand, leaving few needs for further interpretation. Several technological and clinical issues have been recently raised related to each of the three methods and will be briefly reviewed here.
Measurement of plasma clotting time is an old technique to screen for abnormality in hemostasis or, more specifically, coagulation. The original method is to directly observe the plasma sample in a test tube by eyes and determine when a fibrin clot is first detected [9, 10]. The reagents used for the test tube methods are essentially the same as those used for modern automated coagulometers. The test tube method is still accepted as the standard method in determining the international sensitivity index of the reagent thromboplastin for calculating the international normalized ratio (INR) [9]. Apart from the robotic sample and reagent pipetting system, the most significant difference between the test tube method and automated coagulometers is the technique of detecting fibrin clots. Automated coagulometers are equipped with viscometric or optic sensors that replace visual observation for high-throughput performance. The sensors track the viscosity or turbidity change of a plasma sample as the plasma turns into a viscous and turbid gel, that is, clot formation. These two methods measure the viscosity or the turbidity of the fibrin clot, respectively [11]. The viscosity-based method is less susceptible to optical interferents such as hemoglobin, bilirubin, and lipid (HIL) than the optical method [12-14]. There are both pros and cons regarding the viscosity-based method. Anyway, the high success rate in reporting the clotting time suits the coagulation screening purpose [11, 15]. The optic method adopted by manufacturers including Werfen, Sysmex, and Roche detects fibrin clot formation by measuring the turbidity of a plasma sample. An optical sensor tracks the turbidity change and determines the time needed for certain turbidity conditions to take place. PT, for example, is the time point where the rate of the turbidity increase (or equivalently the light transmission decrease) is the maximum (or minimum). For thrombin time, the time point where the turbidity (or light transmission) is one-third of the maximum (or minimum) is reported. At least theoretically, optic coagulometers are more susceptible than the viscosity method to spectral interferences as mentioned above [12-14]. The spectral interference problem inherent to the optical analyzers has been addressed continuously with accompanying effort to overcome. Major spectral interferents have become subjects of less concern by using an alternative detection wavelength for tracking the clot formation [11].
Despite the problem of spectral interference, optical coagulometers are advocated by many laboratories. They can present the turbidimetric curves that closely reflect the process of clot formation in plasma samples. The curve is drawn by plotting the time series of turbidimetric values (e.g., measured every 0.1 seconds) against the time. This visual representation of the clotting process provides plenty of information about the coagulation occurring in a plasma sample. The turbidimetric curve or clotting curve from every coagulation test assumes a sigmoidal shape. Chernysh and Weisel [15] reported that the developing fibrin clot evolved through several discernable steps of microstructure. Each step corresponded to a specific part of a sigmoidal clotting curve. Whereas various clotting curves take the common sigmoidal pattern, the exact curve shape differs between individuals or samples. The unique curve shape reflects the characteristics of the coagulation reaction of the sample. The clotting time does not express the full feature of a clotting curve shape. Rather, the clotting time is one of several features that collectively determine the curve shape. Same clotting time (e.g., PT or APTT) can be reported from different clotting curve shapes (Fig. 1). Shima et al. also presented such examples and introduced new coagulation parameters derived from the clotting curve of screening coagulation tests [16, 17]. A clotting curve can be differentiated to give first and second derivative curves. Because the clotting curve is the quantitative representation of clot formation, the first and second derivative curves can be regarded as the velocity and acceleration of clot formation (Fig. 2). As the clotting curves are sigmoidal, each of the derivative curves has one peak equivalent to the maximum velocity or acceleration of clot formation. The peaks are pointed to the negative or positive direction depending on the light transmittance (turbidimetry) or scattered light (nephelometry) being measured [18]. The two parameters were named min1 and min2. They can be called by other names depending on the manufacturer of the coagulometers. Clinical application of min1 and/or min2 for examining the patients with hemostatic risk is referred to as clot waveform analysis (CWA) [16, 17]. In the same reports above, the clotting curve shape changed with coagulation factor levels (e.g., factor VIII and factor IX). The shape of the clotting curve changed with decreasing FVIII or FIX level so that the clotting time (i.e., APTT) got prolonged, and min1 and/or min2 (or equivalently peak clotting velocity and/or acceleration) decreased gradually. The min1 and min2 even showed curvilinear responses to the factor levels. The findings are in accordance with intuitive expectations considering that the parameters represent clotting velocity and acceleration. The CWA parameters and the corresponding clotting times are not well correlated in general (Fig. 3). Probably, the CWA parameters seem to contain information separate from clotting times. However, they are not totally irrelevant. They all originate from the same clot-forming reaction and show different aspects. The time point corresponding to the first derivative peak (clotting velocity) is chosen for PT, whereas the value itself at that point is the min1. Similarly, the second derivative peak (clotting acceleration) is chosen for APTT, and the peak value is the min2 (Fig. 4).
