This article has been
cited by other articles in ScienceCentral.
Abstract
Immunohistochemistry is a technique that uses antigen-antibody interactions to detect specific proteins in cells. This technique has several essential applications in lymphoma diagnosis, including identifying the cell lineage and phase of maturation, detecting specific genetic alterations, visualizing the degree of cell proliferation, and identifying therapeutic targets. CD3 is a pan T-cell marker expressed on most of the mature T/NK-cell lymphomas, except for anaplastic large cell lymphoma, whereas CD20 is a pan B-cell marker that is expressed on most of the mature B-cell lymphomas. CD79a may be a good alternative to CD20, compensating for its loss owing to the plasmocytic differentiation of tumor cells or history of rituximab administration. CD56, a neuroendocrine marker, is used as an NK cell marker in lymphoma diagnosis. Characteristic translocations occurring in follicular lymphoma (BCL2) and mantle cell lymphoma (CCND1) can be detected by the overexpression of Bcl-2 and cyclin D-1 in immunohistochemistry, respectively. Ki-67 reflects the degree of tumor cell proliferation by indicating cells in cell cycle phases other than G0. With the development of immunotherapy, several antibodies against markers such as programmed death-ligand 1 (PD-L1), CD19, and CD30 have been used as biomarkers to identify therapeutic targets. It is critical to properly fix the specimens to obtain accurate immunohistochemical results. Therefore, all processes, from tissue collection to the final pathological diagnosis, must be performed appropriately for accurate lymphoma diagnosis.
Go to :

Keywords: Lymphoma, Immunohistochemistry, In situ hybridization
INTRODUCTION
Immunohistochemistry (IHC) is a diagnostic technique relying on antigen-antibody interactions to detect specific proteins in cells. Compared to other ancillary techniques, IHC has the advantages of high cost-effectiveness, shorter turnaround time, and the ability to be performed on formalin-fixed paraffin-embedded tissues.
IHC is essential in lymphoma diagnosis because the lymphocyte type (B or T cell) is indistinguishable on hematoxylin and eosin (H&E) stained slides. This technique is usually performed for lymphoma diagnosis for the following reasons: 1) cell lineage and maturation phase can be identified, 2) specific genetic alterations can be detected, 3) the degree of cell proliferation can be visualized, and 4) therapeutic targets can be identified. Markers could be used for various purposes depending on the situation and type of tumor.
In this review, we intend to enhance the readers’ understanding of IHC results in pathology reports by introducing recommended panels for various lymphomas.
Go to :

RECOMMENDED PANELS FOR DLBCL AND ITS VARIANTS
|
Essential |
CD3, CD20, Ki-67, CD10, Bcl-6, MUM-1, Bcl-2, c-myc, EBV ISH |
|
Optional |
CD79a, CD30, CD23, ALK, CD138 |
In current WHO classification of tumours of haematopoietic and lymphoid tissues [
1], diffuse large B-cell lymphoma (DLBCL) consists of DLBCL, not otherwise specified (DLBCL NOS), and various specific variants. Typical IHC results of DLBCL NOS are characterized by large, atypical CD3-negative and CD20-positive lymphocytes with a Ki-67 labeling index greater than 80%. Further, the cell of origin (COO) classification is performed by staining for CD10 (cut off: 30%), Bcl-6 (cut off: 30%), and MUM-1 (multiple myeloma 1) (cut off: 30%) through the Hans algorithm [
2]. Their double-expressor phenotype is verified by staining for Bcl-2 (cut off: 50%) and c-myc (cut off: 40%) [
3]. Additionally, Epstein-Barr virus in situ hybridization (EBV ISH) staining aids in the detection of EBV-positive DLBCL (EBV DLBCL) (
Fig. 1).
 | Fig. 1
Representative immuno-histochemical staining images of various type of diffuse large B-cell lymphomas.
Abbreviations: ABC, activated B-cell type; DLBCL, diffuse large B-cell lymphoma; GCB, germinal center B-cell type; EBV DLBCL, EBV-positive diffuse large B-cell lymphoma.

|
CD10 is a marker normally expressed in centroblasts and centrocytes of reactive germinal centers. Therefore, according to the Hans algorithm, the tumor is classified as germinal center B-cell (GCB) type if there is >30% CD10-positive tumor cell population, regardless of the results from the other two markers. MUM-1 is normally expressed in plasma cells or post-GCBs; therefore, MUM-1 expression in DLBCL suggests the possibility of a non-GCB activated B-cell (ABC) type. Similar to CD10, Bcl-6 is a marker expressed in centroblasts and centrocytes of the germinal center, as well as in many ABC-DLBCLs. Therefore, when Bcl-6 expression is positive (≥30%), it is classified as a GCB only when MUM-1 expression is negative (<30%). In GCB-DLBCL, if the sample is CD10-positive, Bcl-2-negative, and Ki-67 labeling index is higher than 95%, the possibility of Burkitt’s lymphoma must be considered and confirmed through
MYC fluorescence in situ hybridization (FISH). To diagnose double hit lymphoma (high-grade B-cell lymphoma with
MYC and
BCL2 and/or
BCL6 rearrangements), all patients with DLBCL would have FISH testing for
MYC rearrangements, ideally. Then, the patients found to have MYC rearrangements should have subsequent FISH for
BCL2 and
BCL6 rearrange-ments. A compromise would be to use c-myc IHC to screen patients for further testing with FISH [
4], however, since the results of c-myc IHC do not correlate well with
MYC translocation [
5], author do not recommend using c-myc IHC as a tool for screening patients in need of
MYC FISH.
