Abstract
The incidence of hematologic malignancy increases with age; thus, the number of older patients who require intensive chemotherapy is expected to increase with the aging population. In Korea, 61.8%, 59.3%, 47.0%, and 46.7% of newly diagnosed cases of multiple myeloma, myelodysplastic syndrome, myeloproliferative disorder, and non-Hodgkin lymphoma, respectively, occurred in patients aged >65 years in 2018. Health status among older patients, defined by frailty, age-related syndrome of physiological decline and increased vulnerability, is associated with adverse health outcomes. Health status is highly heterogeneous among older patients, and treatment outcomes vary according to frailty and physiologic age rather than chronologic age. Comprehensive geriatric assessment (CGA) is a multidimensional and multidisciplinary diagnostic and treatment process that identifies multiple domains, including functional status, cognition, comorbidities, medications, socioeconomic status, and nutritional status, to develop a coordinated plan to improve treatment-related outcomes and quality of life. Frailty can be assessed with CGA findings, and CGA is considered the “gold standard of care” for frail, older patients. Through CGA, unidentified problems can be assessed, and pre-emptive and non-oncologic interventions can be delivered. CGA is an objective and reliable tool for predicting further treatment-related complications and identifying patients for whom intensive chemotherapy with curative intent is appropriate. CGA should be considered a routine practice before starting treatment planning in older patients diagnosed with hematologic malignancies who require intensive chemotherapy. Further study is needed to allocate individualized treatment plans or multidisciplinary geriatric interventions according to CGA results.
Korea is the fastest-aging country worldwide. This was attributed to the “baby boom” following World War II and extremely low birth rates in recent years. Korea is expected to become a super-aged society by 2025, defined as a population in which >20% are ≥65 years of age [1]. Since hematologic malignancy increases with age, older adults represent the growing majority of patients diagnosed with hematologic disorders. The number of older patients receiving intensive chemotherapy is expected to increase as the Korean population ages. In Korea, 61.8%, 59.3%, 47.0%, and 46.7% of newly diagnosed cases of multiple myeloma, myelodysplastic syndrome, myeloproliferative disorder, and non-Hodgkin lymphoma, respectively, occurred in patients aged >65 years in 2018 [2]. Intensive chemotherapy, defined as administering anticancer drugs at high doses or over several months, is typically used to treat cancer or cause remission. Treatment with intensive chemotherapy requires a physiological reserve to endure the treatment and may not be tolerated in individuals with poor health status and consequence to extensive complications. Because of the heterogeneity of health status in the older population, standard oncological performance evaluation is insufficient for assessing the physiological reserve necessary to endure intensive chemotherapy. This represents a dilemma in determining the best treatment and care for older patients with hematologic malignancies, prompting the emergence of geriatric hematology. The present review will provide insights into frailty, comprehensive geriatric assessment (CGA), and clinical usefulness of CGA in older patients with hematological malignancies receiving intensive chemotherapy.
Aging processes and chronic conditions worsen functional capacity, quality of life, and life expectancy [3]. Since symptoms and functional capacity vary among the aging population, estimating complications or outcomes after treatment based only on chronological age or conventional oncologic evaluation is difficult. People age at different rates; thus, determining methods for quantifying aging rates is important.
Frailty is a common clinical syndrome in older adults and is characterized by decreased physiological reserves and marked vulnerability to adverse outcomes after potentially stressful events [4]. Since the Karnofsky Performance Status or International Prognostic Index are commonly used validated performance scale among hematologic malignancies, performance status is not synonymous with frailty [5, 6]. Frailty can be defined according to two major concepts as phenotypic frailty or deficit accumulation frailty. Phenotypic frailty is defined as meeting three or more of the following characteristics: weight loss, exhaustion, weakness, slow gait speed, or decreased physical activity [7]. Deficit accumulation frailty is defined by the accumulation of illnesses, symptoms, functional and cognitive decline, and socioeconomic status, which are added together to calculate deficit accumulation [8]. In Korea, the prevalence of frailty in community-dwelling older adults ranges from 7.7% to 17.0% according to frailty phenotype assessment and from 17.5% to 26.3% according to frailty index assessment [1].