The CWA is not a test directed to a single target of measure. Rather, it can be regarded as providing data reflecting comprehensive coagulation function as PT and/or APTT as screening coagulation tests do. The CWA data is produced from every clotting time-based coagulation test. Thus, clinical implication and application of CWA can be sought in any medico-surgical condition and scheme where a hemostatic issue can be raised. The CWA parameters have been studied mainly in bleeding diseases. CWA parameters showed responsiveness to various hemostatic stimulation. Nogami et al. [19] reported a reagent formulation to measure the coagulation potential of emicizumab spiked plasma. The min1 correlated well with the plasma emicizumab level as well as FVIII plus emicizumab level. The min1 also responded to bypassing agents, including recombinant factor VII (rFVII) or activated prothrombin complex concentrate (aPCC) in the emicizumab milieu. Clinically, min1 was used to assess the effects of emicizumab and/or bypassing agent in various subgroups of hemophilia A [20-22]. Beyond pharmacodynamic studies, CWA was a useful screening tool for hemophilia A [23]. Furthermore, FVIII specific inhibitors were easily differentiated from lupus anticoagulants when mixing APTT data was interpreted by CWA [24]. The most fundamental approach of CWA is probably the attempt to analyze the shape itself of the clotting curve. In this regard, an early report published in 2002 introduced the slanted baseline of clotting curve as a useful marker for disseminated intravascular coagulation [25]. Shimonishi et al. [26] represented the contour of the clotting velocity curve (the first derivative curve) by a set of indices derived from the numerical curve data. From the indices collected from reconstituted disease model plasma samples, a numerical template for the indices to which patient clotting curve can be compared. By this approach, a rough estimation of FVIII level could be made to diagnose hemophilia A. The beauty of the CWA is that the data is easily accessible both in terms of data availability and cost of analysis, as long as the clinical laboratory uses optic coagulometers. The CWA data is produced simultaneously every time the common screening coagulation tests such as PT and APTT are conducted. On the other hand, most relevant manufacturers provide the CWA data mainly as research parameters. The clinical implication and application still remain unexplored in many hemostatic and thrombotic diseases. Moreover, the standardization issues have not been fully addressed, although there has been a report discussing the recommendation of analyzers and reagents [18].
Using chromogenic substrate is a fully established method of measuring coagulation or anticoagulation factors [3-7]. The chromogenic two-stage FVIII assay (CSA) has also been in common use as one of the two main FVIII assays, the other one being the one-stage assay (OSA) based on APTT [6, 7]. The experimental procedure of the CSA consists of two steps, the incubation step and the measurement step, which is the most crucial difference between CSA and OSA. The CSA reagent contains coagulation factor IXa (FIXa), factor X (FX), and a trace amount of thrombin. Patient plasma is added to the reagent and incubated so that FVIII in patient plasma is activated to FVIIIa by thrombin and mediates the conversion of FX to FXa by FIXa. Because FVIII is preactivated fully, the subsequent reaction is not affected by FVIII to FVIIIa conversion. Theoretically, the variable efficiency of FVIII activation can affect the OSA result. The FXa produced in the first stage of CSA is measured by chromogenic FXa substrate. The amount of FXa depends on the FVIII content of patient plasma and can be calibrated to FVIII levels. Fewer coagulation factors participate in CSA reaction than in OSA. This means CSA is subject to less chance than OSA that coagulation factors other than FVIII affect the assay result. The discrepancy between the two assay methods has become a problem at hand as the trend of FVIII replacement therapy of hemophilia changed from plasma-derived FVIII to recombinant FVIII with some modifications such as B-domain deletion or extended half-life [27-30]. The OSA often underestimates FVIII activity of recombinant FVIII with these modifications that are potency-labeled with CSA. The clinical laboratories are encouraged to adopt CSA or drug-specific standards for OSA calibration to address the discrepancy problem.
Apart from the concern whether the CSA is always the better choice for potency labeling, the CSA is unique in that it contains an excess amount of FIXa and FX as the reagent constituents. Thus, the reagent can be modified to fit some particular purposes. This property endows the CSA a powerful advantage in emicizumab era of hemophilia management. Emicizumab is a bispecific antibody with one Fab arm targeting FIXa in the other targeting FX [31, 32]. The mechanism of action of emicizumab is similar to FVIII in that it approximates FIXa and FX in proper conformation such that FIXa can activate FX. However, emicizumab is not a natural substance directly involved in the pathogenesis of hemophilia A and, on many occasions, is needed to be excluded from the measurement of FVIII activity. For example, FVIII is used to manage acute bleeding in surgery or trauma patients on emicizumab prophylaxis [33, 34]. The infused FVIII cannot be measured accurately by OSA due to the FVIII-like activity of emicizumab. In this case, the emicizumab can be regarded as an interfering substance of the FVIII activity assay. The interfering effects of emicizumab have been demonstrated in APTT and APTT based coagulation factor assays (FVIII, FIX, FXI, and FXII) [35]. As for CSA, the interfering effects of emicizumab can be circumvented by using bovine FIXa and/or FX for reagent. Because emicizumab is xenospecific for human FIXa and FX, it does not bind bovine FIXa and/or FX to enable FX activation [36-38]. The conformed arrangement of FIXa and FX with FX activation is mediated only by endogenous and/or infused FVIII. Thus, CSA reagents using bovine FIXa and/or FX can selectively measure FVIII activity in the presence of emicizumab in a sample. It should be noted that not all commercial CSA reagents are made of bovine FIXa and/or FX [38]. CSA reagents composed of human FIXa and FX cannot differentiate FVIII activity from emicizumab interference. Theoretically, the difference of chromogenic FVIII activity between human FIXa/FX and bovine FIXa/FX reagents can be regarded as the FVIII equivalent value of emicizumab level in a sample. However, for this purpose, emicizumab specific OSA with an emicizumab-dedicated calibrator that has recently been developed is a better option [36].
