Abstract
Immune-mediated thrombotic thrombocytopenic purpura (iTTP) is a potentially life-threatening thrombotic microangiopathy caused by autoantibody-mediated severe ADAMTS13 deficiency. TTP should be suspected in patients with microangiopathic hemolytic anemia and thrombocytopenia without a definite cause. Early detection of iTTP and prompt treatment with plasma exchange and corticosteroids are essential. Rituximab administration should be considered for refractory or relapsed iTTP, and can be used as a first-line adjuvant or preemptive therapy. Treatment with caplacizumab, a novel anti-von Willebrand factor nanobody, resulted in a faster time to platelet count response, significant reduction in iTTP-related deaths, and reduced incidence of refractory iTTP. TTP survivors showed a higher rate of chronic morbidities, including cardiovascular disease and neurocognitive impairment, which can lead to a poor quality of life and higher mortality rate. Meticulous long-term follow-up of TTP survivors is crucial.
Thrombotic microangiopathy (TMA) is a potentially fatal syndrome characterized by microangiopathic hemolytic anemia (MAHA), consumptive thrombocytopenia, and variable ischemic end-organ injury. TMA is a heterogeneous group of diseases, including thrombotic thrombocytopenic purpura (TTP), atypical hemolytic uremic syndrome, and secondary TMA caused by abnormal coagulation, infection, malignancy, transplantation, drug, autoimmune disease, or pregnancy (Table 1) [1, 2].
TTP is defined as a severe deficiency (activity <10%) of ADAMTS13 (a disintegrin and metalloproteinase with thrombospondin type 1 motif, member 13). ADAMTS13 is a plasma metalloprotease that cleaves von Willebrand factor (vWF), which mediates platelet adhesion to damaged vascular endothelium and platelet aggregation. ADAMTS13 deficiency leads to the accumulation of unusually large vWF multimers in the bloodstream and subsequent exacerbation of platelet adhesion, aggregation, and formation of microthrombi in small arterioles and capillaries [3-7]. TTP is divided into congenital (inherited) and immune-mediated (acquired) TTP. Congenital TTP (also called Upshaw–Schulman syndrome) is caused by biallelic mutations in the ADAMTS13 gene [8]. Immune-mediated TTP (iTTP) is an autoimmune disorder resulting from the production of autoantibodies against ADAMTS13 [1-3]. TTP is a rare disease with an annual incidence of approximately 2–6 cases/million/year [9, 10]. TTP typically presents in adulthood, with a median age at onset around the fourth decade of life. iTTP is 2 to 3 times more frequent in women [3]. Congenital TTP accounts for approximately 3–5% of all cases of TTP [9].
A TTP-like syndrome can be observed in patients with critical conditions, which cause vascular endothelial injury, such as sepsis, trauma, and cancer. Damage to the vascular endothelium leads to the enhanced release of unusually large vWF. Excessive unusually large vWF become anchored to endothelial cells of targeted organs and recruit activated platelets to assemble unusually large vWF-platelet complexes. Both TTP-like syndrome and TTP produce similar pathological conditions of microvascular thrombosis. However, the former involves normal hemostasis in endothelial injury and the latter involves pathologic hemostasis without endothelial injury [11].
Recently, remarkable progress has been made in understanding the pathophysiology of iTTP. This has been successfully translated to patient management. This study aimed to review recent advances in the diagnosis, treatment, and long-term follow-up of patients with iTTP.
ADAMTS13 activity <10% confirms the diagnosis of TTP. ADAMTS13 activity should be tested in all patients with MAHA and thrombocytopenia without a definite cause. A blood sample for the ADAMTS13 assay must be collected before the initiation of treatment to avoid the false-negative result. ADAMTS13 assay is not immediately available in most centers in Korea, and several days are required to obtain the results. TTP is a medical emergency, and a delay in appropriate therapy is associated with substantial morbidity and mortality. Immediate initiation of treatment may be lifesaving before the ADAMTS13 activity level is known. Patients with TTP uniformly present with severe thrombocytopenia (<30×109/L) and MAHA with evidence of schistocytes on a peripheral blood smear. Parameters of hemolysis are also present, including elevated reticulocyte count, elevated lactate dehydrogenase (LDH) level, elevated unconjugated bilirubin level, and undetectable haptoglobin concentration. Coombs’ test is usually negative, and coagulation parameters are usually normal [3].
Early recognition of TTP is difficult because of its diverse clinical manifestations. Approximately 60% of patients with TTP have neurological symptoms that range from headache to stroke, seizures, or coma [9, 12-16]. Mesenchymal ischemia is present in 35% of patients [9]. Approximately 25% of patients have cardiac symptoms, ranging from electrocardiographic abnormalities to myocardial infarction [17]. Although acute renal injury is common in TTP, severe renal failure is rare in iTTP [18]. The Korean TTP registry reported symptoms, such as fever (56%), bleeding (45%), and severe neurological symptoms (20%), upon admission [16].
Clinical prediction of TTP is important to make initial management decisions before ascertaining the ADAMTS13 activity level. The French and PLASMIC scores have been developed and validated to estimate the probability of severe ADAMTS13 deficiency (Table 2) [19, 20]. These prediction scores incorporate basic clinical and laboratory parameters. Both scores showed that severe thrombocytopenia and mild renal impairment were strongly associated with a severe ADAMTS13 deficiency. The PLASMIC score includes not only platelet count and creatinine levels, but also the evidence of hemolysis, presence of active cancer, history of transplantation, mean corpuscular volume, and international normalized ratio. When the PLASMIC score was applied to the external validation cohort, 82% of patients with a PLASMIC score of 6 or 7 had severe ADAMTS13 deficiency compared with 4% of patients with a score of 0–4 [20]. A PLASMIC score ≥5 suggests the requirement of immediate treatment, whereas a low probability score requires an alternative diagnosis. However, it is important to recognize that clinical prediction scores are accurate only when applied to a population with TMA without an obvious cause, and these models cannot definitively confirm TTP.