In hematology, frailty assessment aids clinicians in improving prognostication to decrease treatment-related morbidity and mortality [9]. Because frailty is a clinical state of increased vulnerability to developing adverse health-related outcomes when exposed to stressors, it is highly correlated with negative outcomes after intensive chemotherapy, which is a substantial stressor. Frailty is associated with shorter survival in patients with myeloma and allogeneic hematopoietic cell transplantation [10, 11]. Thus, measuring frailty in such patients provides essential insights for clinicians concerning prognosis and appropriate non-oncologic geriatric interventions for modifiable risk factors [12].
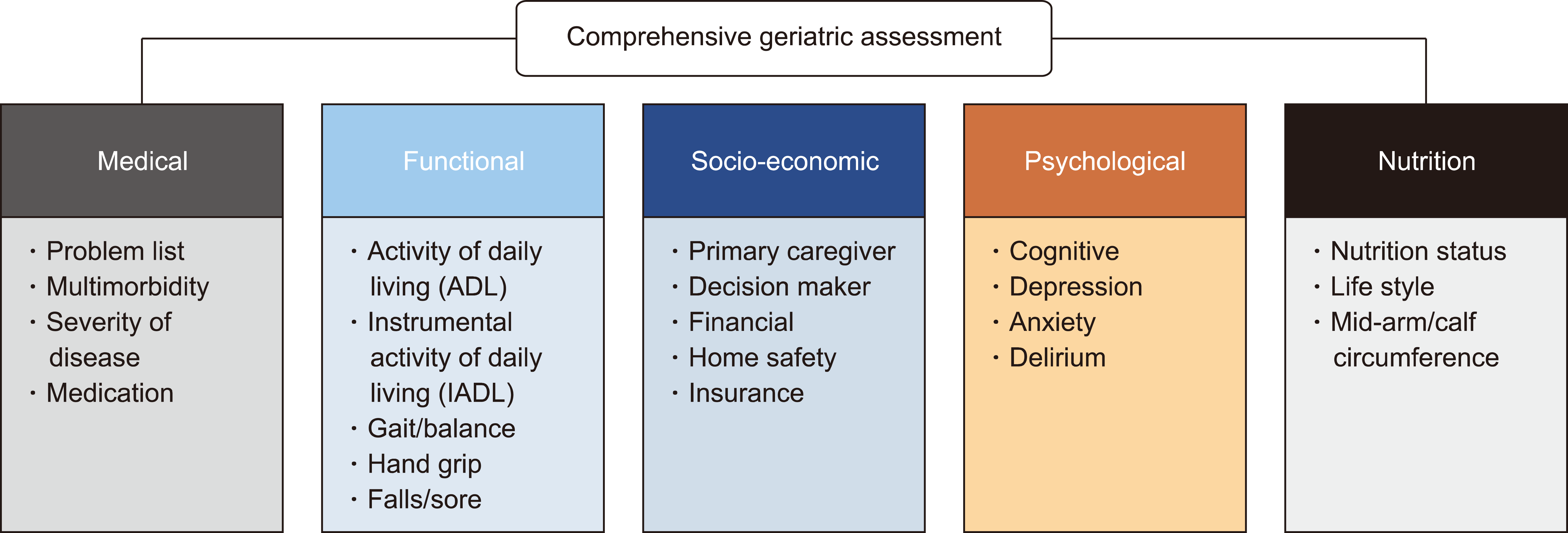
CGA is a multidimensional, interdisciplinary diagnostic process focused on determining older persons’ medical, physical, psychological, functional, social, and environmental capabilities to develop a patient-centered, coordinated, and integrated treatment and long-term management plan. Detailed CGA evaluation tools differ depending on the institution, healthcare setting, and needs. The definition of CGA, which includes both the assessment of needs in multiple domains and the development of a treatment plan, is consistent [13, 14]. CGA usually consists of reviews for 1) acute and chronic disease, 2) medication, 3) functional assessment, 4) psychological assessment including cognitive function and emotional status, and 5) nutrition status (Fig 1).
Patients are assessed for acute and chronic diseases to identify specific diseases that may limit treatment efficacy or increase the risk of treatment-related adverse outcomes. If a patient’s chronic disease is improperly managed, medical optimization may be required to stabilize the patient before and during chemotherapy.
Adverse drug reactions (ADR) are prevalent in older adults, and their prevalence increases with age [15]. Medications with a higher risk of ADRs are commonly referred to as potentially inappropriate medications (PIM) for older adults. Specific medication lists for PIMs vary according to quality criteria, mainly because distinct prescription patterns vary between countries. The two most frequently used quality criteria for PIMs are the Beers Criteria, developed by the American Geriatric Society, and the Screening Tool of Older Persons’ potentially inappropriate Prescriptions (STOPP) criteria developed by the British Geriatrics Society [16, 17]. Many anticholinergic drugs are classified as PIMs, and their use in older patients is discouraged. Some medication lists for PIMs and anticholinergic drugs have been developed and validated for use in Korea [18-20]. Considering that polypharmacy is also related to increased ADRs, reviewing a patient’s medication lists and indications is an essential component of CGA. After medication review, reducing or ceasing medications may be necessary to ensure safe and effective use. It is essential to minimize unnecessary medications because chemotherapy can reduce kidney or liver function or cause drug interactions with existing medications.
Functional assessment usually comprises components such as activities of daily living (ADL), instrumental activities of daily living (IADL), and physical functions (gait speed, balance, and handgrip strength). ADL includes basic tasks that must be accomplished daily for an individual to thrive. In general, ADL assessment includes evaluation of personal hygiene, continence management, dressing, feeding, and ambulation [21]. IADL is complex but nevertheless reflects a person’s ability to live independently. IADL includes the evaluation of competencies related to transportation, shopping, preparing meals, managing households, managing medication, communicating with others, and managing finances [22]. Gait speed and grip strength are easily obtained objective measures associated with mortality or unplanned hospitalization in older patients with hematologic malignancies [23]. Assessment of physical function is sometimes preferred because the evaluation is simple and objective. Functional assessment is important in Korea because most older adults (78.2%) live in single households, alone, or with a spouse [1]. Patients should be admitted to a long-term care facility or hospital if functional capacity is reduced due to treatment-related toxicity and deconditioning. Therefore, it is essential to evaluate functional vulnerability in older adults using CGA to assess whether treatment regimens will be well tolerated and predict the need for rehabilitation before and after treatment.