FVIII activity also composes the primary data for quantifying FVIII inhibitor titer. The standard method to measure FVIII inhibitor is Nijmegen modification of Bethesda assay [39, 40]. To describe briefly, a plasma sample containing inhibitor is mixed with an equal volume of pooled normal plasma (PNP). The inhibitor titer is calculated from the extent to which the PNP FVIII activity (OSA) is suppressed. One Bethesda unit (BU) is defined as the amount of inhibitor that neutralizes half of FVIII contained in the PNP. FVIII inhibitor does not affect emicizumab, and emicizumab in plasma can mask the effect of FVIII inhibitor; that is, FVIII activity can be measured falsely high despite the presence of FVIII inhibitor. Thus, the emicizumab interference in the presence of an FVIII inhibitor can cause a false negative inhibitor assay result. FVIII inhibitor patients are priority candidates for emicizumab prophylaxis. After initiating the emicizumab prophylaxis, the FVIII inhibitor titer cannot be followed up by the standard FVIII inhibitor assay. To address this limitation, the standard FVIII assay has been modified by replacing the FVIII OSA with CSA using bovine FIXa and/or FX [31]. The CSA with bovine FIXa and/or FX measures endogenous or infused FVIII exclusively unaffected by emicizumab interference. The inhibitor-induced suppression of FVIII activity is well reflected in CSA results regardless of the presence of emicizumab in a sample.
Immunoassay is probably the most widely adopted in vitro diagnostic assay method. As mentioned above, many modern coagulometers are equipped with immunoturbidimetry and quantitate hemostatic molecules such as d-dimer and von Willebrand factor (VWF) [41-43]. There have been some recent developments in immunoassays for von Willebrand disease (VWD) diagnosis. The immunoassays on VWF are unique in that they have some functional implications in addition to measuring a simple molar concentration. Von Willebrand factor or VWF is a large glycoprotein produced and secreted by endothelial cells. The essential function of VWF is to bind platelets and collagen. Thus it has a hemostatic role in connecting platelets and subendothelial tissue around an injury. VWF is also the carrier protein of coagulation factor VIII and is indispensable for FVIII stability [44, 45]. VWD is well known as the number one inherited bleeding disease.
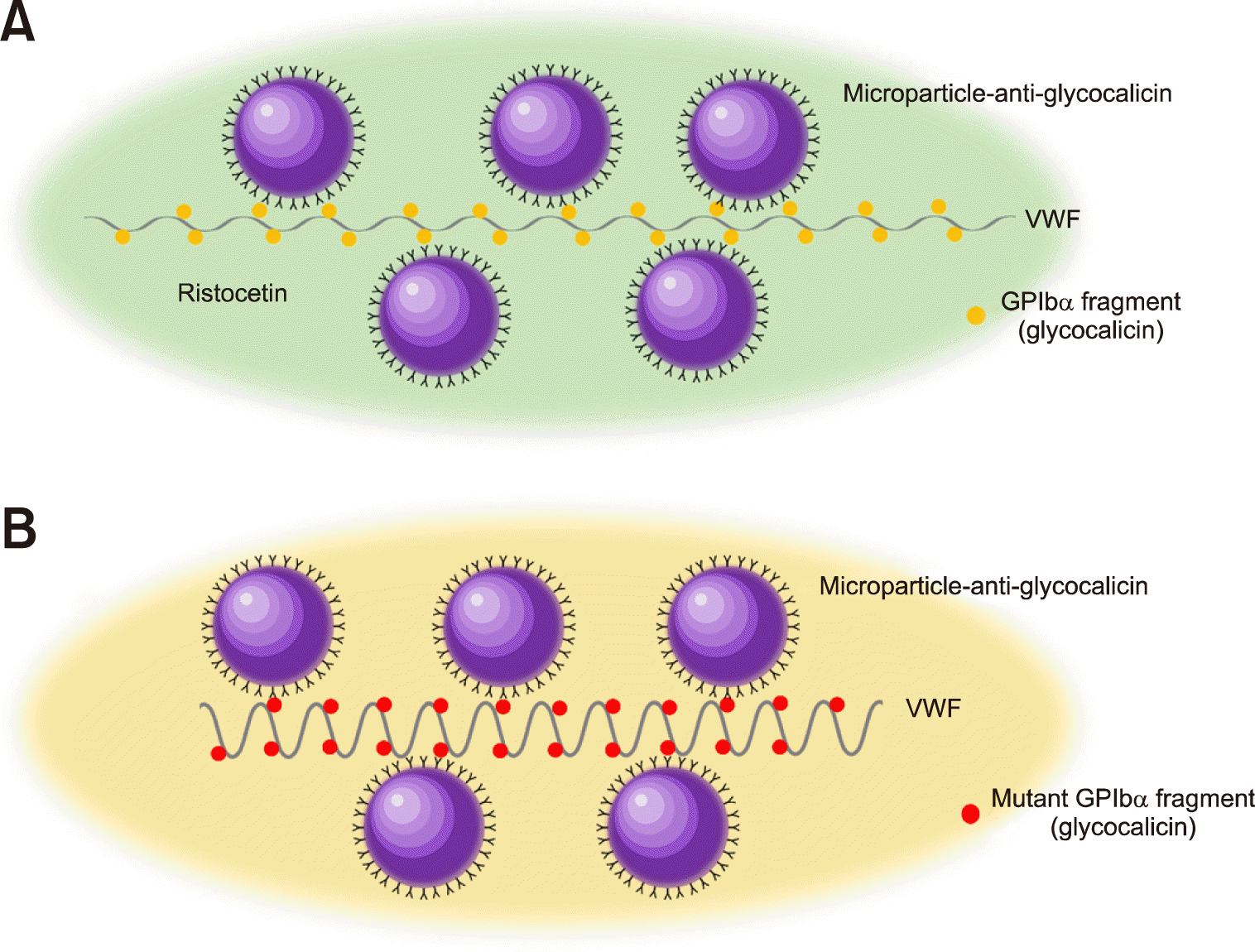
The laboratory tests for VWD diagnosis are VWF:Ag level, ristocetin cofactor activity (VWF:RCO), collagen-binding activity (VWF:CB), and FVIII binding assay (VWF:FVIIIB) [46-48]. VWF:Ag assay is simply an immunoassay to measure physical VWF concentration in principle. In contrast, VWF:RCO measures the most important function of VWF, which is binding platelets. VWF in static solution does not bind platelets because the GPIb binding site on VWF is concealed by peptide folds around. However, when VWF is under shear stress like turbulent blood flow (e.g., blood flow through an injured vessel), it is stretched, and GPIb binding sites are exposed, allowing platelet binding. A similar reaction can be chemically induced by adding ristocetin to platelet-rich plasma. Ristocetin binds VWF and causes a conformational change to expose GPIb binding site. Ristocetin-induced platelet aggregation is tracked by a platelet aggregometer, and the slopes of the aggregation curves can be calibrated to VWF concentrations. This is the basic assay principle of VWF:RCO. The original method involves a lot of manual work, and its assay performance is limited by poor reproducibility and analytic sensitivity. Recently, the VWF:RCO assay has been modified by replacing the platelets with microparticles coated with anti-glycocalicin, which is the extracellular fragment of GPIbα. The use of microparticle enabled automated VWF:RCO assay, in which the microparticle aggregation rather than platelet aggregation was detected by turbidimetry (Fig. 5A) [48, 49]. The microparticle aggregation is partly mediated by the binding of glycocalicin to ristocetin-treated VWF. This differs from simple immunoturbidimetric assays where the binding occurs only between antigens and antibodies. Alternatively, ristocetin can be eliminated from the reaction by using the glycocalicin with a gain of function (GOF) mutation (Fig. 5B) [50]. Gain of function means that the mutated glycocalicin binds VWF with such a high affinity that ristocetin is no longer needed, as in the case of platelet type VWD.
For other functional assays, a separate analyzer relying on a different assay principle or a manual procedure should be considered. VWF:CB assay is similar to VWF:RCO assay in that both receptor-ligand and antigen-antibody binding are needed for a measurable reaction to develop [51]. For VWF:CB, an old enzyme-link immunosorbent assay (ELISA) kit was available. Also, an automated VWF:CB assay has recently been developed by Werfen. However, the assay is based on the chemiluminescence immunoassay method and is not run by the immunoturbidimetric method [52].
VWF:CB assay is not as widely introduced in clinical laboratories as VWF:Ag and VWF:RCO assay. Collagen tends to bind large VWF multimers, and collagen binding activity is known to reflect VWF multimer distribution. FVIII carrier function of VWF can be examined by a FVIII binding assay to diagnose type 2N VWD [47]. The assay principle is also a modified form of ELISA with anti-FVIII as the detection antibody. The current diagnostic capability of modern high-throughput coagulometers for VWD is at most VWF:Ag, VWF:RCO, and FVIII:C. Differential diagnosis of VWD subtypes relying on these three tests is by no means complete. Automated VWF:Ag, VWF:RCO, and VWF:CB immunoassays that can be conducted in a single chemiluminescence analyzer, with analytical performance comparable to conventional methods, are now in the market [52].
The data produced and reported daily from common high-throughput coagulometers are often overlooked as a quantitative marker with simple cutoffs of decision making. Many hemostasis tests are unique in that they are functional assays, and the results can take different forms depending on how the data are interpreted. Although they are based on long-established assay principles, the new way of data analysis and continuous improvement of assay components make new kinds of contributions to managing hemostatic and thrombotic diseases.
REFERENCES
1. Lippi G, Plebani M, Favaloro EJ. 2014; Technological advances in the hemostasis laboratory. Semin Thromb Hemost. 40:178–85. DOI: 10.1055/s-0033-1364206. PMID: 24443219. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84896730641&origin=inward.