EBV ISH is distinct from the IHC technique, although they have similar methods of interpreting stained slides. Therefore, we will discuss it together in this review. If EBV ISH is positive in >80% of tumor cells and the patient is not immunodeficient, it is classified as EBV DLBCL NOS. Occasionally, small numbers of EBV-positive cells may be present as bystander B cells in EBV-negative lymphomas [
6].
Occasionally, DLBCL is suspected based on H&E staining; however, the tumor cells are negative for CD3 and CD20. In such cases, the possibilities of a poorly-differentiated carcinoma or small round cell tumor should be considered as a differential diagnosis in routine practice. However, CD20-negative DLBCL and anaplastic large-cell lymphoma (ALCL) are included in the differential diagnosis when considering the possibility of lymphoma. ALCL will be addressed in the T-cell lymphoma section below.
If a tumor suspected of DLBCL is CD20-negative, any history of rituximab administration should be verified. If there is a history of rituximab administration, the presence of CD79a markers should be verified additionally to identify the B-cell lineage. In patients with no history of rituximab administration, the possibility of plasmablastic lymphoma (PBL) or anaplastic lymphoma kinase (ALK)-positive large B-cell lymphoma should be considered. For the diagnosis of PBL, plasma cell differentiation should be confirmed through CD138 staining. The EBV expression can also aid in the diagnosis. In ALK-positive large B-cell lymphoma, pan-B-cell markers including CD20, CD79a, and paired box 5 (PAX5) are usually negative or weakly positive. However, plasma cell markers are positive and ALK is strongly positive, confirming a B-cell lineage.
Most specific variants of DLBCL show an IHC pattern similar to that of DLBCL NOS. However, primary mediastinal (thymic) large B-cell lymphoma (PMLBL) cells express CD30 and CD23, which can aid in the diagnosis. Since the sensitivities for CD30 and CD23 are approximately 80% and 70% [
7,
8], respectively, they are not expressed in all PMLBLs. When both markers are negative, PMLBL and DLBCL NOS are differentiated based on tumor location, patient age, and characteristic histological findings of PMLBL. However, the differential diagnosis is sometimes ambiguous.
Go to :

RECOMMENDED PANELS FOR SMALL/LOW GRADE B-CELL LYMPHOMAS
|
Essential |
CD3, CD20, Ki-67, Bcl-2, CD10 |
|
Optional |
CD5, Cyclin D-1, SOX11, CD23, CD21, CD138, Kappa, Lambda, CD79a, IgD, IgG, IgM |
There is no proper term to describe a group of mature B-cell lymphomas and cannot be classified under DLBCL or its variants. This is attributed to the fact that all small cell lymphomas cannot be considered low-grade lymphomas, and “small B-cell lymphoma” can be confused with “small lymphocytic lymphoma”. For convenience, I have used the term “small/low-grade B-cell lymphomas in this review”. The four most basic differential diagnoses of small/low-grade B-cell lymphoma are follicular lymphoma, chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), mantle cell lymphoma (MCL), and marginal zone lymphoma (MZL).
Follicular lymphoma has a pathognomonic feature with the co-expression of CD10 and Bcl-2 (
Fig. 2). The CD10 expression implies that the tumor cells originate from centrocytes or centroblasts, similar to GCB-DLBCL. Bcl-2 should be negative in normal centroblasts or centrocytes, but BCL2 translocation causes aberrant expression of Bcl-2. A few small/low-grade B-cell lymphomas exist wherein both CD10 and Bcl-2 are expressed; these do not include follicular lymphoma. One of the most difficult aspects of follicular lymphoma diagnosis is that a significant number of these lymphomas have follicular architecture without CD10 expression. In particular, the higher the grade of follicular lymphoma, the higher is the rate of CD10 negativity [
9,
10]. This leads to difficulties in pathological diagnosis. Although there are alternative germinal center markers such as LMO2, GCET1, and HGAL (also called GCET2), these stains are not recommended [
11]. The Ki-67 labeling index in follicular lymphoma is generally known to be <20% in low-grade (grades 1 and 2) and >30% in high-grade (grades 3A and 3B); however, the Ki-67 labeling index alone is not used for grading.