Detection of anti-ADAMTS13 antibodies confirms the diagnosis of iTTP. However, a negative anti-ADAMTS13 antibody assay does not exclude iTTP because antibodies become detectable during the follow-up period or relapse. In cases that have persistent severe ADAMTS13 deficiency with no detectable anti-ADAMTS13 antibodies during remission, ADAMTS13 gene sequencing should be performed to rule out congenital TTP [3].
Before the use of plasma exchange (PEX), the survival rate of patients with iTTP was 10–20%. Therapeutic PEX has improved survival rates from <20% to >80%. The Canadian Apheresis Group conducted a prospective randomized study, comparing PEX with plasma infusion for acute TTP treatment. They reported that patients receiving PEX had a higher response rate, lower early mortality rate (3.9%), and improved survival at 6 months (78%) [21]. The effectiveness of PEX is related to the supply of ADAMTS13 with removal of unusually large vWF multimers and anti-ADAMTS13 autoantibodies. Given the high risk of early death in TTP, PEX treatment should be initiated at the earliest early. The optimal PEX regimen has not yet been determined. However, according to the Canadian apheresis trial group and guidelines from the British Committee for Standards in Haematology, PEX is usually started by a 1.5-plasma volume exchange for the first 3 days, followed by a 1.0 plasma volume exchange [12, 22]. Daily PEX should be continued for a minimum duration of 2 days after platelet count normalization. More intensive exchanges, such as twice-daily PEX, should be considered in resistant disease.
Corticosteroids suppress anti-ADAMTS-13 autoantibodies [23]. In addition to PEX, corticosteroids should be administered to all patients with an acute episode of iTTP. Although the benefits of corticosteroids were not well determined in a randomized clinical trial, the use of corticosteroids may be supported by the autoimmune nature of the disease. Corticosteroids have been administered as either intravenous pulse methylprednisolone (1 g/day for 3 days, followed by 1 mg/kg/day prednisone) or oral prednisolone (1 mg/kg/day) (Fig. 1) [22].
The criteria for clinical response, exacerbation, remission, and relapse of TTP have been defined by the International Working Group (IWG) for TTP in 2017 [2]. ‘Clinical response’ was defined as a sustained platelet count increment (platelet count >150×109/L) and LDH <1.5 times the upper limit of normal (ULN). ‘Refractory iTTP’ is defined as a lack of platelet count increment (platelet count <50×109/L) with persistently high LDH levels (>1.5×ULN) despite undergoing PEX five times or a decrease in platelet count after an initial improvement while receiving PEX. ‘Exacerbation’ refers to early recurrence of iTTP within 30 days of discontinuing PEX. ‘Clinical remission’ of iTTP is defined as a sustained clinical response for more than 30 days after discontinuation of PEX. ‘Relapse’ is defined as a decrease in platelet count to below the lower limit with or without clinical symptoms, after clinical remission [2]. Relapses occur in 30–50% of patients with iTTP [3]. Despite advances in iTTP management, refractory disease and recurrence remain critical issues.
Rituximab, a monoclonal antibody targeting CD20 on B cells, has been used to treat iTTP by suppression of ADAMTS13 autoantibody production. Several groups have conducted studies to demonstrate the efficacy of rituximab for refractory or recurrent iTTP [24-28]. A UK group reported that clinical remission was achieved in all 25 patients who received rituximab, and relapses were not observed during a median follow-up of 19 months [24]. In a prospective study involving 40 patients with refractory (N=20) or relapsed iTTP (N=20), rituximab treatment resulted in remission in 63.6% of refractory patients and 90% of relapsed patients at 8 weeks [26]. The Korean Society of Hematology Thrombosis Working Party conducted a nationwide survey to validate whether rituximab improved outcomes in Korean patients with iTTP. Rituximab successfully induced clinical remission in 10 of 12 patients (83%) who were refractory to PEX. During a median follow-up of 79 weeks, there was no relapse in 10 responders [28]. Although rituximab therapy for iTTP has not yet been approved by the Korean Ministry of Food and Drug Safety (MFDS), rituximab should be considered for refractory or relapsed patients with iTTP for “off-label use” after obtaining approval from each Institutional Review Board and the MFDS.
Front-line rituximab in conjunction with standard treatment for acute iTTP significantly reduced the relapse rate (10% compared with 57% in historical controls) [29]. Earlier upfront administration of rituximab within 3 days may reduce the time to remission, number of PEX days, and days of hospitalization [30]. Rituximab can be used as a preemptive therapy to prevent relapse after the detection of ADAMTS13 deficiency during follow-up [31, 32]. The development of severe ADAMTS13 deficiency during remission is significantly associated with a high risk of relapse [32-35]. A French group reported the efficacy of preemptive rituximab for iTTP patients with lower ADAMTS13 activity (<10%) during remission. There was a significant reduction in the relapse rate in the preemptive treatment cohort, compared with that in historical controls [32].
Measurements of ADAMTS-13 at regular intervals during treatment and remission (e.g., weekly during treatment, monthly, and then every 3 months during the follow-up period, extending to every 6–12 months) may provide data about the risk of relapse and persistence of subclinical disease activity [2].
Other immunomodulators, such as vincristine, cyclosporine, and cyclophosphamide, have been used as second-line therapies for patients with refractory iTTP, pre-rituximab era [36]. The clinical response rate was 50–87% in patients who received vincristine. Additionally, bortezomib, a proteasome inhibitor, was found to be effective in the treatment of refractory or relapsed iTTP by depleting plasma cells [36, 37]. Among 12 patients with iTTP who received bortezomib as a salvage treatment, 11 survived and maintained remission [37].