2. Lippi G, Cadamuro J, Danese E, et al. 2018; Internal quality assurance of HIL indices on Roche Cobas c702. PLoS One. 13:e0200088. DOI: 10.1371/journal.pone.0200088. PMID: 29979722. PMCID: PMC6034854. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85049512643&origin=inward.

3. Abildgaard U, Lie M, Odegård OR. 1977; Antithrombin (heparin cofactor) assay with "new" chromogenic substrates (S-2238 and Chromozym TH). Thromb Res. 11:549–53. DOI: 10.1016/0049-3848(77)90208-0. PMID: 72424. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0017599989&origin=inward.

4. Goodnight SH Jr, Schaeffer JL, Sheth K. 1980; Measurement of antithrombin III in normal and pathologic states using chromogenic substrate S-2238. Comparison with immuno-electrophoretic and factor Xa inhibition assays. Am J Clin Pathol. 73:639–47. DOI: 10.1093/ajcp/73.5.639. PMID: 7377131. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0019313202&origin=inward.

5. Dang QD, Di Cera E. 1997; Chromogenic substrates selective for activated protein C. Blood. 89:2220–2. DOI: 10.1182/blood.V89.6.2220. PMID: 9058748. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0030893304&origin=inward.

6. Prasa D, Sturzebecher J. 1998; Determination of activated factor IX in factor IX concentrates with a chromogenic substrate. Thromb Res. 92:99–102. DOI: 10.1016/S0049-3848(98)00117-0. PMID: 9792118. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0344474670&origin=inward.

7. Bowyer AE, Hillarp A, Ezban M, Persson P, Kitchen S. 2016; Measuring factor IX activity of nonacog beta pegol with commercially available one-stage clotting and chromogenic assay kits: a two-center study. J Thromb Haemost. 14:1428–35. DOI: 10.1111/jth.13348. PMID: 27107268. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84990198558&origin=inward.

8. Whicher JT, Price CP, Spencer K. 1983; Immunonephelometric and immunoturbidimetric assays for proteins. Crit Rev Clin Lab Sci. 18:213–60. DOI: 10.3109/10408368209085072. PMID: 6339164. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0020648763&origin=inward.

9. Tripodi A, Chantarangkul V, Negri B, Clerici M, Mannucci PM. 1998; International collaborative study for the calibration of a proposed reference preparation for thromboplastin, human recombinant, plain. On behalf of the Subcommittee on Control of Anticoagulation. Thromb Haemost. 79:439–43. DOI: 10.1055/s-0037-1615004. PMID: 9493604. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0031936408&origin=inward.
10. van den Besselaar AMHP, van Rijn CJJ, Abdoel CF, et al. 2020; Paving the way for establishing a reference measurement system for standardization of plasma prothrombin time: harmonizing the manual tilt tube method. J Thromb Haemost. 18:1986–94. DOI: 10.1111/jth.14873. PMID: 32356308. PMCID: PMC7496835. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85087175635&origin=inward.

11. Favaloro EJ, Lippi G. 2019; Recent advances in mainstream hemostasis diagnostics and coagulation testing. Semin Thromb Hemost. 45:228–46. DOI: 10.1055/s-0038-1676579. PMID: 30912101. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85063881193&origin=inward.

12. Magnette A, Chatelain M, Chatelain B, Ten Cate H, Mullier F. 2016; Pre-analytical issues in the haemostasis laboratory: guidance for the clinical laboratories. Thromb J. 14:49. DOI: 10.1186/s12959-016-0123-z. PMID: 27999475. PMCID: PMC5154122. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85003480080&origin=inward.

13. Woolley A, Golmard JL, Kitchen S. 2016; Effects of haemolysis, icterus and lipaemia on coagulation tests as performed on Stago STA- Compact-Max analyser. Int J Lab Hematol. 38:375–88. DOI: 10.1111/ijlh.12498. PMID: 27306848. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84978374732&origin=inward.
14. Nougier C, Jousselme E, Sobas F, Pousseur V, Négrier C. 2020; Effects of hemolysis, bilirubin, and lipemia interference on coagulation tests detected by two analytical systems. Int J Lab Hematol. 42:88–94. DOI: 10.1111/ijlh.13147. PMID: 31846202. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85077078664&origin=inward.