Socioeconomic status was also evaluated through CGA to identify the patient’s primary caregiver, medical decision-maker, and financial or insurance status. The patient’s socioeconomic status and the range of resources available to them can determine whether to treat the patient as well as the treatment intensity. Cognitive function and emotional status (e.g., depression or anxiety) were also evaluated to determine suitable treatments. Confusion or delirium, a state of brain dysfunction that may be a side effect of the malignancy itself or intensive chemotherapy, can complicate treatment. Evaluation of cognitive function makes it possible to predict the likelihood of developing delirium during intensive treatment and provides an opportunity for non-pharmacological delirium prevention activities or education for patients and their caregivers [24].
Assessment of nutritional status is essential because malnutrition is a frequent problem in older patients with cancer and negatively affects clinical prognosis and quality of life. Malnutrition plays a crucial role in the pathogenesis of frailty and sarcopenia, and nutritional interventions may effectively reduce or revert either condition. Numerous nutritional screening tools have been established and validated for older patients, including the Mini Nutritional Assessment (MNA) and short form of MNA (MNA-SF) [25]. The MNA is composed of simple measurements and brief questions that can be completed in 10 minutes.
Even in patients with good performance status, geriatric impairments were discovered by CGA, with a median prevalence of 17–68% [14]. These systematic, multidimensional assessments have an additive value for informing clinical decision-making, treatment allocation, and the implementation of non-oncological geriatric interventions before and during intensive chemotherapy to improve resilience and tolerance.
A previous systematic review showed that impairment in geriatric domains is common among older patients with hematologic malignancies, even in those with good performance status. CGA could detect unrecognized vulnerabilities.
CGA can benefit prognostication in mortality, treatment-related toxicity, and treatment non-completion [14]. Among the 13 reported studies, the median prevalence estimates of CGA were 26% for ADLs (range, 8–59%), 44% for IADLs (range, 21–81%), 19% for cognitive impairment (range, 0–38%), 32% for depressive symptoms (range, 19–94%), and 39% for impaired objective physical capacity (range, 12–76%) [26]. In a previous study by Ribi et al. [27], patients with aggressive B-cell lymphoma were assessed using CGA. Those with impaired functional status and summary scores contained a higher proportion of treatment non-responders and were more likely to die before the median follow-up or during intensive chemotherapy. A study by Ong et al. [28] showed that CGA score was associated with baseline dose reduction during intensive chemotherapy. This dose reduction was independently associated with poorer progression-free survival and overall survival among patients with diffuse large B-cell lymphoma (DLBCL). This study also emphasized physiological heterogeneity among older patients with DLBCL (41% fit, 21% unfit, and 38% frail, as assessed by CGA). In addition, a previous study of very old patients with DLBCL suggested that these patients did not have improved survival following full-dose standard chemotherapy and that their completion rate for intensive treatment was low [29]. These findings demonstrate that it is necessary to accurately evaluate physiologic age rather than chronologic age to determine the optimal balance between treatment intensity and disease control and treatment-related complications according to individuals’ tolerability. Thus, CGA enhances the understanding of treatment benefits and can guide personalized supportive care [30]. The importance of CGA in geriatric hematology is expected to increase as the population ages.
With the rapid population aging in Korea, hematologic malignancy is expected to increase. Thus, the appropriate evaluation of older patients expected to receive intensive chemotherapy to treat hematologic malignancies is emerging as an essential clinical issue. This review discusses the clinical definitions of frailty, the utility of frailty as a physiologic reserve measurement, and the relevance of performing CGAs in older patients. Our review demonstrated that CGA could be effectively used to identify geriatric impairments that may be modifiable and to predict mortality. CGA results are associated with the risk of treatment-related adverse outcomes and survival, thus, can be used as prognostic indicators in older adults with hematologic malignancies. Further research is needed to extend and apply these findings to individualized treatment allocations or multidisciplinary geriatric interventions.
REFERENCES
1. Baek JY, Lee E, Jung HW, Jang IY. 2021; Geriatrics fact sheet in Korea 2021. Ann Geriatr Med Res. 25:65–71. DOI: 10.4235/agmr.21.0063. PMID: 34187140. PMCID: PMC8272996. PMID: a96b154a77dc4335a5ebd4aab3fd28dc. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85110520397&origin=inward.

