Abstract
Malignant gastrointestinal stromal tumors (GISTs) are rare neoplasms originating from the gastrointestinal tract that rarely occur in patients below 40 years of age. To our knowledge, there have been no previous reports of satellite and metastatic nodules in GIST. We present a case of a young patient with a huge malignant gastric GIST accompanied by spontaneous bleeding and satellite and metastatic nodules, successfully treated surgically, without preoperative chemotherapy administration. A 28-year-old man was admitted to Haeundae Paik Hospital with melena. A huge bulging gastric mass with ulceration and bleeding was observed on endoscopy. A subepithelial lesion on the stomach body, abutting the pancreatic body and tail, with regional lymph node enlargement was confirmed by EUS and CT. Radical total gastrectomy was performed, the invasion surrounding the pancreatic tail and spleen were surgically dissected, and enlarged lymph nodes around the celiac trunk and the common hepatic artery were removed. The pathology results showed a malignant GIST with two satellite nodules and a metastatic tumor nodule at the left paracardial lymph node site. After complete resection of the malignant GIST, adjuvant chemotherapy with imatinib was initiated. Follow-up CT and endoscopy performed 6 months after surgery confirmed no recurrence of the disease.
Malignant gastrointestinal stromal tumors (GISTs) are rare neoplasms derived from the GI tract. GISTs account for 0.1% to 3% of all malignant GI tumors, and 60-70% of GISTs are found in the stomach.1,2 Typically, GISTs occur in individuals >50 years of age, with only 5-20% found in adults <40 years of age.1 Mitotic counts >5 mitoses per 5 mm2 indicate high-grade malignancy for GIST and a maximal tumor diameter >5 cm and biomarker Ki-67 positivity are major risk factors for gastric GIST with overt bleeding. GI bleeding, such as melena, hematemesis, and hematochezia, is the most frequent presenting symptom in cases of GIST-related emergency (48.9%).3 In addition, GI bleeding itself is a poor prognostic factor for overall survival in patients with GIST.4 Lymph node metastasis in GISTs is more common in patients below 40 years of age.5
Herein, we report a case of a huge exophytic GIST with spontaneous bleeding and satellite and metastatic nodules in a young patient who was successfully treated surgically without preoperative chemotherapy.
A 28-year-old man presented to the emergency department with melena. His vital signs, including blood pressure and heart rate, were stable, but anemia was observed with a hemoglobin concentration of 9.6 g/dL. Upper GI endoscopy was performed on suspicion of upper GI bleeding, based on the emergency room examination findings. Upper GI endoscopy showed a huge bulging subepithelial tumor >5 cm in diameter in the mid-body posterior wall of the stomach, with a central ulcer and spurting bleeding. Endoscopic hemostasis was performed using a mechanical clip. An approximately 7.2×7.3 cm heterogeneous and hypoechoic lesion, with a well-circumscribed, poorly defined margin that was thought to arise from the fourth layer of the stomach wall (muscularis propria) was identified on EUS performed with a radial endoscope (UM-3R miniprobe; Olympus, Tokyo, Japan). Hypoechoic foci and hyperechoic spots with diffusely heterogeneous hypervascular patterns were also observed. A suspected 7 mm-sized reactive lymph node with round, sharply demarcated, homogeneous, hypoechoic features was also observed (Fig. 1). A CT scan showed an exophytic mass in the stomach body, abutting the body and tail of the pancreas and the spleen (Fig. 2). During upper GI endoscopy, biopsies were performed at the ulcer site, but the pathology findings did not lead to a definitive diagnosis.
Therefore, an exploratory laparotomy was planned in based on the suspicion of the presence of a malignant GIST. Intraoperatively, a subepithelial tumor approximately 11 cm in size was observed on the upper body of the stomach next to the splenic hilum, with invasion around the pancreatic tail (Fig. 3A). The capsulation of the GIST lesions attached to the surrounding organs (pancreas and spleen) was not clear. However, the borders were clearly separated from the surrounding tissue, and minimal tissue infiltration had occurred. Therefore, dissection of the surrounding tissue was performed with total gastrectomy. A clear dissection and curative-intent surgery were possible without requiring a combined resection of the pancreas or spleen (Fig. 4). Subsequently, a radical total gastrectomy with Roux-en-Y esophagojejunostomy was performed. Enlarged lymph nodes were observed around the celiac trunk and common hepatic artery and these were removed by lymph node dissection.
