INTRODUCTION
SUBJECTS AND METHODS
1. Inhibitory effect of TUDCA on NF-κB signaling in gastric epithelial cells
1) Cell culture and TUDCA administration
2) RNA extraction and real-time reverse transcription-PCR (RT-PCR)
3) Electrophoretic mobility shift assay (EMSA)
4) Immunoblot assay
2. Ethanol-induced gastritis and the effect of pretreatment with TUDCA/UDCA
1) Mice
2) Induction of ethanol-induced gastritis and pretreatment with TUDCA/UDCA
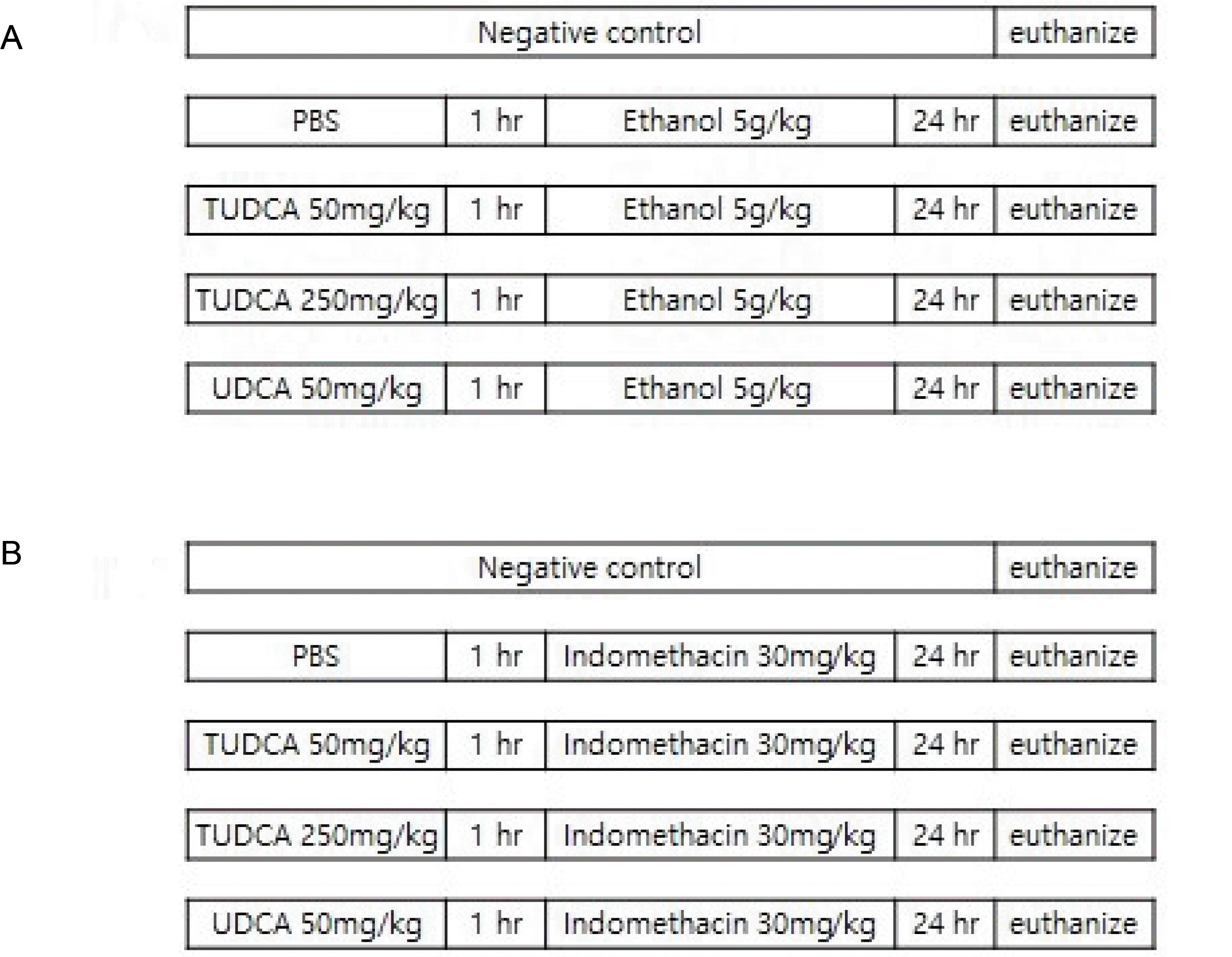 | Fig. 1Experimental protocol. (A) Ethanol-induced gastritis model (n=5 for each group). Group 1, negative control group, received filtered water; Group 2, ethanol (5 g/kg) after a pretreatment with PBS; Group 