2. Korea Central Cancer Registry. National Cancer Center. 2020. Annual report of cancer statistics in Korea in 2018. Ministry of Health and Welfare;Sejong, Korea:
3. Murray CJL, Barber RM, et al. GBD 2013 DALYs and HALE Collaborators. 2015; Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990-2013: quantifying the epidemiological transition. Lancet. 386:2145–91. DOI: 10.1016/S0140-6736(15)61340-X. PMID: 26321261. PMCID: PMC4673910. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84949086655&origin=inward.
4. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. 2013; Frailty in elderly people. Lancet. 381:752–62. DOI: 10.1016/S0140-6736(12)62167-9. PMID: 23395245. PMCID: PMC4098658. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84874422422&origin=inward.

5. Ziepert M, Hasenclever D, Kuhnt E, et al. 2010; Standard International prognostic index remains a valid predictor of outcome for patients with aggressive CD20+ B-cell lymphoma in the rituximab era. J Clin Oncol. 28:2373–80. DOI: 10.1200/JCO.2009.26.2493. PMID: 20385988. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=77952477025&origin=inward.
6. Shaffer BC, Ahn KW, Hu ZH, et al. 2016; Scoring system prognostic of outcome in patients undergoing allogeneic hematopoietic cell transplantation for myelodysplastic syndrome. J Clin Oncol. 34:1864–71. DOI: 10.1200/JCO.2015.65.0515. PMID: 27044940. PMCID: PMC4966345. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84974539642&origin=inward.
7. Fried LP, Tangen CM, Walston J, et al. 2001; Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 56:M146–56. DOI: 10.1093/gerona/56.3.M146. PMID: 11253156. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=2442699834&origin=inward.