Caplacizumab, an anti–vWF humanized single-variable domain immunoglobulin (nanobody), targets the A1 domain of vWF, inhibits vWF-mediated platelet aggregation, and subsequently prevents microvascular thrombosis [38]. The efficacy and safety of caplacizumab in patients with iTTP have been evaluated in the phase 2 TITAN and phase 3 HERCULES trials [39, 40]. The median time to normalization of platelet count was shorter with caplacizumab than with a placebo (2.69 days vs. 2.88 days, P=0.01) in the HERCULES trial. The caplacizumab group required less PEX and had a shorter hospital stay than the placebo group [40]. The percentage of patients with a composite outcome of TTP-related death, recurrence of TTP, or a major thromboembolic event was lower with caplacizumab than with a placebo (12% vs. 49%, P<0.001) [40]. In the integrated analysis of data from both trials, a significant reduction in the number of deaths (0 vs. 4; P<0.05) and a significantly lower incidence of refractory TTP (0 vs. 8; P<0.05) were observed in patients who received caplacizumab [41]. The most common adverse event associated with caplacizumab was mucocutaneous bleeding. However, these events were mild or moderate in severity, and resolved spontaneously in most patients [39-41].
Caplacizumab (10 mg intravenous loading bolus, followed by 10 mg daily subcutaneously) was administered for 30 days after the last daily PEX in the phase 2 TITAN trial. The relapse rate after the discontinuation of caplacizumab was 22% in this trial. A subgroup of patients with persistent severe ADAMTS13 deficiency (<10%) after discontinuation of caplacizumab had a higher risk of relapse [39]. Based on these results, the HERCULES trial was designed to monitor ADAMTS13 weekly and use caplacizumab for patients with ADAMTS13 deficiency (<10%) for up to 4 weeks at the end of the treatment period. The relapse rate after discontinuation of caplacizumab was 8% in the HERCULES trial. In patients who receive caplacizumab, monitoring of ADAMTS13 activity may be used to tailor the duration of therapy. Although the threshold for discontinuation of therapy has not been well defined, ADAMTS13 >20% for at least 2 consecutive weeks may be a reasonable point [42].
Recently, the IWG proposed revised consensus outcome definitions that incorporate ADAMTS13 activity and the effects of anti-VWF therapy [43]. In this proposal, clinical remission is defined as a sustained clinical response with either no PEX and no anti-vWF therapy for ≥30 days or with the attainment of ADAMTS13 remission (partial or complete). Partial ADAMTS13 remission is defined as ADAMTS13 activity ≥20% to less than the lower limit of normal (LLN). Complete ADAMTS13 remission refers to ADAMTS13 activity ≥LLN. ADAMTS13 relapse is termed when ADAMTS13 levels decrease to <20% after ADAMTS13 remission [43].
Recombinant human ADAMTS13 (rhADAMTS13) has been shown to normalize the cleavage of vWF from congenital TTP plasma [44]. The safety and pharmacokinetics of rhADAMTS13 have been investigated in patients with severe congenital TTP. In a phase I trial, rhADAMTS13 was safe, non-immunogenic, and well tolerated. The pharmacokinetic profile was comparable to that of plasma infusion [45]. This drug has been granted a fast-track designation by the US Food and Drug Administration for the treatment of congenital TTP. In vitro addition of rhADAMTS13 to plasma can override the inhibitor and cleave vWF in iTTP [44]. A clinical trial (NCT03922308) is ongoing to evaluate the efficacy of rhADAMTS13 in iTTP.
Anfibatide, a snake C-type lectin derived from the venom of Agkistrodon acutus, binds to the platelet receptor GPIb and prevents its adhesion to vWF. Anfibatide inhibited the interaction between vWF multimers and platelets without a significant increase in bleeding tendency in healthy controls [46]. A randomized phase II study (NCT04021173) is ongoing to evaluate the safety and efficacy of anfibatide for iTTP.
With prompt and effective treatment, most patients with iTTP survive an acute episode. Although the major concern following recovery from an acute episode of iTTP is the risk of relapse, a higher rate of chronic morbidities, poor quality of life, and a higher mortality rate are problems in TTP survivors [47-51]. Patients enrolled in the Oklahoma TTP-HUS Registry had an increased prevalence of hypertension, cognitive impairment, and major depression compared with the reference populations. These patients also had a higher risk of premature death [47]. Several groups have also shown TTP survivors have a higher incidence of other health complications, including major adverse cardiovascular events, neurocognitive deficits, and autoimmune disorders than the general population [48-51]. These chronic morbidities may have a significant impact on quality of life. Moreover, TTP survivors without significant disabilities or additional health problems consistently scored lower across all domains of health-related quality of life surveys than the general population [48, 49]. Cardiovascular disease is the leading cause of death among iTTP survivors. Upreti et al. [50] demonstrated that patients with iTTP had a five-fold higher stroke prevalence than age- and sex-matched controls. Another study reported that the prevalence of major adverse cardiovascular events during iTTP remission was 28.6% at a median follow-up of 7.6 years, with the first major adverse cardiovascular events occurring 1–2 decades earlier than in the general population [51]. Aggressive screening and management of cardiovascular risk factors may reduce the incidence of cardiovascular events in iTTP survivors. Meticulous long-term follow-up of iTTP survivors is crucial in identifying the development of additional health problems.
PEX and corticosteroids, the mainstay of treatment for acute episodes of iTTP in Korea, have dramatically improved the survival of patients with iTTP. However, mortality rates have been reported to be 10–20%. A subset of patients remains refractory to PEX, and relapses after discontinuation of PEX. Prompt initiation of treatment is crucial for reducing early death. Targeted therapies based on iTTP pathogenesis will help overcome these unmet needs. Rituximab has shown to be effective for the treatment of refractory or relapsed iTTP, and may be useful as a first-line or pre-emptive therapy. Caplacizumab shortened the time to normalization of platelet count and reduced refractoriness, recurrence, and death during the acute phase of iTTP. ADAMTS13 activity is an important predictor of recurrence and can guide the duration of anti-vWF therapy. iTTP survivors are at risk of several long-term complications, including cardiovascular disease, depression, and neurocognitive deficits, which can lead to poor quality of life and shorter life expectancy. We need to investigate the mechanisms, risk factors, and management of these complications to improve the long-term outcomes of iTTP.
REFERENCES
1. George JN, Nester CM. 2014; Syndromes of thrombotic microangiopathy. N Engl J Med. 371:654–66. DOI: 10.1056/NEJMra1312353. PMID: 25119611. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84906077328&origin=inward.