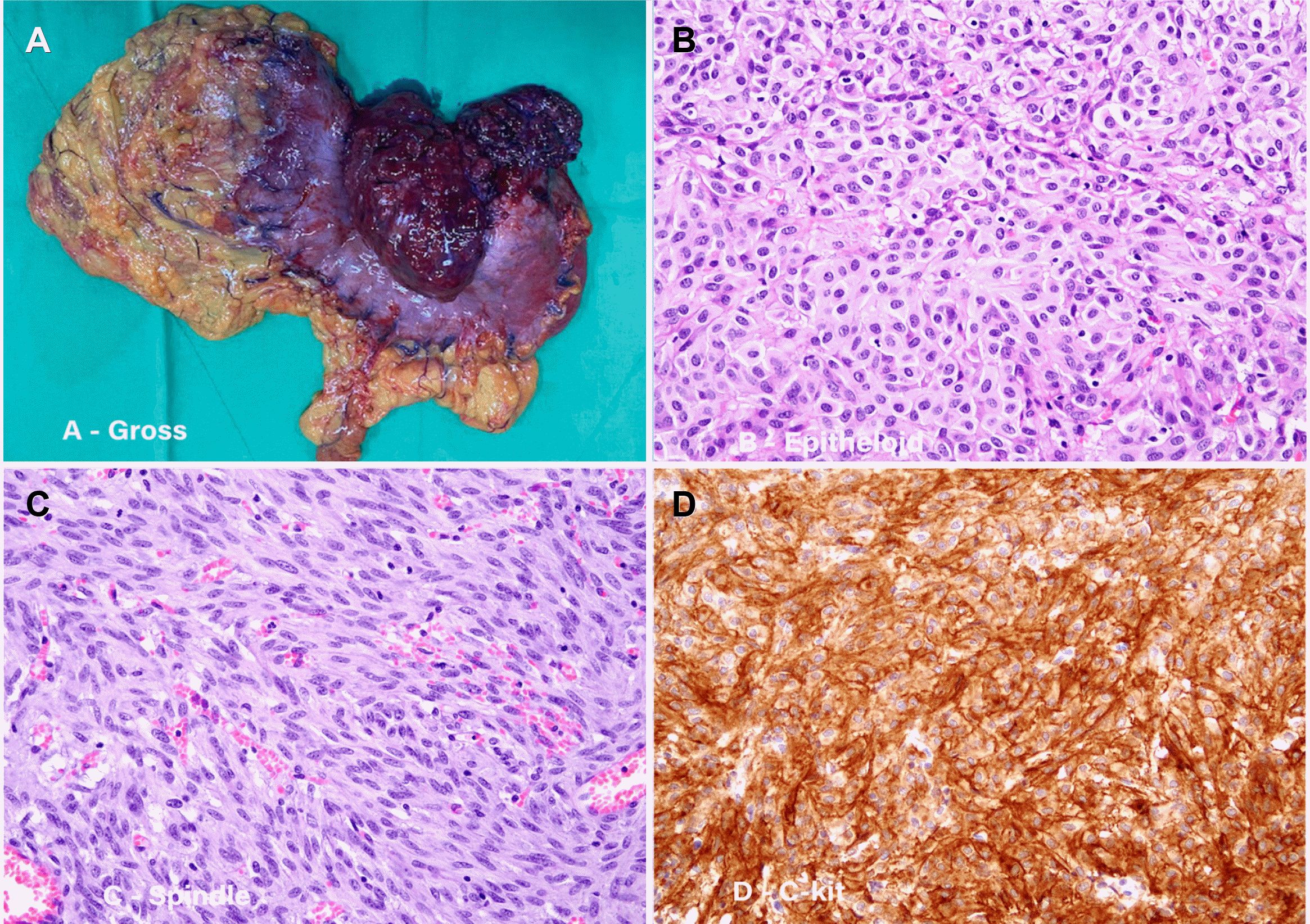
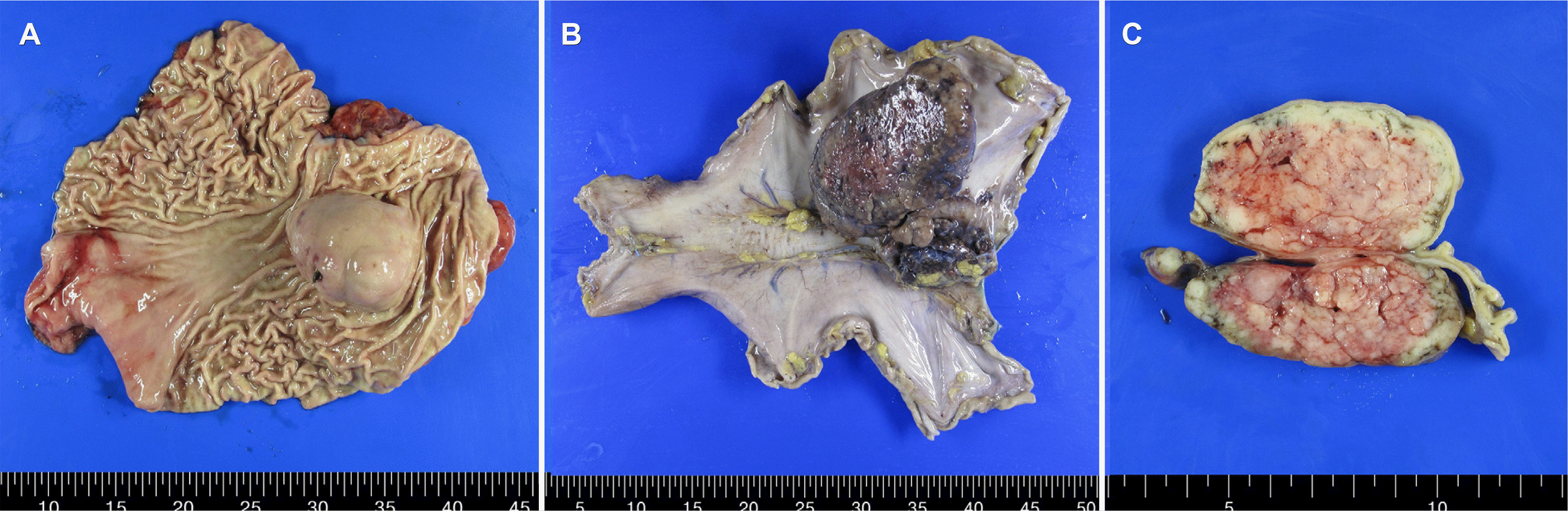
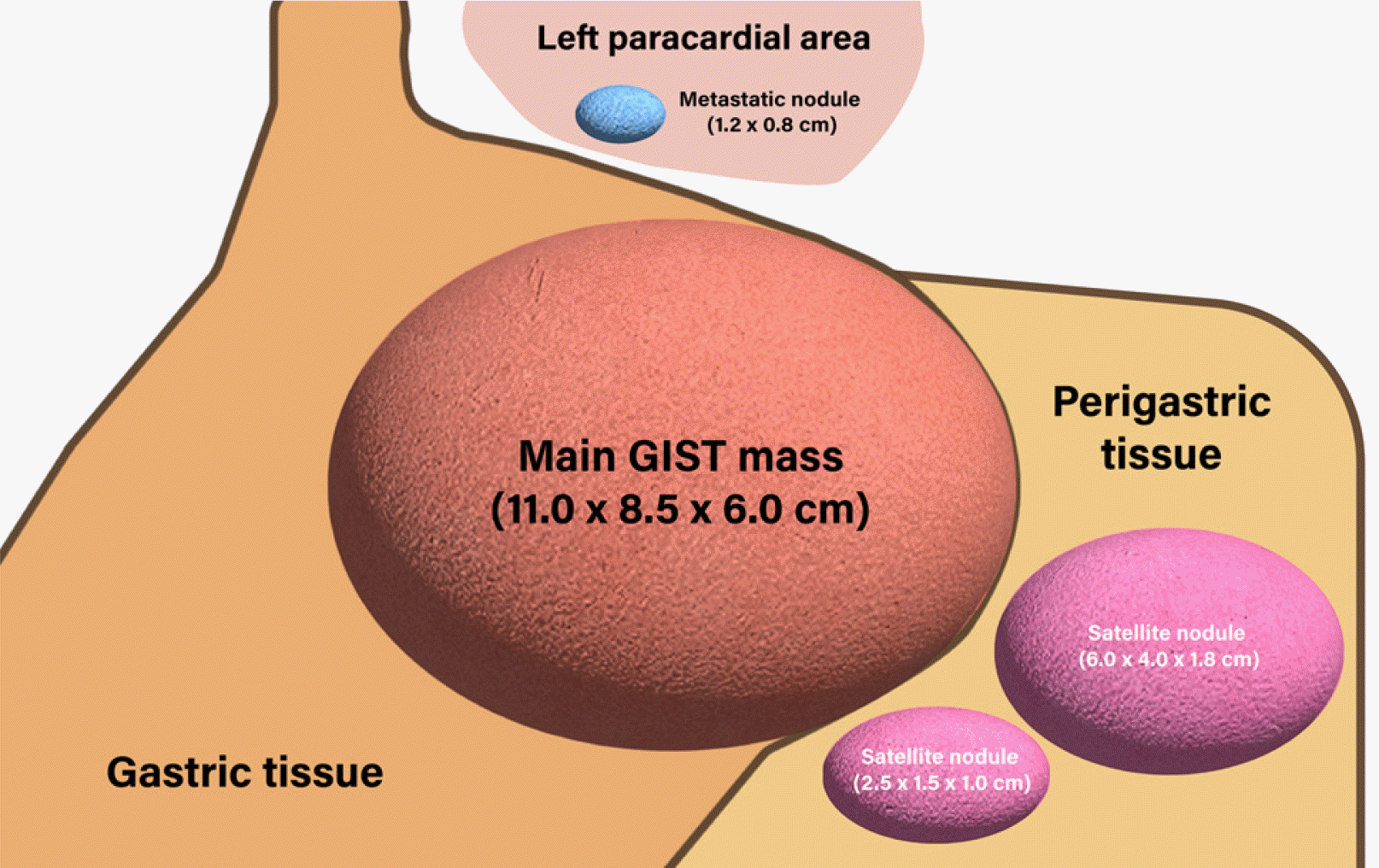
Gross examination revealed a dumbbell-shaped mass with intraluminal and external components in the upper body and posterior wall. The mass measured 11.0×8.5×6.0 cm. Two satellite nodules, measuring 6.0×4.0×1.8 cm and 2.5×1.5×1.0 cm were identified on the serosal surface in the adjacent perigastric tissues (Fig. 5). The cut surface of the mass was well-defined, tan-white, and fleshy. The mass spanned the entire gastric wall thickness and showed small mucosal ulcerations. A frozen biopsy of the resected mass demonstrated features of a malignant GIST. Histopathological analysis revealed that the tumor was composed of