3, ethanol (5 g/kg) after a pretreatment with TUDCA (50 mg/kg); Group 4, ethanol (5 g/kg) after pretreatment with TUDCA (250 mg/kg); and Group 5, ethanol (5 g/kg) after pretreatment with UDCA (50 mg/kg). TUDCA or UDCA was dissolved in PBS and administered one hour before ethanol administration via oral gavage. (B) Indomethacin-induced gastritis model (n=5 for each group). Group 1, negative control group received filtered water; Group 2, indomethacin (30 mg/kg in 5% NaHCO3) after a pretreatment with PBS; Group 3, indomethacin (30 mg/kg in 5% NaHCO3) after a pretreatment with TUDCA (50 mg/kg); Group 4, indomethacin (30 mg/kg in 5% NaHCO3) after a pretreatment with TUDCA (250 mg/kg); and Group 5, indomethacin (30 mg/kg in 5% NaHCO3) after a pretreatment with UDCA (50 mg/kg). TUDCA or UDCA was dissolved in PBS and administered one hour before indomethacin administration via oral gavage. PBS, phosphate-buffered saline; TUDCA, tauroursodeoxycholic acid; UDCA, ursodeoxycholic acid. |
3) Macroscopic scores
4) Microscopic scores
3. NSAID-induced gastritis and effect of pretreatment with TUDCA/UDCA
1) Mice
2) Induction of indomethacin-induced gastritis and pretreatment with TUDCA/UDCA
3) Macroscopic scores
4) Microscopic scores
4. Statistical analysis
RESULTS
1. Inhibitory effect of TUDCA on NF-κB signaling in gastric epithelial cells
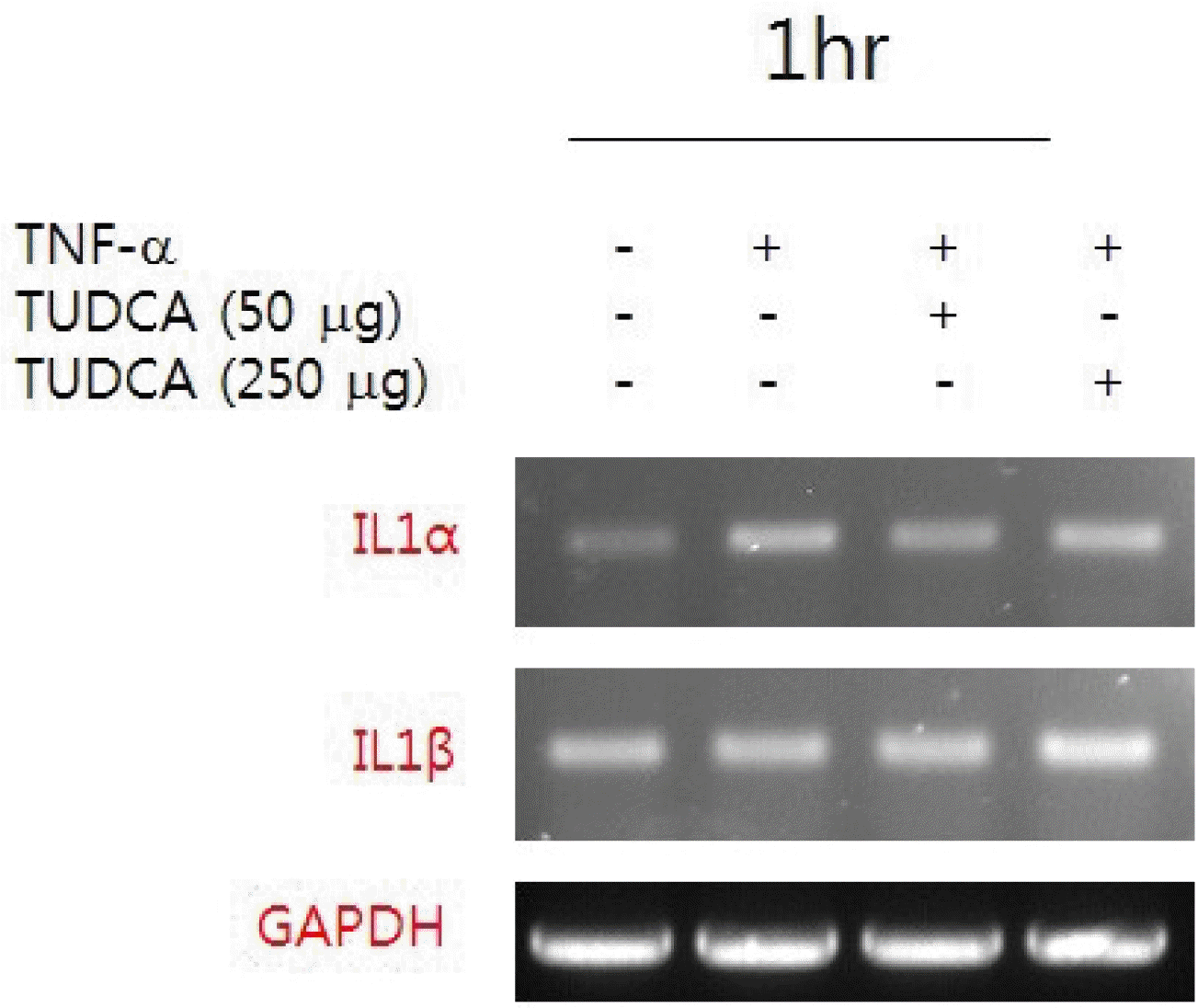
1) RT-PCR