2. Scully M, Cataland S, Coppo P, et al. 2017; Consensus on the standardization of terminology in thrombotic thrombocytopenic purpura and related thrombotic microangiopathies. J Thromb Haemost. 15:312–22. DOI: 10.1111/jth.13571. PMID: 27868334. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85011711363&origin=inward.

3. Joly BS, Coppo P, Veyradier A. 2017; Thrombotic thrombocytopenic purpura. Blood. 129:2836–46. DOI: 10.1182/blood-2016-10-709857. PMID: 28416507. PMCID: PMC9038040. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85019687342&origin=inward.

4. Moake JL, Rudy CK, Troll JH, et al. 1982; Unusually large plasma factor VIII: von Willebrand factor multimers in chronic relapsing thrombotic thrombocytopenic purpura. N Engl J Med. 307:1432–5. DOI: 10.1056/NEJM198212023072306. PMID: 6813740. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0020428664&origin=inward.

5. Furlan M, Robles R, Lämmle B. 1996; Partial purification and characterization of a protease from human plasma cleaving von Willebrand factor to fragments produced by in vivo proteolysis. Blood. 87:4223–34. DOI: 10.1182/blood.V87.10.4223.bloodjournal87104223. PMID: 8639781. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0029925856&origin=inward.

6. Tsai HM. 1996; Physiologic cleavage of von Willebrand factor by a plasma protease is dependent on its conformation and requires calcium ion. Blood. 87:4235–44. DOI: 10.1182/blood.V87.10.4235.bloodjournal87104235. PMID: 8639782. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0029878123&origin=inward.

7. Zheng XL, Chung D, Takayama TK, Majerus EM, Sadler JE, Fujikawa K. 2001; Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombo-cytopenic purpura. J Biol Chem. 276:41059–63. DOI: 10.1074/jbc.C100515200. PMID: 11557746. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0035798582&origin=inward.

8. Levy GG, Nichols WC, Lian EC, et al. 2001; Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 413:488–94. DOI: 10.1038/35097008. PMID: 11586351. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0035807348&origin=inward.

9. Mariotte E, Azoulay E, Galicier L, et al. 2016; Epidemiology and pathophysiology of adulthood-onset thrombotic microangiopathy with severe ADAMTS13 deficiency (thrombotic thrombocytopenic purpura): a cross-sectional analysis of the French national registry for thrombotic microangiopathy. Lancet Haematol. 3:e237–45. DOI: 10.1016/S2352-3026(16)30018-7. PMID: 27132698. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84963657077&origin=inward.