15. Chernysh IN, Weisel JW. 2008; Dynamic imaging of fibrin network formation correlated with other measures of polymerization. Blood. 111:4854–61. DOI: 10.1182/blood-2007-08-105247. PMID: 18272815. PMCID: PMC2384121. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=46749153500&origin=inward.

16. Shima M, Matsumoto T, Fukuda K, et al. 2002; The utility of activated partial thromboplastin time (aPTT) clot waveform analysis in the investigation of hemophilia A patients with very low levels of factor VIII activity (FVIII:C). Thromb Haemost. 87:436–41. DOI: 10.1055/s-0037-1613023. PMID: 11916076. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0036125865&origin=inward.

17. Matsumoto T, Shima M, Takeyama M, et al. 2006; The measurement of low levels of factor VIII or factor IX in hemophilia A and hemophilia B plasma by clot waveform analysis and thrombin generation assay. J Thromb Haemost. 4:377–84. DOI: 10.1111/j.1538-7836.2006.01730.x. PMID: 16420569. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33645582371&origin=inward.

18. Shima M, Thachil J, Nair SC, Srivastava A. Scientific and Standardization Committee. 2013; Towards standardization of clot waveform analysis and recommendations for its clinical applications. J Thromb Haemost. 11:1417–20. DOI: 10.1111/jth.12287. PMID: 23648068. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84893514460&origin=inward.

19. Nogami K, Matsumoto T, Tabuchi Y, et al. 2018; Modified clot waveform analysis to measure plasma coagulation potential in the presence of the anti-factor IXa/factor X bispecific antibody emicizumab. J Thromb Haemost. 16:1078–88. DOI: 10.1111/jth.14022. PMID: 29645406. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85047426922&origin=inward.

20. Nakajima Y, Nogami K, Yada K, et al. 2020; Emicizumab improves ex vivo clotting function in patients with mild/moderate hemophilia A. Thromb Haemost. 120:968–76. DOI: 10.1055/s-0040-1710315. PMID: 32384547. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85085962315&origin=inward.

21. Takeyama M, Furukawa S, Yada K, et al. 2021; Ex vivo prediction of comprehensive coagulation potential using simulated blood concentrations of emicizumab in patients with acquired hemophilia A. Thromb Haemost. 121:1289–98. DOI: 10.1055/s-0041-1725009. PMID: 33641138. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85102007390&origin=inward.

22. Furukawa S, Nogami K, Shimonishi N, Nakajima Y, Matsumoto T, Shima M. 2020; Prediction of the haemostatic effects of bypassing therapy using comprehensive coagulation assays in emicizumab prophylaxis-treated haemophilia A patients with inhibitors. Br J Haematol. 190:727–35. DOI: 10.1111/bjh.16574. PMID: 32162680. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85081739388&origin=inward.

23. Milos M, Coen Herak D, Mahmoud Hourani Soutari N, et al. 2021; Overall hemostasis potential and aPTT-clot waveform analysis as powerful laboratory diagnostic tools for identification of hemophilia A patients with unexpected bleeding phenotype. Int J Lab Hematol. 43:273–80. DOI: 10.1111/ijlh.13347. PMID: 32964648. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85091267698&origin=inward.

24. Shimonishi N, Ogiwara K, Oda Y, et al. 2021; Inhibitor index in the clot waveform analysis-based mixing test differentiates among hemophilia A without and with inhibitors, and lupus anticoagulant. Thromb Haemost. 121:792–9. DOI: 10.1055/s-0040-1721776. PMID: 33412612. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85099277802&origin=inward.

25. Toh CH, Samis J, Downey C, et al. 2002; Biphasic transmittance waveform in the APTT coagulation assay is due to the formation of a Ca(++)-dependent complex of C-reactive protein with very-low-density lipoprotein and is a novel marker of impending disseminated intravascular coagulation. Blood. 100:2522–9. DOI: 10.1182/blood.V100.7.2522. PMID: 12239165. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0036785406&origin=inward.

26. Shimonishi N, Ogiwara K, Oda Y, et al. 2021; A novel assessment of factor VIII activity by template matching utilizing weighted average parameters from comprehensive clot waveform analysis. Thromb Haemost. 121:164–73. DOI: 10.1055/s-0040-1715838. PMID: 32828071. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85099292567&origin=inward.

27. Pavlova A, Delev D, Pezeshkpoor B, Müller J, Oldenburg J. 2014; Haemophilia A mutations in patients with non-severe phenotype associated with a discrepancy between one-stage and chromogenic factor VIII activity assays. Thromb Haemost. 111:851–61. DOI: 10.1160/TH13-08-0690. PMID: 24452774. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84899768541&origin=inward.

28. Trossaërt M, Regnault V, Sigaud M, Boisseau P, Fressinaud E, Lecompte T. 2008; Mild hemophilia A with factor VIII assay discrepancy: using thrombin generation assay to assess the bleeding phenotype. J Thromb Haemost. 6:486–93. DOI: 10.1111/j.1538-7836.2007.02861.x. PMID: 18047548. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=39549098650&origin=inward.

29. Lyall H, Hill M, Westby J, Grimley C, Dolan G. 2008; Tyr346-->Cys mutation results in factor VIII:C assay discrepancy and a normal bleeding phenotype - is this mild haemophilia A? Haemophilia. 14:78–80. DOI: 10.1111/j.1365-2516.2007.01557.x. PMID: 18034822. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=37749007969&origin=inward.
30. Peyvandi F, Oldenburg J, Friedman KD. 2016; A critical appraisal of one-stage and chromogenic assays of factor VIII activity. J Thromb Haemost. 14:248–61. DOI: 10.1111/jth.13215. PMID: 26663865. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84976209150&origin=inward.

31. Oldenburg J, Mahlangu JN, Kim B, et al. 2017; Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med. 377:809–18. DOI: 10.1056/NEJMoa1703068. PMID: 28691557. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85028497303&origin=inward.

32. Mahlangu J, Oldenburg J, Paz-Priel I, et al. 2018; Emicizumab prophylaxis in patients who have hemophilia A without inhibitors. N Engl J Med. 379:811–22. DOI: 10.1056/NEJMoa1803550. PMID: 30157389. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85052877162&origin=inward.