cells with epithelioid and spindle morphology arranged in vague fascicles and sheets. The tumor cells showed mild nuclear atypia with a mitotic count of 7 per 5 mm2. The epithelioid cells had round nuclei, abundant eosinophilic cytoplasm (Fig. 3B), and the spindle cells showed elongated nuclei with eosinophilic cytoplasm (Fig. 3C). The tumor invaded the serosal layer. Immunohistochemically, the tumor cells were positive for the receptor tyrosine kinase, c-KIT, discovered on GIST 1 (DOG-1) biomarker, and the human hematopoietic progenitor cell antigen, CD34, but negative for smooth muscle actin and S100 (Fig. 3D). The next-generation sequencing (NGS) test was additionally performed for confirming gene mutations including the KIT gene, PDGFRA gene, and succinate dehydrogenase (SDH) subunits (A, B, C, and D). As a result, the PDGFRA mutation was confirmed, but the rest of the mutations were not confirmed. These findings were consistent with the diagnosis of sporadic GIST. The tumor was completely resected, with no evidence of lymph node metastasis in the 37 regional lymph nodes. However, among the tissues obtained through the lymph node dissection, a metastatic nodule was identified in one of the two tissue samples collected from the left paracardial lymph node site. The nodule was initially suspected to be a lymph node, but the pathological examination showed no findings suggestive of the same; therefore, it was finally confirmed to be a metastatic nodule (Fig. 5).