10. Reese JA, Muthurajah DS, Kremer Hovinga JA, Vesely SK, Terrell DR, George JN. 2013; Children and adults with thrombotic thrombo-cytopenic purpura associated with severe, acquired ADAMTS13 deficiency: comparison of incidence, demographic and clinical features. Pediatr Blood Cancer. 60:1676–82. DOI: 10.1002/pbc.24612. PMID: 23729372. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84881538470&origin=inward.

11. Chang JC. 2018; Hemostasis based on a novel 'two-path unifying theory' and classification of hemostatic disorders. Blood Coagul Fibrinolysis. 29:573–84. DOI: 10.1097/MBC.0000000000000765. PMID: 30063477. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85055072846&origin=inward.

12. Scully M, Yarranton H, Liesner R, et al. 2008; Regional UK TTP registry: correlation with laboratory ADAMTS 13 analysis and clinical features. Br J Haematol. 142:819–26. DOI: 10.1111/j.1365-2141.2008.07276.x. PMID: 18637802. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=48749102188&origin=inward.

13. Blombery P, Kivivali L, Pepperell D, et al. 2016; Diagnosis and management of thrombotic thrombocytopenic purpura (TTP) in Australia: findings from the first 5 years of the Australian TTP/thrombotic microangiopathy registry. Intern Med J. 46:71–9. DOI: 10.1111/imj.12935. PMID: 26477687. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84969389725&origin=inward.

14. Fujimura Y, Matsumoto M. 2010; Registry of 919 patients with thrombotic microangiopathies across Japan: database of Nara Medical University during 1998-2008. Intern Med. 49:7–15. DOI: 10.2169/internalmedicine.49.2706. PMID: 20045995. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=74549138555&origin=inward.

15. Kremer Hovinga JA, Vesely SK, Terrell DR, Lämmle B, George JN. 2010; Survival and relapse in patients with thrombotic thrombocytopenic purpura. Blood. 115:1500–11. DOI: 10.1182/blood-2009-09-243790. PMID: 20032506. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=77949903692&origin=inward.

16. Jang MJ, Chong SY, Kim IH, et al. 2011; Clinical features of severe acquired ADAMTS13 deficiency in thrombotic thrombocytopenic purpura: the Korean TTP registry experience. Int J Hematol. 93:163–9. DOI: 10.1007/s12185-011-0771-5. PMID: 21287408. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=79952249613&origin=inward.

17. Benhamou Y, Boelle PY, Baudin B, et al. 2015; Cardiac troponin-I on diagnosis predicts early death and refractoriness in acquired thrombotic thrombocytopenic purpura. Experience of the French Thrombotic Microangiopathies Reference Center. J Thromb Haemost. 13:293–302. DOI: 10.1111/jth.12790. PMID: 25403270. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84922919803&origin=inward.

18. Zafrani L, Mariotte E, Darmon M, et al. 2015; Acute renal failure is prevalent in patients with thrombotic thrombocytopenic purpura associated with low plasma ADAMTS13 activity. J Thromb Haemost. 13:380–9. DOI: 10.1111/jth.12826. PMID: 25523333. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84923667200&origin=inward.

19. Coppo P, Schwarzinger M, Buffet M, et al. 2010; Predictive features of severe acquired ADAMTS13 deficiency in idiopathic thrombotic microangiopathies: the French TMA reference center experience. PLoS One. 5:e10208. DOI: 10.1371/journal.pone.0010208. PMID: 20436664. PMCID: PMC2859048. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=77956532403&origin=inward.

20. Bendapudi PK, Hurwitz S, Fry A, et al. 2017; Derivation and external validation of the PLASMIC score for rapid assessment of adults with thrombotic microangiopathies: a cohort study. Lancet Haematol. 4:e157–64. DOI: 10.1016/S2352-3026(17)30026-1. PMID: 28259520. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85014108297&origin=inward.

21. Rock GA, Shumak KH, Buskard NA, et al. 1991; Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. N Engl J Med. 325:393–7. DOI: 10.1056/NEJM199108083250604. PMID: 2062330. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0026048114&origin=inward.

22. Scully M, Hunt BJ, Benjamin S, et al. 2012; Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br J Haematol. 158:323–35. DOI: 10.1111/j.1365-2141.2012.09167.x. PMID: 22624596. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84863841323&origin=inward.

23. Cataland SR, Kourlas PJ, Yang S, et al. 2017; Cyclosporine or steroids as an adjunct to plasma exchange in the treatment of immune- mediated thrombotic thrombocytopenic purpura. Blood Adv. 1:2075–82. DOI: 10.1182/bloodadvances.2017009308. PMID: 29296854. PMCID: PMC5728286. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85048363060&origin=inward.
24. Scully M, Cohen H, Cavenagh J, et al. 2007; Remission in acute refractory and relapsing thrombotic thrombocytopenic purpura following rituximab is associated with a reduction in IgG antibodies to ADAMTS-13. Br J Haematol. 136:451–61. DOI: 10.1111/j.1365-2141.2006.06448.x. PMID: 17233847. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33846018020&origin=inward.