8. Rockwood K, Mitnitski A. 2007; Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 62:722–7. DOI: 10.1093/gerona/62.7.722. PMID: 17634318. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=34548395418&origin=inward.

9. Koll TT, Rosko AE. 2018; Frailty in hematologic malignancy. Curr Hematol Malig Rep. 13:143–54. DOI: 10.1007/s11899-018-0454-x. PMID: 29730710. PMCID: PMC6023762. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85046490145&origin=inward.

10. Buckstein R, Wells RA, Zhu N, et al. 2016; Patient-related factors independently impact overall survival in patients with myelo-dysplastic syndromes: an MDS-CAN prospective study. Br J Haematol. 174:88–101. DOI: 10.1111/bjh.14033. PMID: 26991631. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84976498280&origin=inward.

11. Muffly LS, Kocherginsky M, Stock W, et al. 2014; Geriatric assessment to predict survival in older allogeneic hematopoietic cell transplantation recipients. Haematologica. 99:1373–9. DOI: 10.3324/haematol.2014.103655. PMID: 24816237. PMCID: PMC4116837. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84905160745&origin=inward.

12. Rezaei-Shahsavarloo Z, Atashzadeh-Shoorideh F, Gobbens RJJ, Ebadi A, Ghaedamini Harouni G. 2020; The impact of interventions on management of frailty in hospitalized frail older adults: a systematic review and meta-analysis. BMC Geriatr. 20:526. DOI: 10.1186/s12877-020-01935-8. PMID: 33272208. PMCID: PMC7712609. PMID: 6d5bf04ffca9416f9ae373c8c3994de2. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85097032053&origin=inward.

13. Parker SG, McCue P, Phelps K, et al. 2018; What is Comprehensive Geriatric Assessment (CGA)? An umbrella review. Age Ageing. 47:149–55. DOI: 10.1093/ageing/afx166. PMID: 29206906. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85040583840&origin=inward.

14. Scheepers ERM, Vondeling AM, Thielen N, van der Griend R, Stauder R, Hamaker ME. 2020; Geriatric assessment in older patients with a hematologic malignancy: a systematic review. Haematologica. 105:1484–93. DOI: 10.3324/haematol.2019.245803. PMID: 32381581. PMCID: PMC7271571. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85085854909&origin=inward.

15. Beijer HJ, de Blaey CJ. 2002; Hospitalisations caused by adverse drug reactions (ADR): a meta-analysis of observational studies. Pharm World Sci. 24:46–54. DOI: 10.1023/A:1015570104121. PMID: 12061133. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0036000276&origin=inward.
16. By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. 2019; American Geriatrics Society 2019 updated AGS Beers CriteriaⓇ for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 67:674–94. DOI: 10.1111/jgs.15767. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85071354580&origin=inward.
17. Hamilton H, Gallagher P, Ryan C, Byrne S, O'Mahony D. 2011; Potentially inappropriate medications defined by STOPP criteria and the risk of adverse drug events in older hospitalized patients. Arch Intern Med. 171:1013–9. DOI: 10.1001/archinternmed.2011.215. PMID: 21670370. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=79959296825&origin=inward.