The patient underwent adjuvant chemotherapy with imatinib after complete resection of the malignant GIST. Scheduled follow-up CT and upper GI endoscopy performed 6 months after surgery demonstrated no evidence of tumor recurrence.
Malignant gastric GIST is a rare type of neoplasm, especially in adults <40 years of age.1,2 Although gastric GISTs are found incidentally in 22-31% of cases, GI bleeding is the most common cause of admission for GIST-related emergencies.4 Upper GI endoscopy and CT are commonly used for GIST diagnosis, and EUS helps to identify the internal features of GIST, distinguish tumor tissue from other subepithelial lesions, and determine lymph node metastasis. However, with ongoing bleeding, immediate intervention through angiography or surgery may be needed.
GISTs are often localized, <5 cm in size, and the incidence of lymph node metastasis is extremely low.6 Therefore, the standard treatment for gastric GISTs is a wedge resection of the tumor. The main goal is to avoid tumor rupture and achieve clear excision margins (R0) with as few post-surgical complications as possible.7 Although lymph node metastasis in GISTs is rare, dissection should be considered when lymph node metastasis is suspected.8 Malignant gastric GISTs can be attached to the spleen or pancreas, but this is often not true infiltration, and excessive attempts to isolate the tumor can lead to tumor rupture or R1 resection.7 Therefore, en bloc resections of attached organs are also considered in attempted curative surgery. For larger GISTs, open surgery and more extensive resection are required. Depending on the size and location of the tumor, total gastrectomy may be required, as in this case. Miettinen and Lasota9 reported that if the gastric GIST is between 5 cm and 10 cm in size and the mitotic rate is >5 mitoses per 5 mm2, the risk of metastasis is 55%. Considering the high possibility of metastasis in tumors of this size, adjuvant chemotherapy with imatinib should be administered.
In the present case of a malignant GIST in a 28-year-old man, a huge exophytic mass over 11 cm was observed on the upper body of the stomach with enlarged regional lymph nodes. Therefore, considering the location and size of the mass and the possibility of lymph node metastasis, it was determined that a wide resection was necessary, and we performed a total gastrectomy with lymph node dissection. Although the mass was in contact with the pancreas and spleen, dissection was successfully performed without tumor rupture, allowing for R0 resection without additional pancreatectomy or splenectomy. If the tumor size or its location does not allow for an R0 surgical excision or is expected to increase post-surgical morbidity, neoadjuvant therapy with imatinib is recommended to reduce the tumor size and facilitate resection.10 Nonetheless, in our case, due to acute GI bleeding accompanied by melena, emergency surgery was chosen over neoadjuvant therapy. Adjuvant chemotherapy with imatinib was administered after surgery because of the mass size, mitotic count, and the presence of a satellite nodule. When observed in the operating room, the additional lesion appeared to be a lymph node, but histological evaluation confirmed that it was a metastatic nodule.
In conclusion, we report a case of a huge malignant gastric GIST, accompanied by spontaneous bleeding and satellite and metastatic nodules in a young patient. The tumor was surgically removed successfully without preoperative chemotherapy and combined adjacent organ resections.
REFERENCES
1. Miettinen M, Lasota J. 2001; Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular g endothelial features and differential diagnosis. Virchows Arch. 438:1–12. DOI: 10.1007/s004280000338. PMID: 11213830.

2. Tran T, Davila JA, El-Serag HB. 2005; The epidemiology of malignant gastrointestinal stromal tumors: an analysis of 1,458 cases from 1992 to 2000. Am J Gastroenterol. 100:162–168. DOI: 10.1111/j.1572-0241.2005.40709.x. PMID: 15654796.

3. Sorour MA, Kassem MI, Ghazal Ael-H, El-Riwini MT, Abu Nasr A. 2014; Gastrointestinal stromal tumors (GIST) related emergencies. Int J Surg. 12:269–280. DOI: 10.1016/j.ijsu.2014.02.004. PMID: 24530605.

4. Pih GY, Jeon SJ, Ahn JY, et al. 2020; Clinical outcomes of upper gastrointestinal bleeding in patients with gastric gastrointestinal stromal tumor. Surg Endosc. 34:696–706. DOI: 10.1007/s00464-019-06816-9. PMID: 31062158.