25. Froissart A, Buffet M, Veyradier A, et al. 2012; Efficacy and safety of first-line rituximab in severe, acquired thrombotic thrombo-cytopenic purpura with a suboptimal response to plasma exchange. Experience of the French Thrombotic Microangiopathies Reference Center. Crit Care Med. 40:104–11. DOI: 10.1097/CCM.0b013e31822e9d66. PMID: 21926591. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84055166960&origin=inward.

26. Clark WF, Rock G, Barth D, et al. 2015; A phase-II sequential case-series study of all patients presenting to four plasma exchange centres with presumed relapsed/refractory thrombotic thrombocytopenic purpura treated with rituximab. Br J Haematol. 170:208–17. DOI: 10.1111/bjh.13408. PMID: 25855259. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84934434760&origin=inward.

27. Page EE, Kremer Hovinga JA, Terrell DR, Vesely SK, George JN. 2016; Rituximab reduces risk for relapse in patients with thrombotic thrombocytopenic purpura. Blood. 127:3092–4. DOI: 10.1182/blood-2016-03-703827. PMID: 27060171. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84976337802&origin=inward.

28. Kim SH, Hong J, Byun JM, et al. 2019; A survey on the use of rituximab in Korean patients with acquired thrombotic thrombocytopenic purpura. Res Pract Thromb Haemost (ISTH Annual Meeting Abstracts). 3(Suppl):abst PB1606. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85014108297&origin=inward.
29. Scully M, McDonald V, Cavenagh J, et al. 2011; A phase 2 study of the safety and efficacy of rituximab with plasma exchange in acute acquired thrombotic thrombocytopenic purpura. Blood. 118:1746–53. DOI: 10.1182/blood-2011-03-341131. PMID: 21636861. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=80051866867&origin=inward.

30. Westwood JP, Webster H, McGuckin S, McDonald V, Machin SJ, Scully M. 2013; Rituximab for thrombotic thrombocytopenic purpura: benefit of early administration during acute episodes and use of prophylaxis to prevent relapse. J Thromb Haemost. 11:481–90. DOI: 10.1111/jth.12114. PMID: 23279219. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84874984825&origin=inward.

31. Hie M, Gay J, Galicier L, et al. 2014; Preemptive rituximab infusions after remission efficiently prevent relapses in acquired thrombotic thrombocytopenic purpura. Blood. 124:204–10. DOI: 10.1182/blood-2014-01-550244. PMID: 24869941. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84904127650&origin=inward.

32. Jestin M, Benhamou Y, Schelpe AS, et al. 2018; Preemptive rituximab prevents long-term relapses in immune-mediated thrombotic thrombocytopenic purpura. Blood. 132:2143–53. DOI: 10.1182/blood-2018-04-840090. PMID: 30201758. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85056619574&origin=inward.

33. Ferrari S, Scheiflinger F, Rieger M, et al. 2007; Prognostic value of anti-ADAMTS 13 antibody features (Ig isotype, titer, and inhibitory effect) in a cohort of 35 adult French patients undergoing a first episode of thrombotic microangiopathy with undetectable ADAMTS 13 activity. Blood. 109:2815–22. DOI: 10.1182/blood-2006-02-006064. PMID: 17164349. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33947594746&origin=inward.
34. Peyvandi F, Lavoretano S, Palla R, et al. 2008; ADAMTS13 and anti-ADAMTS13 antibodies as markers for recurrence of acquired thrombotic thrombocytopenic purpura during remission. Haematologica. 93:232–9. DOI: 10.3324/haematol.11739. PMID: 18223285. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=40849114959&origin=inward.

35. Jin M, Casper TC, Cataland SR, et al. 2008; Relationship between ADAMTS13 activity in clinical remission and the risk of TTP relapse. Br J Haematol. 141:651–8. DOI: 10.1111/j.1365-2141.2008.07107.x. PMID: 18397340. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=43449135029&origin=inward.

36. Sayani FA, Abrams CS. 2015; How I treat refractory thrombotic thrombocytopenic purpura. Blood. 125:3860–7. DOI: 10.1182/blood-2014-11-551580. PMID: 25784681. PMCID: PMC4473115. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84929994371&origin=inward.

37. Eskazan AE. 2016; Bortezomib therapy in patients with relapsed/refractory acquired thrombotic thrombocytopenic purpura. Ann Hematol. 95:1751–6. DOI: 10.1007/s00277-016-2804-x. PMID: 27590601. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84984923647&origin=inward.

38. Callewaert F, Roodt J, Ulrichts H, et al. 2012; Evaluation of efficacy and safety of the anti-VWF Nanobody ALX-0681 in a preclinical baboon model of acquired thrombotic thrombocytopenic purpura. Blood. 120:3603–10. DOI: 10.1182/blood-2012-04-420943. PMID: 22948047. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84868565430&origin=inward.

39. Peyvandi F, Scully M, Kremer Hovinga JA, et al. 2016; Caplacizumab for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 374:511–22. DOI: 10.1056/NEJMoa1505533. PMID: 26863353. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84959293895&origin=inward.

40. Scully M, Cataland SR, Peyvandi F, et al. 2019; Caplacizumab treatment for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 380:335–46. DOI: 10.1056/NEJMoa1806311. PMID: 30625070. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85060365954&origin=inward.