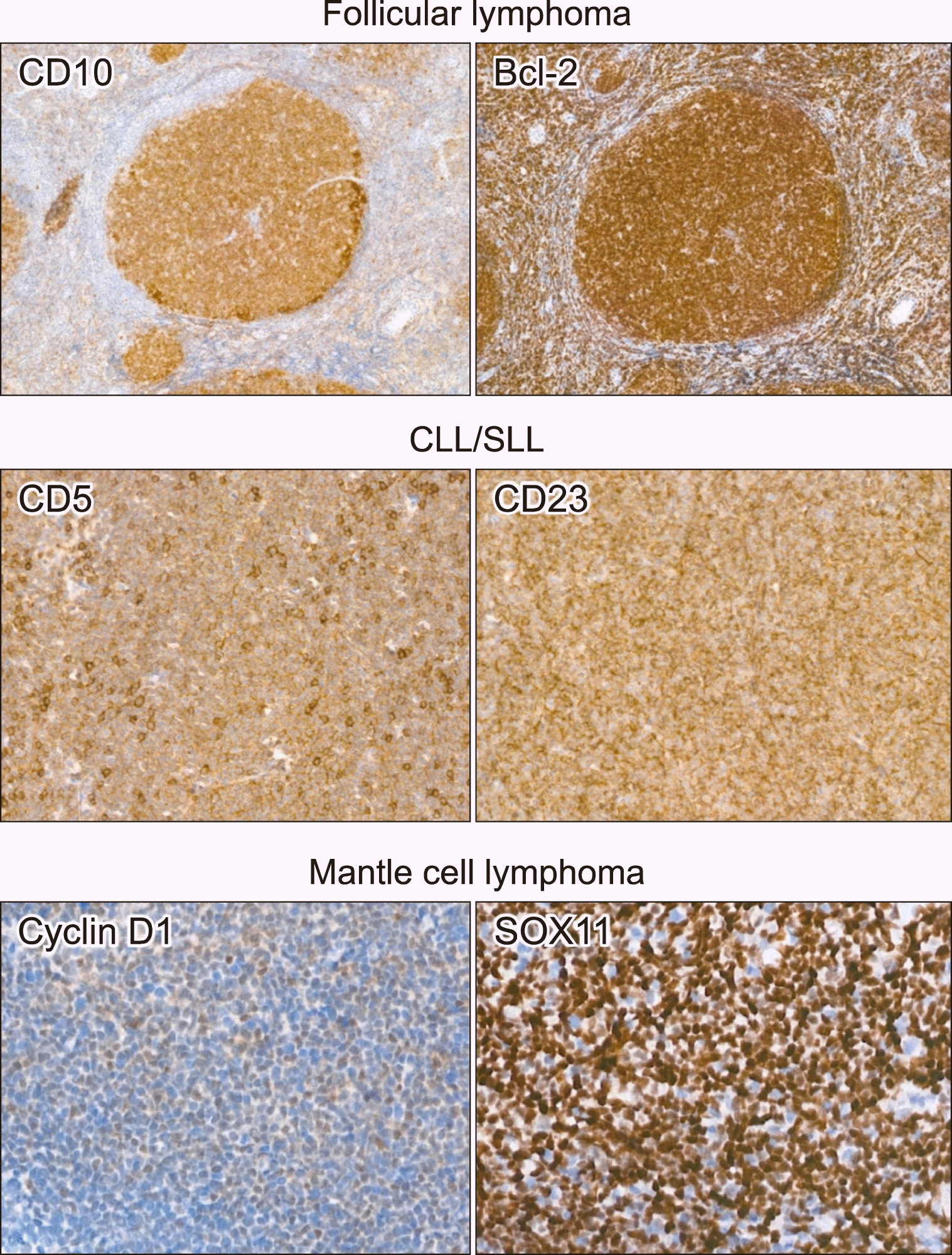 | Fig. 2
Representative images of immunohistochemical staining of small/low grade B-cell lymphomas. Tumor cells are positive for CD10 and Bcl-2 in follicular lymphoma, positive for CD5 and CD23 in chronic lymphocytic leukemia/small lymphocytic lymphoma, positive for cyclin D-1 and SOX11 in mantle cell lymphoma.
Abbreviation: CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma.

|
If small/low-grade B-cell lymphoma tests positive for CD10-negative and Bcl-2-positive, the following lymphomas should be differentially diagnosed.
Although CLL/SLL is not as common in Asia as in the West, it must be included in the differential diagnosis of small/low-grade B-cell lymphomas. The most characteristic findings of IHC are that these tumor cells are CD3-negative and CD20-positive and that CD5 and CD23 are co-expressed in tumor cells (
Fig. 2). CD5 is originally a T-cell marker, and since reactive T cells are mixed in tumor areas of virtually all B-cell lymphomas, it is important to determine if CD5 is stained in a diffuse pattern for CLL/SLL diagnosis. Although CD23 expression is highly sensitive in CLL/SLL, it can be expressed in other small/low-grade B-cell lymphomas. Therefore, it is risky to diagnose CLL/SLL solely based on CD23 expression.