2) EMSA
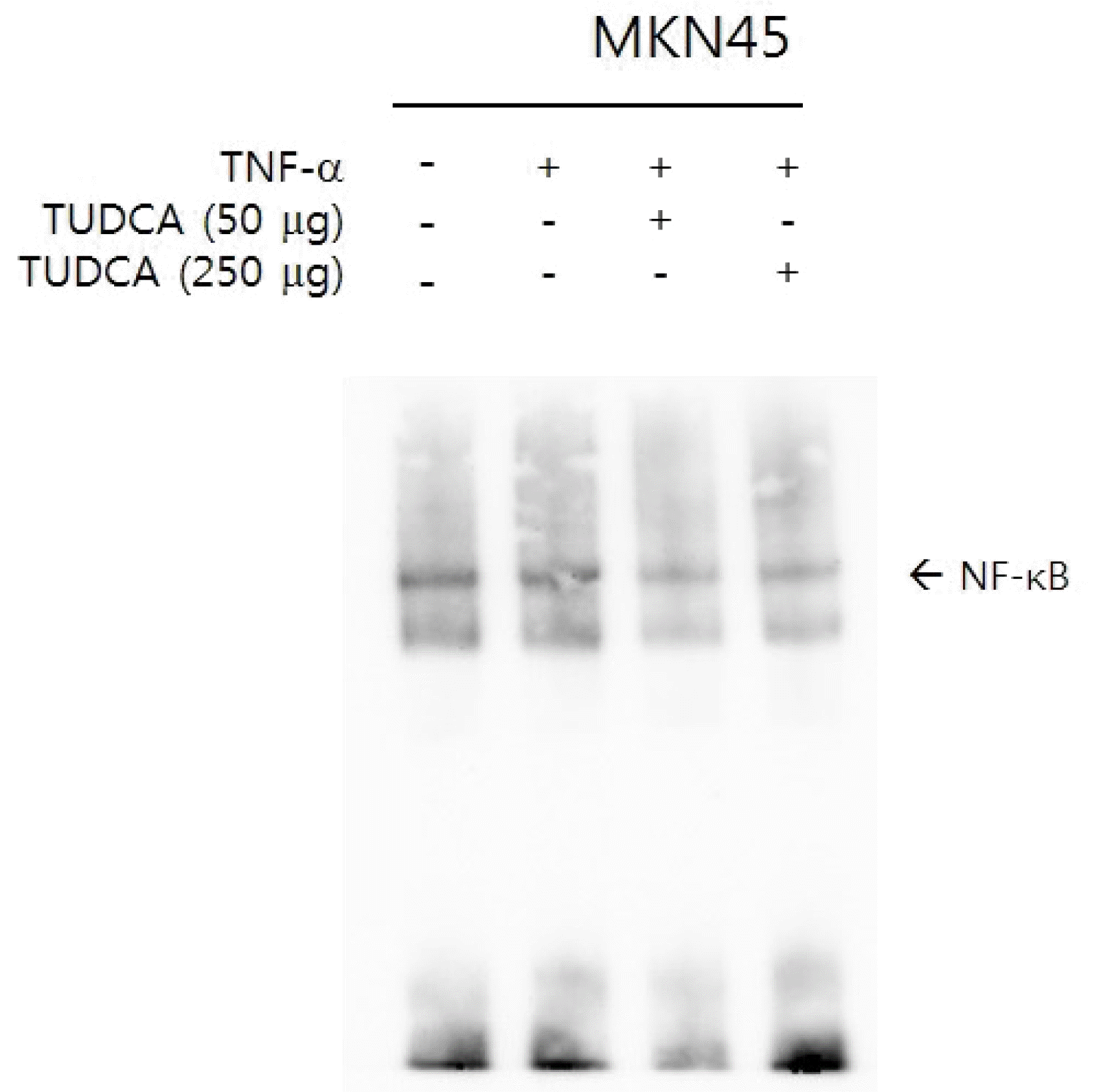
3) Immunoblot assay
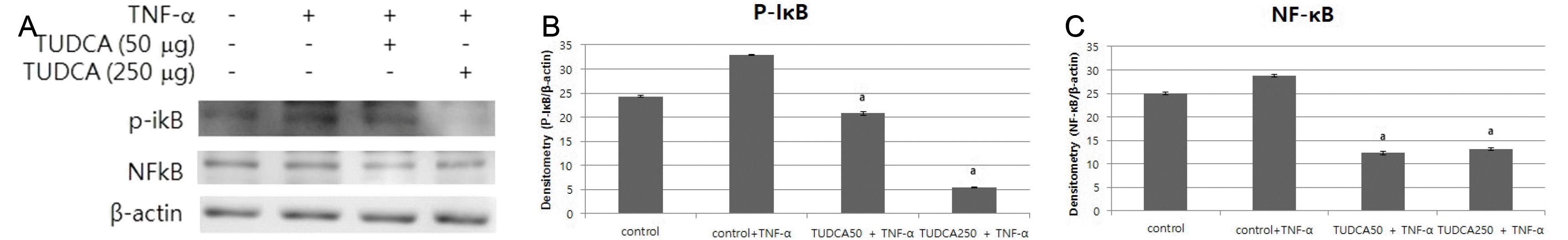 | Fig. 4(A) Pretreatment of MKN-45 cells with various concentrations of TUDCA suppressed TNF-α-induced IκBα phosphorylation. (B) Pretreatment of MKN-45 cells with TUDCA (50 and 250 μg/mL) suppressed IκBα phosphorylation. (C) Pretreatment of MKN-45 cells with TUDCA (50 and 250 μg/mL) suppressed NF-κB signaling. TUDCA, tauroursodeoxycholic acid; TNF, tumor necrosis factor. ap<0.05 compared with TNF-α alone. |
2. Ethanol-induced gastritis and the effect of pretreatment with TUDCA/UDCA
1) Macroscopic scores