41. Peyvandi F, Cataland SR, Scully M, et al. 2021; Caplacizumab prevents refractoriness and mortality in acquired thrombotic thrombo-cytopenic purpura: integrated analysis. Blood Adv. 5:2137–41. DOI: 10.1182/bloodadvances.2020001834. PMID: 33881463. PMCID: PMC8095153. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85106281530&origin=inward.

42. Mazepa MA, Masias C, Chaturvedi S. 2019; How targeted therapy disrupts the treatment paradigm for acquired TTP: the risks, benefits, and unknowns. Blood. 134:415–20. DOI: 10.1182/blood.2019000954. PMID: 31217190. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85070622102&origin=inward.

43. Cuker A, Cataland SR, Coppo P, et al. 2021; Redefining outcomes in immune TTP: an international working group consensus report. Blood. 137:1855–61. DOI: 10.1182/blood.2020009150. PMID: 33529333. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85103778501&origin=inward.

44. Plaimauer B, Kremer Hovinga JA, Juno C, et al. 2011; Recombinant ADAMTS13 normalizes von Willebrand factor-cleaving activity in plasma of acquired TTP patients by overriding inhibitory antibodies. J Thromb Haemost. 9:936–44. DOI: 10.1111/j.1538-7836.2011.04224.x. PMID: 21294825. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=79955643808&origin=inward.

45. Scully M, Knobl P, Kentouche K, et al. 2017; Recombinant human ADAMTS-13: first in-human pharmacokinetics and safety in congenital thrombotic thrombocytopenic purpura. Blood. 130:2055–63. DOI: 10.1182/blood-2017-06-788026. PMID: 28912376. PMCID: PMC5680611. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85033472099&origin=inward.
46. Li BX, Dai X, Xu XR, et al. 2021; In vitro assessment and phase I randomized clinical trial of anfibatide a snake venom derived anti-thrombotic agent targeting human platelet GPIbα. Sci Rep. 11:11663. DOI: 10.1038/s41598-021-91165-8. PMID: 34083615. PMCID: PMC8175443. PMID: 2f1cc945ff084274a10fe99842cf9bb1. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85107174595&origin=inward.

47. Deford CC, Reese JA, Schwartz LH, et al. 2013; Multiple major morbidities and increased mortality during long-term follow-up after recovery from thrombotic thrombocytopenic purpura. Blood. 122:2023–9. DOI: 10.1182/blood-2013-04-496752. PMID: 23838348. PMCID: PMC3778546. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84887595203&origin=inward.

48. Lewis QF, Lanneau MS, Mathias SD, Terrell DR, Vesely SK, George JN. 2009; Long-term deficits in health-related quality of life after recovery from thrombotic thrombocytopenic purpura. Transfusion. 49:118–24. DOI: 10.1111/j.1537-2995.2008.01938.x. PMID: 18954401. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=58149129398&origin=inward.

49. Riva S, Mancini I, Maino A, et al. 2020; Long-term neuropsychological sequelae, emotional wellbeing and quality of life in patients with acquired thrombotic thrombocytopenic purpura. Haematologica. 105:1957–62. DOI: 10.3324/haematol.2019.226423. PMID: 31558667. PMCID: PMC7327631. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85082454893&origin=inward.

50. Upreti H, Kasmani J, Dane K, et al. 2019; Reduced ADAMTS13 activity during TTP remission is associated with stroke in TTP survivors. Blood. 134:1037–45. DOI: 10.1182/blood.2019001056. PMID: 31431443. PMCID: PMC7022317. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85072662137&origin=inward.

51. Brodsky MA, Sukumar S, Selvakumar S, et al. 2021; Major adverse cardiovascular events in survivors of immune-mediated thrombotic thrombocytopenic purpura. Am J Hematol. 96:1587–94. DOI: 10.1002/ajh.26341. PMID: 34460124. PMCID: PMC8616844. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85114914084&origin=inward.