33. Aledort L, Mannucci PM, Schramm W, Tarantino M. 2019; Factor VIII replacement is still the standard of care in haemophilia A. Blood Transfus. 17:479–86. DOI: 10.3410/f.737105219.793576025. PMID: 31846611. PMCID: PMC6917528.
34. Castaman G, Santoro C, Coppola A, et al. 2020; Emergency management in patients with haemophilia A and inhibitors on prophylaxis with emicizumab: AICE practical guidance in collaboration with SIBioC, SIMEU, SIMEUP, SIPMeL and SISET. Blood Transfus. 18:143–51. DOI: 10.2450/2019.0186-19. PMID: 31657709. PMCID: PMC7141940. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85078796323&origin=inward.
35. Adamkewicz JI, Chen DC, Paz-Priel I. 2019; Effects and interferences of emicizumab, a humanised bispecific antibody mimicking activated factor VIII cofactor function, on coagulation assays. Thromb Haemost. 119:1084–93. DOI: 10.1055/s-0039-1688687. PMID: 31064025. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85067976440&origin=inward.

36. Müller J, Pekrul I, Pötzsch B, Berning B, Oldenburg J, Spannagl M. 2019; Laboratory monitoring in emicizumab-treated persons with hemophilia A. Thromb Haemost. 119:1384–93. DOI: 10.1055/s-0039-1692427. PMID: 31203578. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85071782898&origin=inward.

37. Jenkins PV, Bowyer A, Burgess C, et al. 2020; Laboratory coagulation tests and emicizumab treatment A United Kingdom Haemophilia Centre Doctors' Organisation guideline. Haemophilia. 26:151–5. DOI: 10.1111/hae.13903. PMID: 31859415. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85076925045&origin=inward.

38. Lowe A, Kitchen S, Jennings I, Kitchen DP, Woods TAL, Walker ID. 2020; Effects of emicizumab on APTT, FVIII assays and FVIII inhibitor assays using different reagents: results of a UK NEQAS proficiency testing exercise. Haemophilia. 26:1087–91. DOI: 10.1111/hae.14177. PMID: 33094895. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85093512558&origin=inward.

39. Kasper C, Aledort L, Counts R, Edson J, Frantantoni J, Green D. 1975; Letter: a more uniform measurement of factor VIII inhibitors. Thromb Diath Haemorrh. 34:869–72. DOI: 10.1055/s-0038-1651378. PMID: 1209552. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0016665839&origin=inward.
40. Verbruggen B, Novakova I, Wessels H, Boezeman J, van den Berg M, Mauser-Bunschoten E. 1995; The Nijmegen modification of the Bethesda assay for factor VIII:C inhibitors: improved specificity and reliability. Thromb Haemost. 73:247–51. DOI: 10.1055/s-0038-1653759. PMID: 7792738. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0028837315&origin=inward.

41. Korte W, Riesen W. 2000; Latex-enhanced immunoturbidimetry allows D-dimer determination in plasma and serum samples. Clin Chem. 46:871–2. DOI: 10.1093/clinchem/46.6.871. PMID: 10839782. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0034125759&origin=inward.

42. Knecht MF, Heinrich F. 1997; Clinical evaluation of an immuno-turbidimetric D-dimer assay in the diagnostic procedure of deep vein thrombosis and pulmonary embolism. Thromb Res. 88:413–7. DOI: 10.1016/S0049-3848(97)00276-4. PMID: 9556229. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0031462097&origin=inward.

43. Christophe O, Rouault C, Obert B, Piétu G, Meyer D, Girma JP. 1995; A monoclonal antibody (B724) to von Willebrand factor recognizing an epitope within the A1 disulphide loop (Cys509- Cys695) discriminates between type 2A and type 2B von Willebrand disease. Br J Haematol. 90:195–203. DOI: 10.1111/j.1365-2141.1995.tb03400.x. PMID: 7540413. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0029076691&origin=inward.

44. Sadler JE. 1998; Biochemistry and genetics of von Willebrand factor. Annu Rev Biochem. 67:395–424. DOI: 10.1146/annurev.biochem.67.1.395. PMID: 9759493. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0031686041&origin=inward.

45. Schneppenheim R, Budde U. 2005; Phenotypic and genotypic diagnosis of von Willebrand disease: a 2004 update. Semin Hematol. 42:15–28. DOI: 10.1053/j.seminhematol.2004.10.002. PMID: 15662612. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=12344262502&origin=inward.

46. Favaloro EJ, Pasalic L, Curnow J. 2016; Laboratory tests used to help diagnose von Willebrand disease: an update. Pathology. 48:303–18. DOI: 10.1016/j.pathol.2016.03.001. PMID: 27131932. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84978664388&origin=inward.

47. Mohammed S, Favaloro EJ. 2017; Laboratory testing for von Willebrand factor: factor VIII binding (for 2N VWD). Methods Mol Biol. 1646:461–72. DOI: 10.1007/978-1-4939-7196-1_34. PMID: 28804848. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85027521321&origin=inward.

48. Patzke J, Favaloro EJ. 2017; Laboratory testing for von Willebrand factor activity by glycoprotein Ib binding assays (VWF:GPIb). Methods Mol Biol. 1646:453–60. DOI: 10.1007/978-1-4939-7196-1_33. PMID: 28804847. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85027507526&origin=inward.

49. Favaloro EJ, Mohammed S. 2014; Towards improved diagnosis of von Willebrand disease: comparative evaluations of several automated von Willebrand factor antigen and activity assays. Thromb Res. 134:1292–300. DOI: 10.1016/j.thromres.2014.09.024. PMID: 25300811. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84915821040&origin=inward.

50. Bodó I, Eikenboom J, Montgomery R, Patzke J, Schneppenheim R, Di Paola J. 2015; Platelet-dependent von Willebrand factor activity. Nomenclature and methodology: communication from the SSC of the ISTH. J Thromb Haemost. 13:1345–50. DOI: 10.1111/jth.12964. PMID: 25858564. PMCID: PMC5576173. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84935687838&origin=inward.