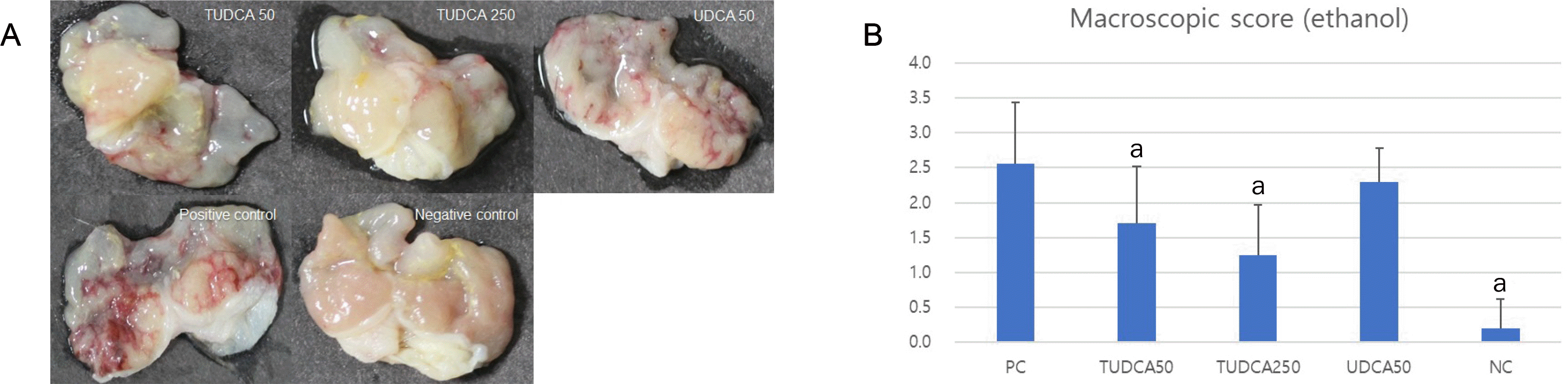 | Fig. 5Pretreatment with 50 and 250 mg/kg TUDCA significantly attenuated the severity of ethanol-induced gastritis. (A) Macroscopic findings of ethanol-induced gastritis. (B) Macroscopic scores of ethanol-induced gastritis. PC, positive control; NC, negative control; TUDCA, tauroursodeoxycholic acid; UDCA, ursodeoxycholic acid; TNF, tumor necrosis factor. ap<0.05 compared with TNF-α alone. |
2) Microscopic scores
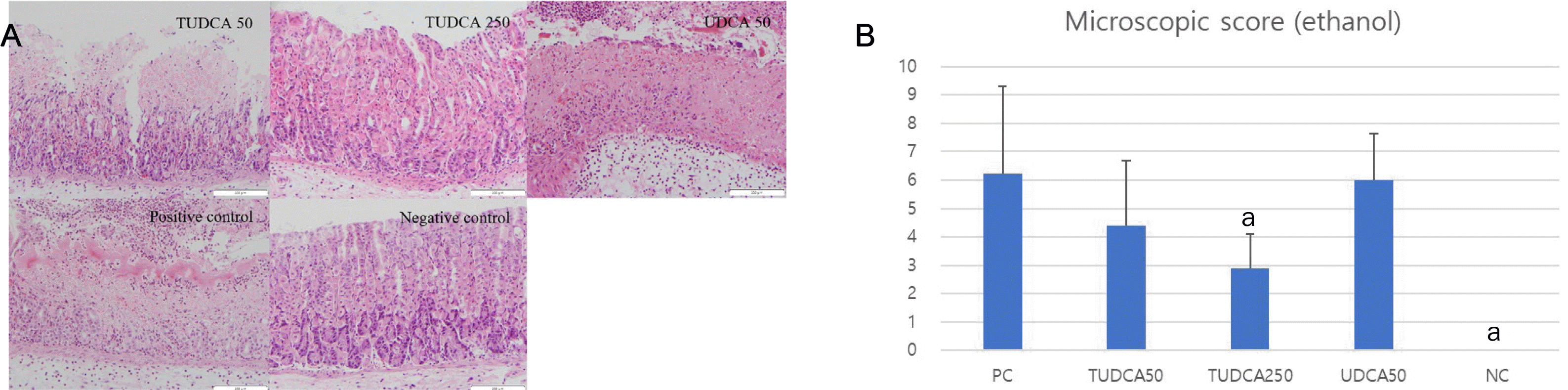 | Fig. 6Pretreatment with 250 mg/kg TUDCA significantly attenuated the severity of ethanol-induced gastritis. (A) Microscopic findings of ethanol-induced gastritis (hematoxylin and eosin, ×200). (B) Microscopic scores of ethanol-induced gastritis. PC, positive control; NC, negative