Fig. 1
Current approach for patients with suspected iTTP in Korea. Plasma exchange (PEX) should be initiated at the earliest when throm-botic thrombocytopenic purpura is suspected. Daily PEX can be discontinued once platelet count is normalized for 2 days. A suboptimal response is defined as a lack of platelet count increment after 5 days of PEX or initial im-provement followed by a decrease in platelet count while receiving PEX. Although rituximab treatment for immune-mediated thrombotic thrombocytopenic purpura (iTTP) has not been approved in Korea, the off-label use of rituximab should be considered for refractory iTTP. Currently, caplacizumab is unavailable in Korea. *ADAMTS13 gene sequencing should be considered in patients who have no detectable anti-ADAMTS13 antibodies and persistent severe ADAMTS13 deficiency during clinical remission.
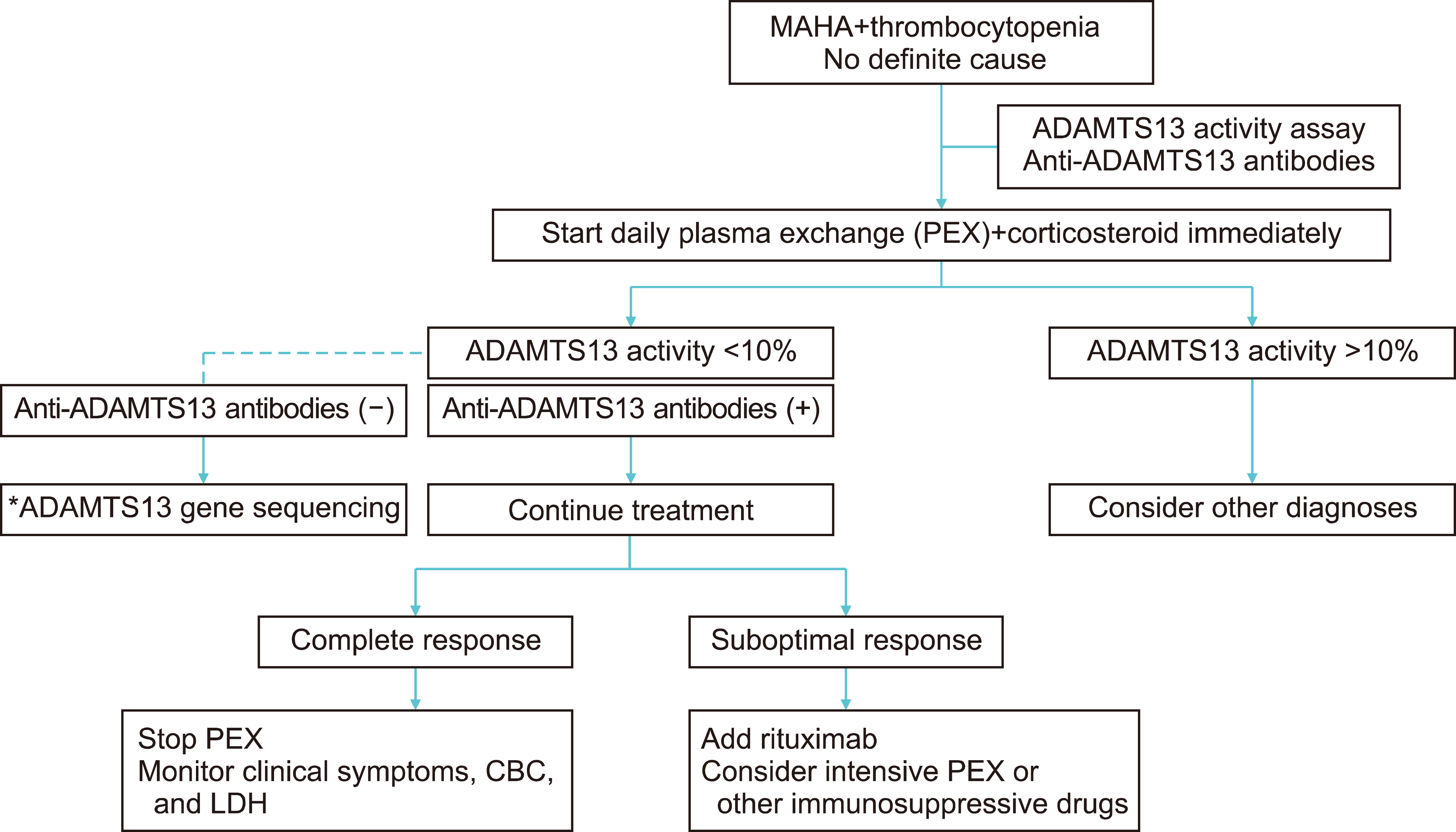
Table 1
Thrombotic microangiopathy syndromes.
| Primary TMAa) |
| TTP |
| Congenital |
| Acquired (immune-mediated) |
| Complement mediated atypical hemolytic uremic syndrome |
| Congenital |
| Acquired |
| Secondary TMA |
| Shiga toxin producing Escherichia coli hemolytic uremic syndrome |
| Disseminated intravascular coagulation |
| Infection associated TMA |
| Cancer associated TMA |
| Drug induced TMA |
| Immune-mediated |
| Toxic |
| Transplant associated TMA |
| Malignant hypertension |
| Autoimmune disease (e.g., systemic lupus erythematosus, systemic sclerosis, antiphospholipid syndrome) associated TMA |
| Pregnancy associated TMA |
| Pre-eclampsia, eclampsia |
| HELLP syndrome (hemolysis, elevated liver enzymes, low platelets) |
Table 2
Clinical scoring systems to estimate the probability of severe ADAMTS13 deficiency in patients with TMA.
| French score | PLASMIC score | |
|---|---|---|
| Platelet count | ≤30×109 L: +1 | <30×109 L: +1 |
| Creatinine level | ≤2.26 mg/dL: +1 | <2.0 mg/dL: +1 |
| Hemolysis variablea) | +1 | |
| No active cancer | +1 | |
| No history of solid-organ or stem-cell transplantation | +1 | |
| MCV <90 Fl | +1 | |
| INR <1.5 | +1 | |
| ANA | +1 | |
| Total score and likelihood of TTP | 0: 2% | 0–4 (low risk): 0–4% |
| 1: 70% | 5 (intermediate risk): 9–24% | |
| 2–3: 94% | 6–7 (high risk): 62–82% |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download