51. Favaloro EJ. 2017; Diagnosis or exclusion of von Willebrand disease using laboratory testing. Methods Mol Biol. 1646:391–402. DOI: 10.1007/978-1-4939-7196-1_29. PMID: 28804843. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85027534798&origin=inward.

52. Favaloro EJ, Mohammed S. 2016; Evaluation of a von Willebrand factor three test panel and chemiluminescent-based assay system for identification of, and therapy monitoring in, von Willebrand disease. Thromb Res. 141:202–11. DOI: 10.1016/j.thromres.2015.12.010. PMID: 26743192. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84951957182&origin=inward.

Fig. 1
Clotting curve shapes determine the clotting times (prothrombin times here), but not vice versa. The slopes and amplitudes of the clotting curves differ significantly and imply variable coagulation potential. However, the clotting times (prothrombin times here) are the same for all six test samples.
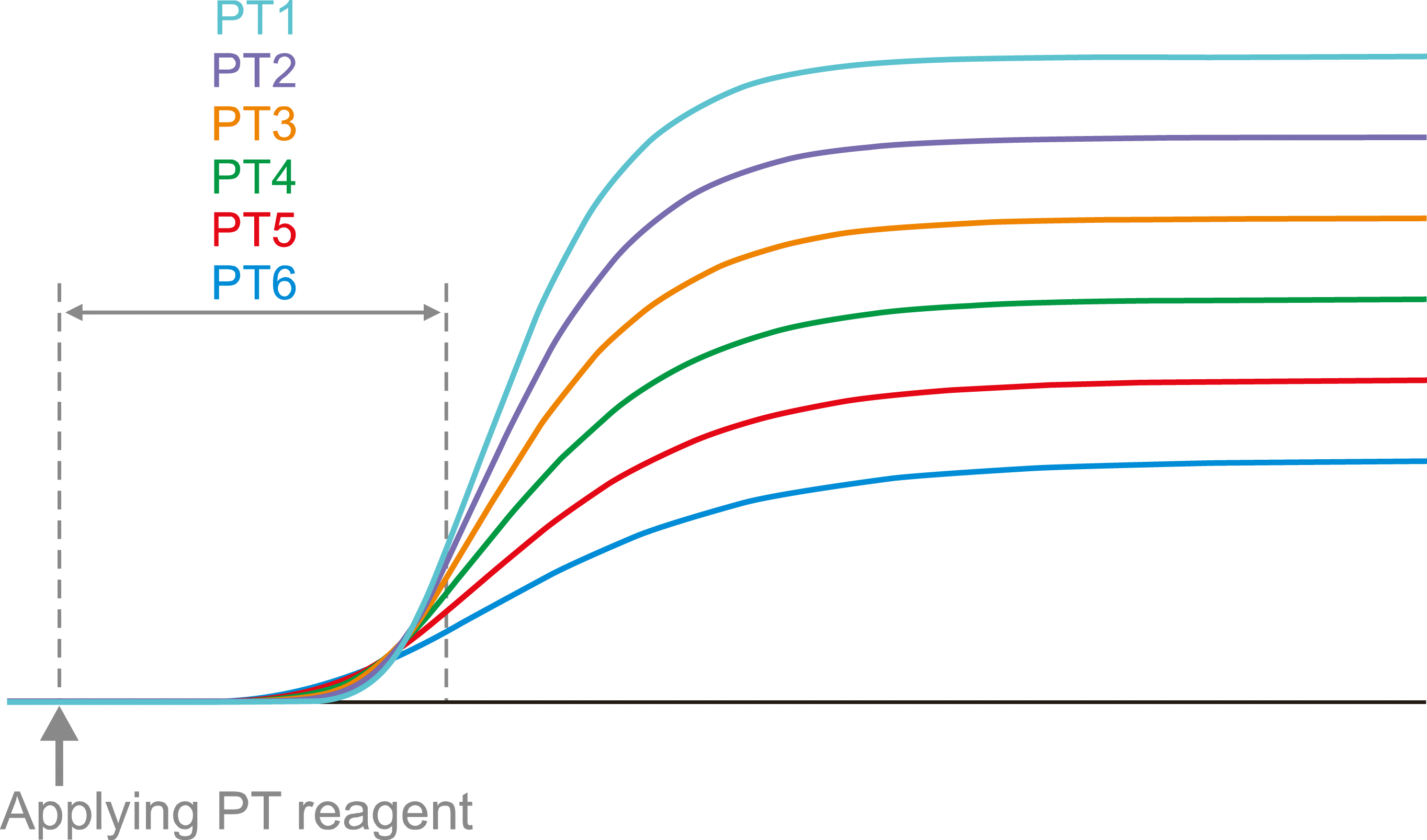
Fig. 2
The clotting curve can be differentiated by a numerical method, and the 1st and 2nd derivative curves can be drawn. If the light transmission is read (turbidimetry) for clot detection, the 1st and the 2nd derivative peaks are directed downward (A). Conversely, if scattered light is read (nephelometry), the 1st and the 2nd derivative peaks are directed upward (B).
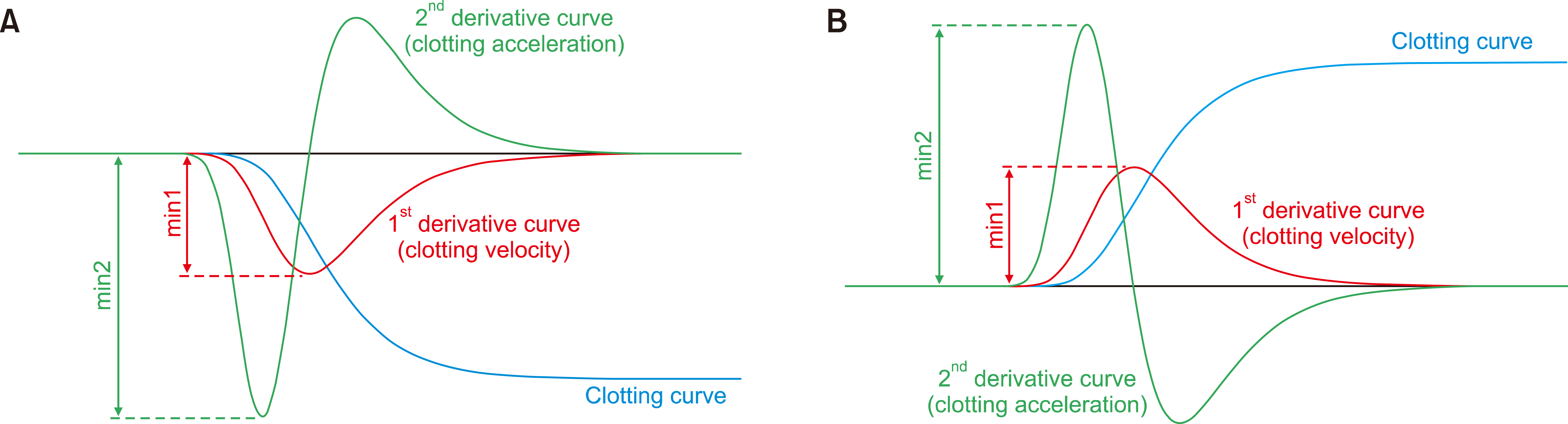