18. Kim MY, Etherton-Beer C, Kim CB, et al. 2018; Development of a consensus list of potentially inappropriate medications for Korean older adults. Ann Geriatr Med Res. 22:121–9. DOI: 10.4235/agmr.2018.22.3.121. PMID: 32743261. PMCID: PMC7387587. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85067959384&origin=inward.

19. Jun K, Hwang S, Ah YM, Suh Y, Lee JY. 2019; Development of an anticholinergic burden scale specific for Korean older adults. Geriatr Gerontol Int. 19:628–34. DOI: 10.1111/ggi.13680. PMID: 31033150. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85065214852&origin=inward.

20. Suh Y, Ah YM, Han E, et al. 2020; Dose response relationship of cumulative anticholinergic exposure with incident dementia: validation study of Korean anticholinergic burden scale. BMC Geriatr. 20:265. DOI: 10.1186/s12877-020-01671-z. PMID: 32727410. PMCID: PMC7391507. PMID: ce742f9517314e008ec3b0f5c19c221c. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85088851783&origin=inward.

21. Mahoney FI, Barthel DW. 1965; Functional evaluation: the Barthel Index. Md State Med J. 14:61–5. DOI: 10.1037/t02366-000. PMID: 14258950.
22. Lawton MP, Brody EM. 1969; Assessment of older people: self- maintaining and instrumental activities of daily living. Gerontologist. 9:179–86. DOI: 10.1093/geront/9.3_Part_1.179. PMID: 5349366. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0014579432&origin=inward.
23. Liu MA, DuMontier C, Murillo A, et al. 2019; Gait speed, grip strength, and clinical outcomes in older patients with hematologic malignancies. Blood. 134:374–82. DOI: 10.1182/blood.2019000758. PMID: 31167800. PMCID: PMC6659254. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85070581520&origin=inward.

24. Inouye SK, Bogardus ST Jr, Baker DI, Leo-Summers L, Cooney LM Jr. 2000; The Hospital Elder Life Program: a model of care to prevent cognitive and functional decline in older hospitalized patients. Hospital Elder Life Program. J Am Geriatr Soc. 48:1697–706. DOI: 10.1111/j.1532-5415.2000.tb03885.x. PMID: 11129764. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0034544273&origin=inward.
25. Vellas B, Guigoz Y, Garry PJ, et al. 1999; The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition. 15:116–22. DOI: 10.1016/S0899-9007(98)00171-3. PMID: 9990575. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0033005224&origin=inward.

26. Hamaker ME, Prins MC, Stauder R. 2014; The relevance of a geriatric assessment for elderly patients with a haematological malignancy-- a systematic review. Leuk Res. 38:275–83. DOI: 10.1016/j.leukres.2013.12.018. PMID: 24439052. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84894277140&origin=inward.
27. Ribi K, Rondeau S, Hitz F, et al. 2017; Cancer-specific geriatric assessment and quality of life: important factors in caring for older patients with aggressive B-cell lymphoma. Support Care Cancer. 25:2833–42. DOI: 10.1007/s00520-017-3698-4. PMID: 28405846. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85017454879&origin=inward.

28. Ong DM, Ashby M, Grigg A, et al. 2019; Comprehensive geriatric assessment is useful in an elderly Australian population with diffuse large B-cell lymphoma receiving rituximab-chemotherapy combinations. Br J Haematol. 187:73–81. DOI: 10.1111/bjh.16049. PMID: 31206608. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85067489159&origin=inward.

29. Carson KR, Riedell P, Lynch R, et al. 2015; Comparative effectiveness of anthracycline-containing chemotherapy in United States veterans age 80 and older with diffuse large B-cell lymphoma. J Geriatr Oncol. 6:211–8. DOI: 10.1016/j.jgo.2015.01.003. PMID: 25614297. PMCID: PMC4605388. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84930475574&origin=inward.

30. Klepin HD. 2019; Ready for prime time: role for geriatric assessment to improve quality of care in hematology practice. Blood. 134:2005–12. DOI: 10.1182/blood.2019001299. PMID: 31805198. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85076284137&origin=inward.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download