The most important markers for MCL diagnosis are CD5 and cyclin D-1. However, unlike CD5, which is also expressed in CLL/SLL, cyclin D-1 becomes positive due to CCND1 translocation. Therefore, cyclin D-1 is a highly specific marker for MCL. Occasionally, there are cyclin D-1-negative MCLs, and in such cases, SOX11 staining can aid in the diagnosis. Monoclonal SOX11 shows sensitivity and specificity comparable to that of cyclin D-1 in MCL [
12]; therefore, SOX11 is often used as a primary marker to differentiate MCL (
Fig. 2). Ki-67 labeling index can vary in MCL. Unlike other B-cell lymphomas that are classified as DLBCL after large cell transformation, MCL is differentially diagnosed even after high-grade transformation. Blastoid and pleomorphic variants are high-grade variants of MCL, wherein the Ki-67 labeling index is as high as that in DLBCL.
The absence of specific IHC markers or genetic alterations in the tumor cells of nodal or extranodal MZLs poses a serious problem in their diagnosis. Therefore, it is often diagnosed when the other lymphomas are excluded, that is, when the tumor is a small B-cell lymphoma, and the tumor cells are all negative for CD10, CD5, CD23, cyclin D-1, and SOX11 markers. Some researchers oppose the use of MZL as a “waste basket diagnosis” as described above [
13], however, there is no other defined category that can contain these cases other than the said MZL category as per the current WHO classification. MZL is of post-GCB origin. Hence, it often demonstrates varying degrees of plasma cell differentiation. In these cases, confirming the clonality of plasma cells through kappa and lambda light-chain staining would be helpful in the diagnosis of MZL. The normal range of the kappa to lambda ratio is 2.8–0.7:1; therefore, monoclonality can be confirmed if kappa-positive cells are more than four times that of lambda-positive cells or if lambda-positive cells are more than twice that of kappa-positive cells. However, this plasmacytic differentiation of MZL obscures the differentiation between MZL and lymphoplasmacytic lymphoma (LPL). Although immunoglobulin (IgG, IgA, IgM) staining can help differentiate between MZL and LPL, it is difficult to obtain a reliable diagnosis using IHC staining.
Go to :

RECOMMENDED PANELS FOR T/NK-CELL LYMPHOMAS
|
Essential |
CD3, CD20, Ki-67, EBV ISH |
|
Optional |
CD4, CD8, CD56, CD30, ALK, TIA-1, granzyme B, PD-1, CXCL13, CD10, Bcl-6, ICOS, CD2, CD5, CD7, CD21, β-F1, TCR-delta |
Although the current WHO classification lists many different types of T/NK-cell lymphoma [
1], the first step in the differential diagnosis is its classification into peripheral T-cell lymphoma, not otherwise specified (PTCL NOS), angioimmunoblastic T-cell lymphoma (AITL), anaplastic large cell lymphoma (ALCL), and extranodal NK/T-cell lymphoma, nasal type (ENKTL).
Although ALCL is a T-cell lymphoma, the CD3 pan-T-cell marker is usually negative. In contrast, CD30 shows strong, diffuse positivity. Thus, based on the ALK staining, positive and negative results are classified as ALK-positive and ALK-negative ALCL, respectively (
Fig. 3). Since CD3 is not expressed in ALK-negative ALCL, additional evidence for the T-cell origin of the tumor is required for ALCL diagnosis. For this purpose, additional T-cell markers such as CD2, CD5, and CD7, and cytotoxic granule markers such as TIA-1 and granzyme B are utilized; the expression of either of these markers in tumor cells would suggest a T-cell origin. CD30 can be expressed at various levels and proportions in different lymphocytes. Since CD30 is also expressed in some PTCL NOS, it is often difficult to differentiate between CD30-positive PTCL and ALK-negative ALCL. In such cases, the most important feature for differentiating ALCL from PTCL is that CD30 is diffusely and strongly expressed in ALCL [
14].