5. Agaimy A, Wünsch PH. 2009; Lymph node metastasis in gastrointestinal stromal tumours (GIST) occurs preferentially in young patients < or = 40 years: an overview based on our case material and the literature. Langenbecks Arch Surg. 394:375–381. DOI: 10.1007/s00423-008-0449-5. PMID: 19104826.

6. Kosmidis CS, Alexandrou V, Koimtzis GD, et al. 2020; Treatment of a gastrointestinal stromal tumor (GIST) Adherent to the spleen and the tail of the pancreas: a case report. Am J Case Rep. 21:e918278. DOI: 10.12659/AJCR.918278. PMID: 32231176. PMCID: PMC7161921.

7. Kong SH, Yang HK. 2013; Surgical treatment of gastric gastrointestinal stromal tumor. J Gastric Cancer. 13:3–18. DOI: 10.5230/jgc.2013.13.1.3. PMID: 23610714. PMCID: PMC3627804.

8. Canda AE, Ozsoy Y, Nalbant OA, Sagol O. 2008; Gastrointestinal stromal tumor of the stomach with lymph node metastasis. World J Surg Oncol. 6:97. DOI: 10.1186/1477-7819-6-97. PMID: 18775061. PMCID: PMC2553079.

9. Miettinen M, Lasota J. 2013; Gastrointestinal stromal tumors. Gastroenterol Clin North Am. 42:399–415. DOI: 10.1016/j.gtc.2013.01.001. PMID: 23639648. PMCID: PMC3644178.