Fig. 3
The clotting time and the CWA parameters present different aspects of a clotting reaction. The clotting curve parameters were collected from randomly selected blood samples referred for PT and APTT. The CWA parameters (|min1| and |min2|) and the clotting times (PT and APTT) were obtained from each plasma sample and dot-plotted on |min1| vs. PT (A), |min2| vs. PT (B), |min1| vs. APTT (C), and |min2| vs. APTT (D) planes. It is apparent by a simple visual ex-amination that both PT and APTT are poorly correlated with |min1| or |min2|. Samples with the same PT or APTT demonstrated a wide range of |min1| or |min2| values.
Fig. 4
PT and APTT are technically defined as the time needed for the clotting velocity and acceleration to reach the maximum after adding each reagent to plasma. Thus, PT is the time point of the min1 and APTT of the min2.
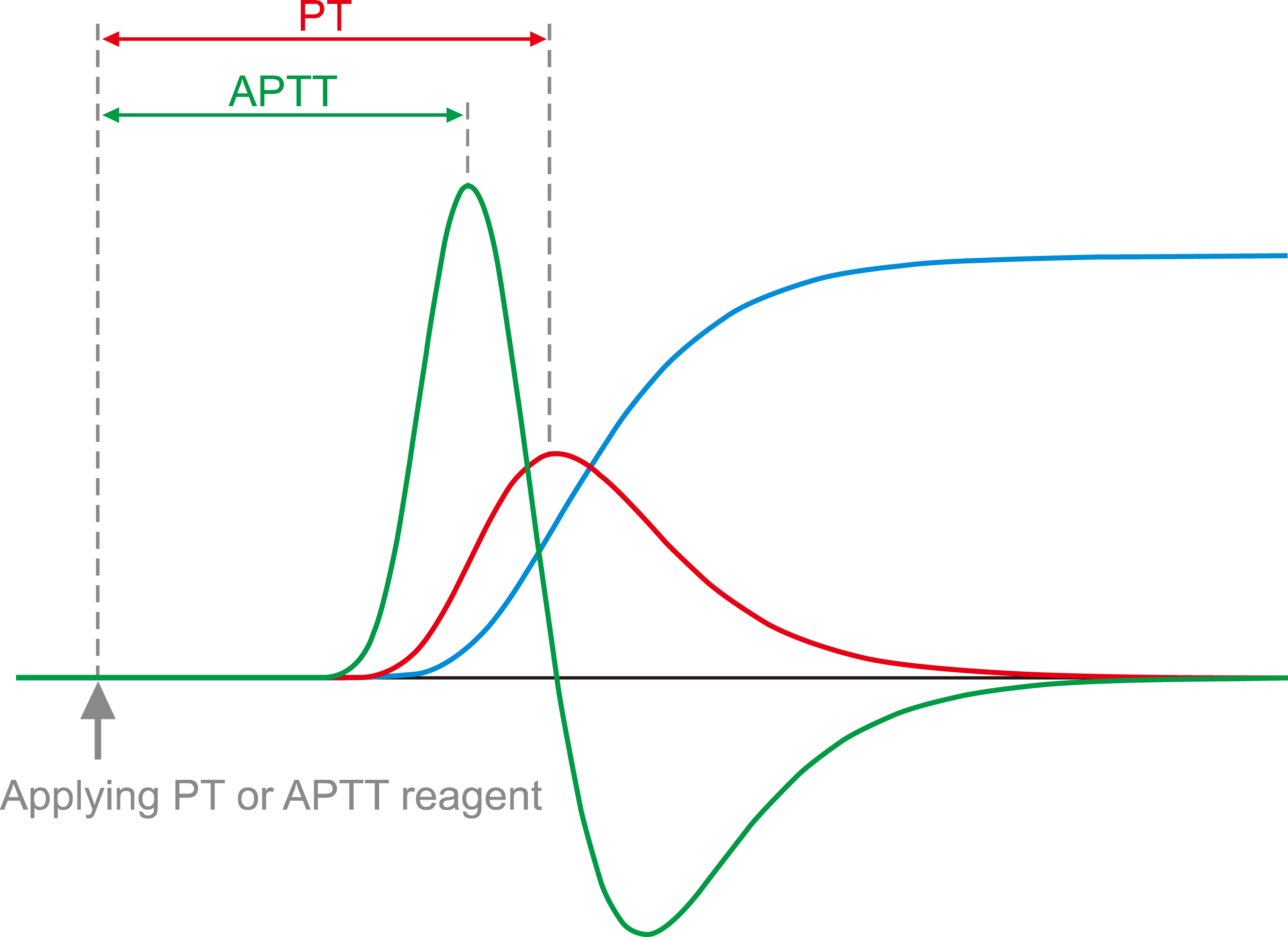
Fig. 5
VWF:RCO can be automated by replacing platelets of the original VWF:RCO with microparticles. In the method developed by Werfen, ristocetin primed plasma VWF binds glycocalicin. The bound glycocalicin molecules can be detected by anti-glycocalicin-coated microparticles (A). Alternatively, the glycocalicin with a GOF mutation of platelet type VWD can be used without ristocetin, as for the reagent from Siemens (B).




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download