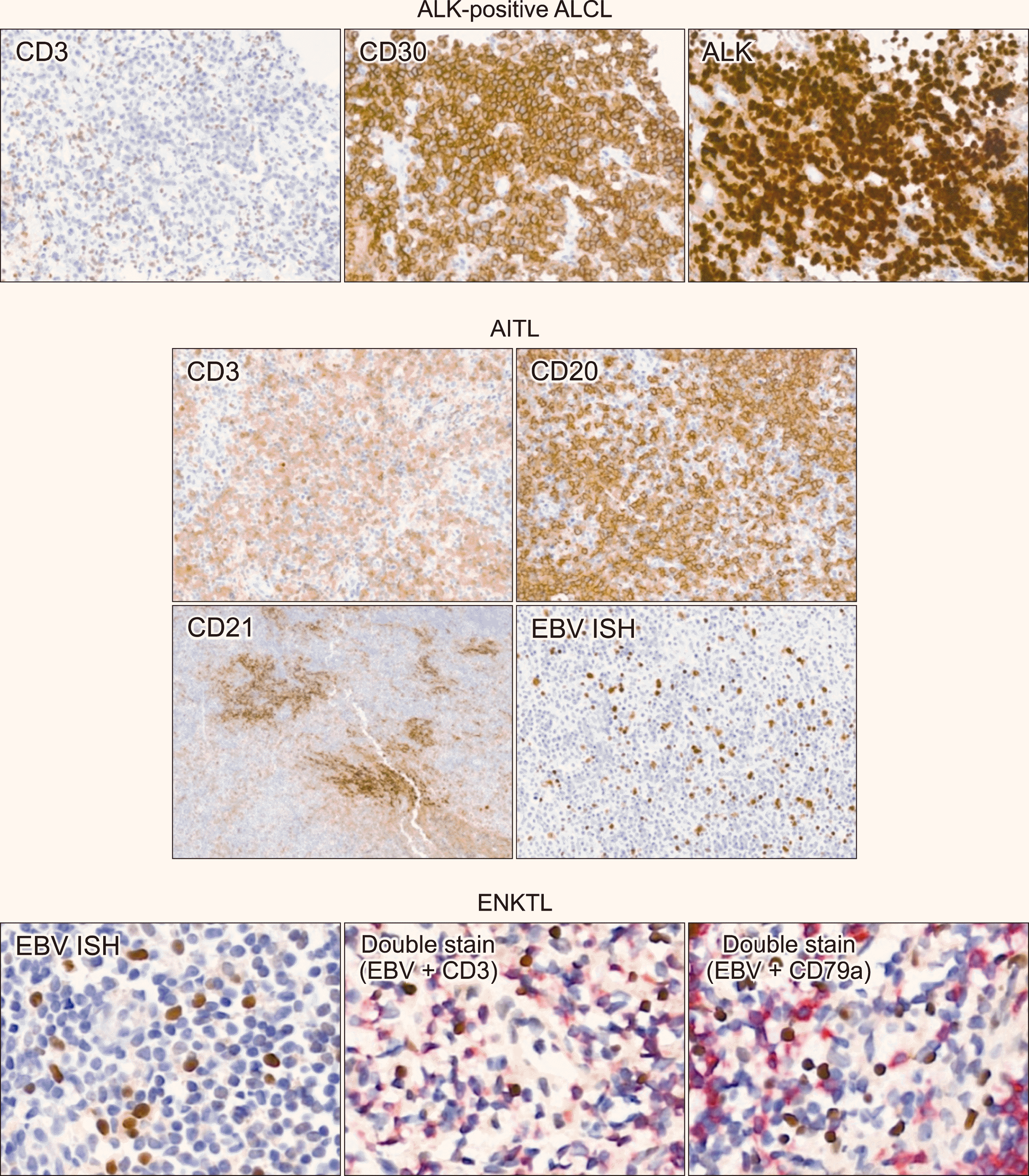 | Fig. 3
Representative images of immunohistochemical staining of T/NK-cell lymphomas. In ALK-positive anaplastic large cell lymphoma, tumor cells are negative for CD3, and positive for CD30 and ALK. In angioimmunoblastic T-cell lymphoma, CD3 and CD20 shows mixed pattern of T- and B-cells. CD21 stain shows expanded follicular dendritic cell meshwork. EBV ISH is positive in scattered large B-cells. In extranodal NK/T-cell lymphoma, EBV positive cells are positive for CD3 and negative for CD79a in double stains.
Abbreviations: AITL, angioimmuno-blastic T-cell lymphoma; ALCL, ana-plastic large cell lymphoma; ENKTL, extranodal NK/T-cell lymphoma.

|
The AITL diagnosis solely based on the negative or positive immunostaining for markers in the sample is often difficult. Furthermore, since the tumor cells do not form a diffuse sheet pattern, it is sometimes difficult to recognize them as T-cell lymphoma with only CD3 and CD20 staining patterns. The AITL tumor cells are CD4 positive and CD8 negative because they are T follicular helper (TFH) cell lymphomas. However, it is difficult to determine what is stained in the tumor cells through the CD4 and CD8 stains. TFH cell markers such as PD-1, CXCL13, CD10, Bcl-6, and ICOS might aid better in the diagnosis. However, the major drawback is that each of the above-mentioned markers shows low sensitivity and specificity. We believe that CD21 is the most important IHC marker for the AITL diagnosis. CD21 is a follicular dendritic cell (FDC) marker. The FDCs support the lymphoid follicle structure, and the characteristic expansion pattern of this FDC meshwork is highly specific for AITL (
Fig. 3). AITL often induces monoclonal B-cell proliferation. Therefore, EBV-positive (or EBV-negative) large B-cell proliferation might be misdiagnosed as (EBV-positive) DLBCL (
Fig. 3). Therefore, AITL must be diagnosed by comprehensively reviewing various IHC results along with various clinical and pathological factors.