10. Koo DH, Ryu MH, Kim KM, et al. 2016; Asian consensus guidelines for the diagnosis and management of gastrointestinal stromal tumor. Cancer Res Treat. 48:1155–1166. DOI: 10.4143/crt.2016.187. PMID: 27384163. PMCID: PMC5080813.

Fig. 1
Images of a gastrointestinal stromal tumor captured during endoscopy and endoscopic ultrasound. (A) Endoscopy image showing a 5 cm exophytic mass with central ulceration and spurting bleeding at the posterior wall of the mid-body of the stomach. (B) Endoscopic ultrasound image showing an approximately 7.2×7.3 cm heterogeneous and hypoechoic lesion with a well-circumscribed margin and hypoechoic foci.
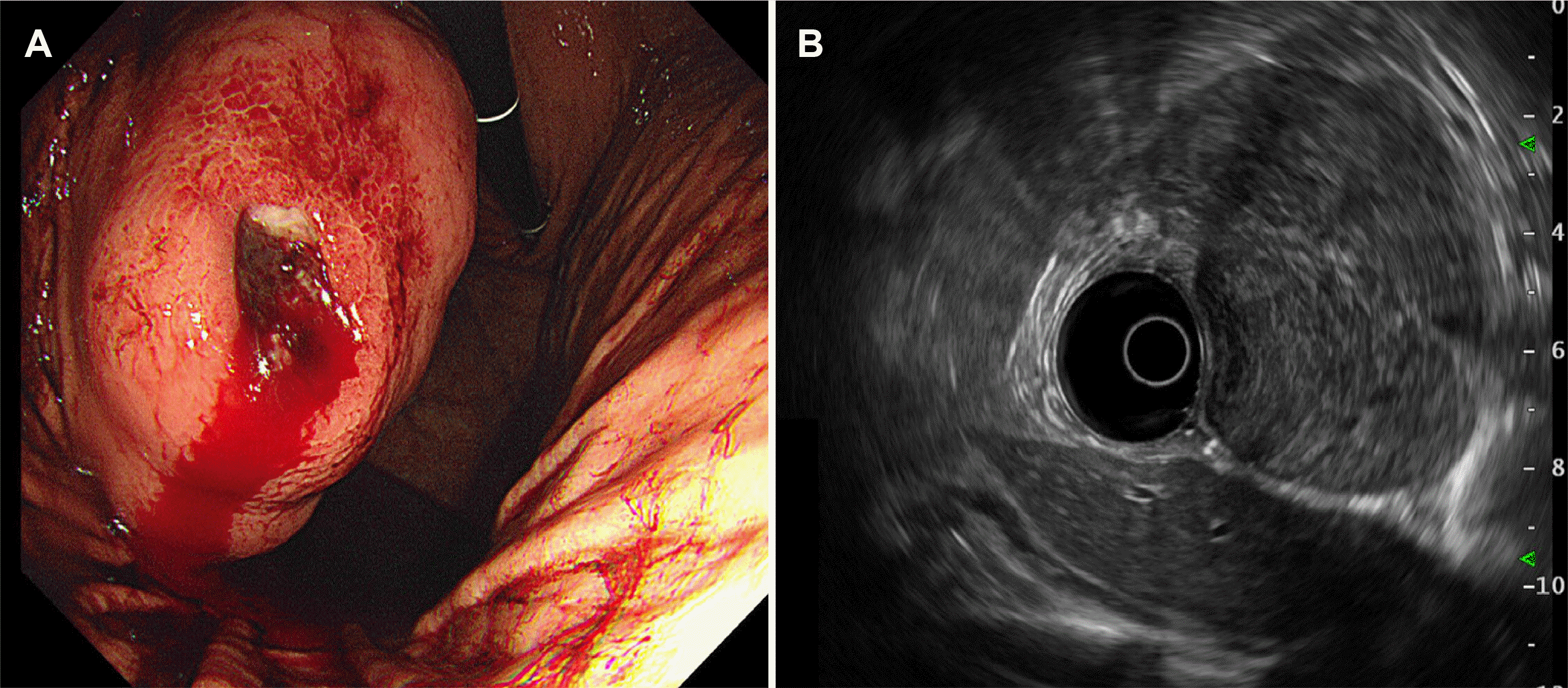
Fig. 2
Computed tomography images of a gastrointestinal stromal tumor. Abdominal and pelvic computed tomography images showing an exophytic mass (M) on the stomach body, abutting the pancreas (P), and spleen (S).
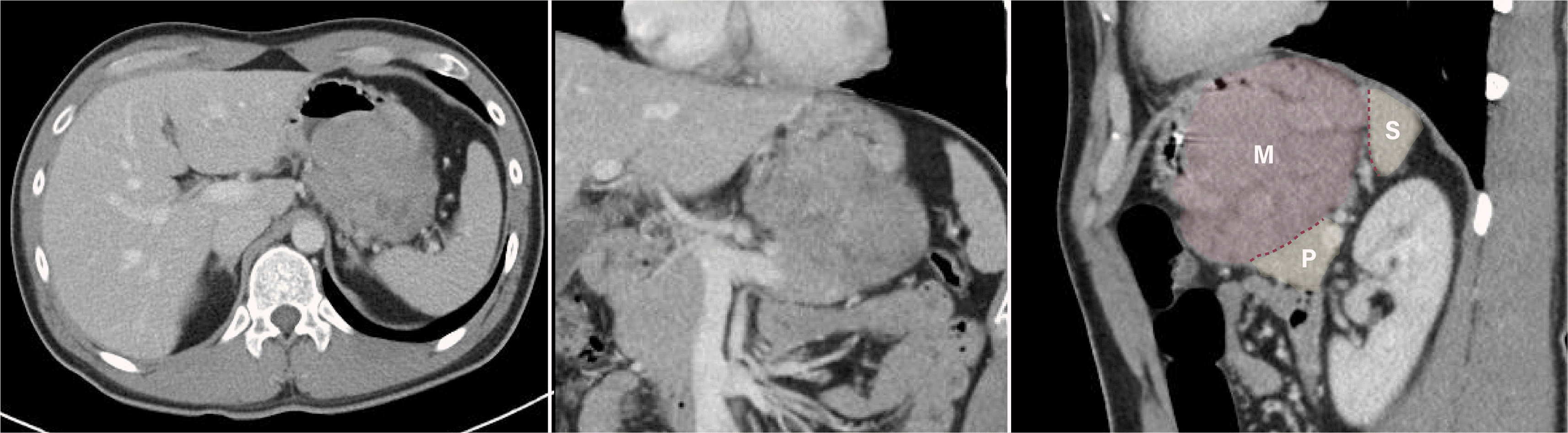
Fig. 3
Surgical and pathology findings. (A) An 11 cm exophytic mass in the upper body of the stomach was observed in the specimen after total gastrectomy. Tumor cells with (B) epithelioid and (C) spindle morphology, arranged in vague fascicles and sheets were observed (hematoxylin and eosin stain [H&E], ×200). (D) Tumor cells, positive for c-KIT (H&E, ×200).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download