A reliable ENKTL diagnosis can be achieved if EBV ISH is included in all T/NK-cell lymphoma panels. In ENKTL, tumor cells are positive for EBV ISH in almost all cases. EBV can only infect B cells and epithelial cells but cannot enter T/NK cells [
15,
16]. Thus, if T cells or NK cells are positive for EBV ISH, it can be considered a T/NK-cell lymphoproliferative disorder. Most ENKTL tumor cells are positive (sometimes weak) for CD3 staining. Depending on the cell lineage, CD56 may or may not be expressed. When the density of tumor cells is extremely low, it is difficult to determine whether the EBV-positive cells are B cells (bystander cells) or T cells (tumor cells). In such cases, the lineage of EBV-positive cells must be identified through double staining, pairing EBV ISH–CD3 and EBV ISH–CD20 (or CD79a) (
Fig. 3).
PTCL NOS comprises the remainder of the PTCL, except for the specific types mentioned above. The tumor cells are CD3-positive, and this staining highlights cytologic atypia of lymphocytes better than H&E staining. Therefore, it is necessary to check whether cytologic atypia is present when observing the CD3 stained slides. For this, quality control checks on immunostaining techniques must be performed at the institutes. Tumor cells express either CD4 or CD8 but occasionally test negative for both markers. It is unlikely that the same cell is both CD4- and CD8-positive; however, both CD4-positive and CD8-positive cells appear diffusely in the low-power view. Therefore, one must exercise caution while determining which marker has stained the tumor cells. CD56 expression varies depending on the type of PTCL sampled. Determining αβ T cells or γδ T cells through β-F1 and T-cell receptor delta (TCR delta) staining can also be helpful in the differential diagnosis. Recently, a treatment targeting CD30 in tumor cells has been introduced [
17,
18], and CD30 staining is often performed in various T-cell lymphomas.
Go to :

RECOMMENDED PANELS FOR PRECURSOR LYMPHOMAS
|
Essential |
CD3, CD20, CD79a, Ki-67, TdT, CD99 |
|
Optional |
CD10, CD19, PAX5, CD5 |
T-lymphoblastic leukemia/lymphoma (T-LBL) and B-lymphoblastic leukemia/lymphoma (B-LBL) are morphologically indistinguishable on H&E-stained slides. If a precursor lymphoid neoplasm is confirmed through terminal deoxynucleotidyl transferase (TdT) and CD99 staining, it is further necessary to determine whether this tumor corresponds to the T- or B-cell lineage. T-LBL usually expresses CD3, but B-LBL often does not express CD20. Therefore, if both CD3 and CD20 are negative, the possibility of B-cell lineage should be confirmed through additional CD79a, PAX5, or CD19 staining (
Fig. 4). In fact, CD3, CD20, and Ki-67 staining are performed before TdT and CD99 in most routine practices. Based on the clinical examinations suspecting LBL and blastoid morphology of the tumor cells, a pathologist must perform TdT and CD99 staining in the appropriate situations.
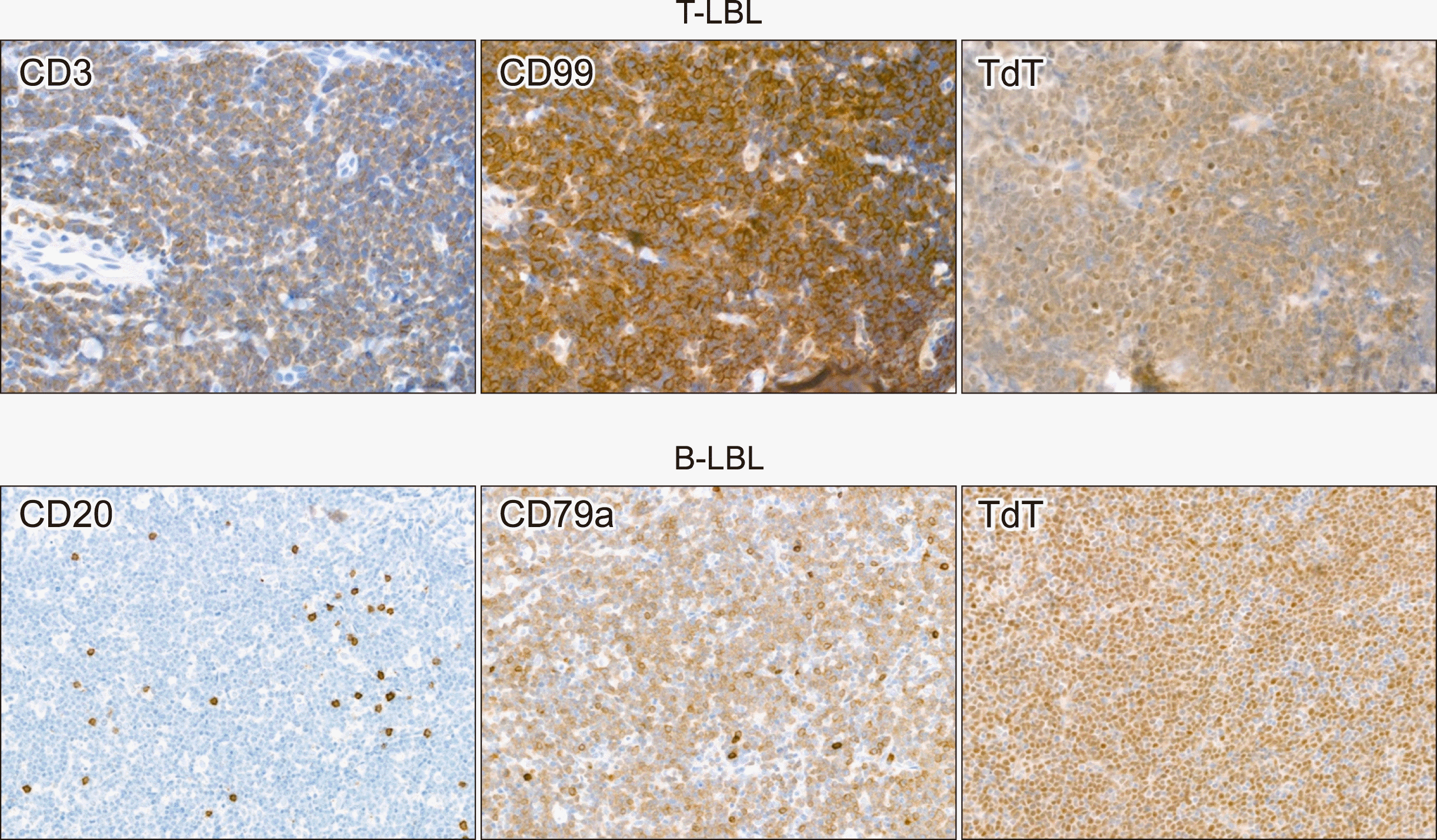 | Fig. 4Representative images of immunohistochemical staining of precursor lymphomas. In T-lympho-blastic leukemia/lymphoma, tumor cells are positive for CD3, CD99 and TdT. In B-lymphoblastic leukemia/ lymphoma, tumor cells are negative for CD20, positive for CD79a and TdT. 
|
Go to :

RECOMMENDED PANELS FOR HODGKIN LYMPHOMAS
|
Essential |
CD3, CD20, Ki-67, CD30, CD15, PAX5, EBV ISH |
|
Optional |
Oct-2, BOB1, EMA |
Hodgkin lymphoma is broadly classified into classic Hodgkin lymphoma (cHL) and nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL). The diagnosis algorithm recommends an initial IHC panel test for cHL diagnosis and additional staining to diagnose NLPHL if the possibility of cHL has been excluded.
The cHL tumor cells have a characteristic morphology (Hodgkin-Reed-Sternberg cells or HRS cells). In IHC, tumor cells strongly express CD30 (membranous and Golgi patterns). Further, CD20 is negative, and PAX5 is weakly positive for these cells (
Fig. 5). CD30 staining has an extremely high sensitivity, so it could be said that CD30 is positive in almost all cHL tumor cells. However, sometimes the intensity of CD30 is not strong; therefore, caution is needed when interpreting these cases. Although CD15 is less sensitive than CD30, cHL can be diagnosed when CD30 and CD15 are expressed in the same tumor sample. The accuracy of CD15 expression in HRS cells must be comprehensively examined since CD15 is often positive for small granulocytes in tumors. EBV ISH positivity varies depending on the cHL variant, and these results do not affect the diagnosis.
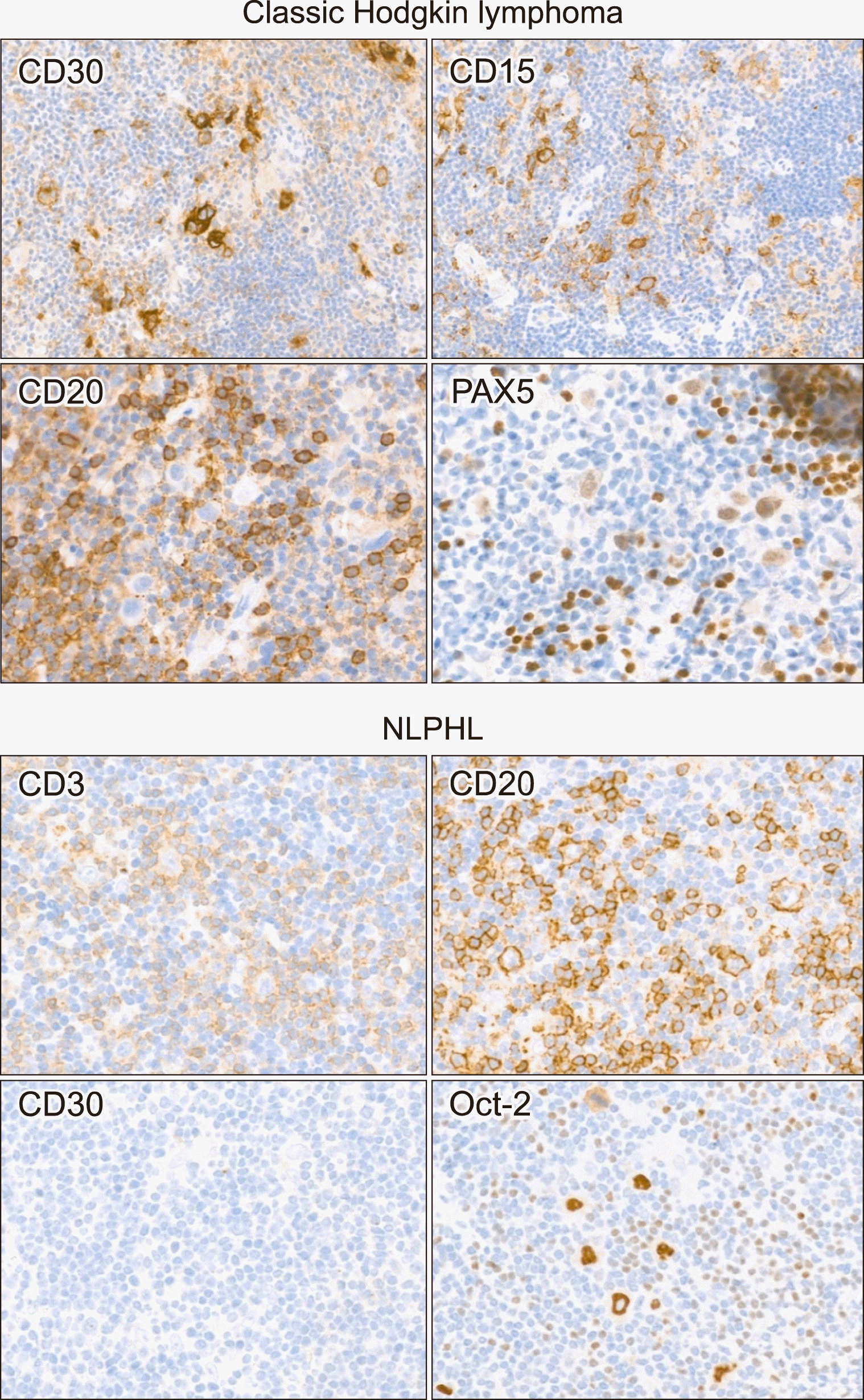 | Fig. 5
Representative immunohistochemical staining images of Hodgkin lymphomas. In classic Hodgkin lymphoma, tumor cells are positive for CD30 and CD15. CD20 is negative and PAX5 is weak positive in large tumor cells. In nodular lymphocyte predominant Hodgkin lymphoma, tumor cells are positive for CD20 and Oct-2. CD30 is negative in tumor cells. CD3 stain shows peritumoral rosette-like T-cells.
Abbreviation: NLPHL, nodular lymphocyte predominant Hodgkin lymphoma.

|
In NLPHL, despite the tumor cell morphology being similar to that of cHL, the tumor cells are CD20-positive and CD30-negative. Differentiation from T-cell/histiocyte-rich large B-cell lymphoma (THRLBL) may be challenging because CD20-positive, scattered, large, and atypical cells are observed in both the lymphomas. Checking the nodular pattern of background B-cells in CD20 staining can help with differentiation (favoring NLPHL). However, various low-power patterns in NLPHL render them difficult to differentiate. NLPHL tumor cells are strongly positive for Oct-2, moderately positive for BOB1, and sometimes express EMA. However, since this pattern appears similarly in THRLBL, it cannot aid in the differential diagnosis of the two diseases (
Fig. 5).
Go to :

CONCLUSION
IHC staining is essential for the diagnosis of various lymphomas. It is crucial that the pathologists select appropriate markers according to the clinical situation of the patient, the H&E staining results, and accurately interpret the IHC results for an optimal lymphoma diagnosis. Therefore, clinicians must collect sufficient samples and properly fix them to obtain accurate IHC results that would aid in a final, reliable pathological diagnosis.
Go to :








 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download