control; TUDCA, tauroursodeoxycholic acid; UDCA, ursodeoxycholic acid; TNF, tumor necrosis factor. ap<0.05 compared with TNF-α alone. |
3. NSAID-induced gastritis and effect of pretreatment with TUDCA/UDCA
1) Macroscopic scores
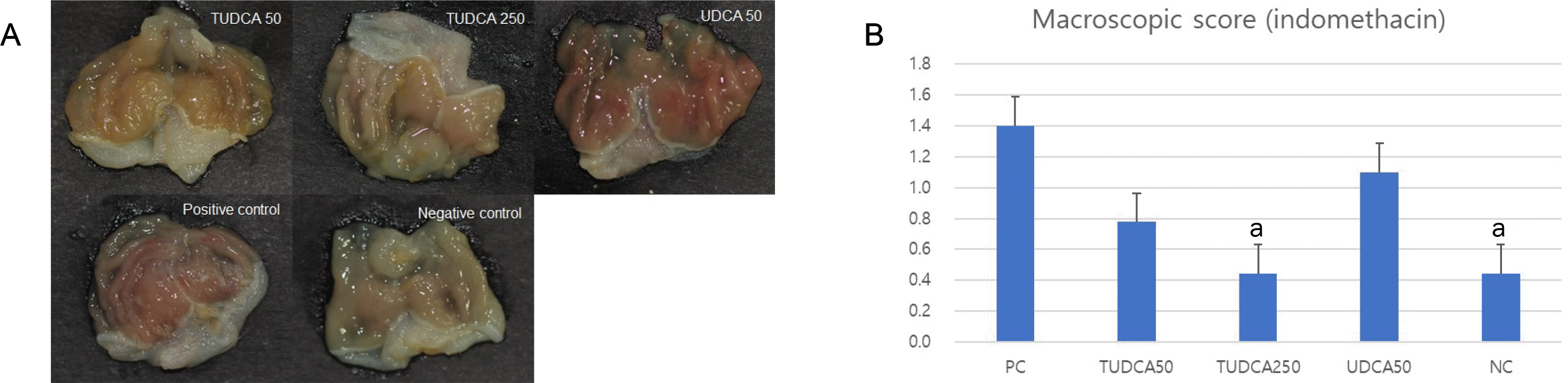 | Fig. 7Pretreatment with 250 mg/kg TUDCA significantly attenuated the severity of indomethacin-induced gastritis. (A) Macroscopic findings of indomethacin-induced gastritis. (B) Macroscopic scores of indomethacin-induced gastritis. PC, positive control; NC, negative control; TUDCA, tauroursodeoxycholic acid; UDCA, ursodeoxycholic acid; TNF, tumor necrosis factor. ap<0.05 compared with TNF-α alone. |
2) Microscopic scores
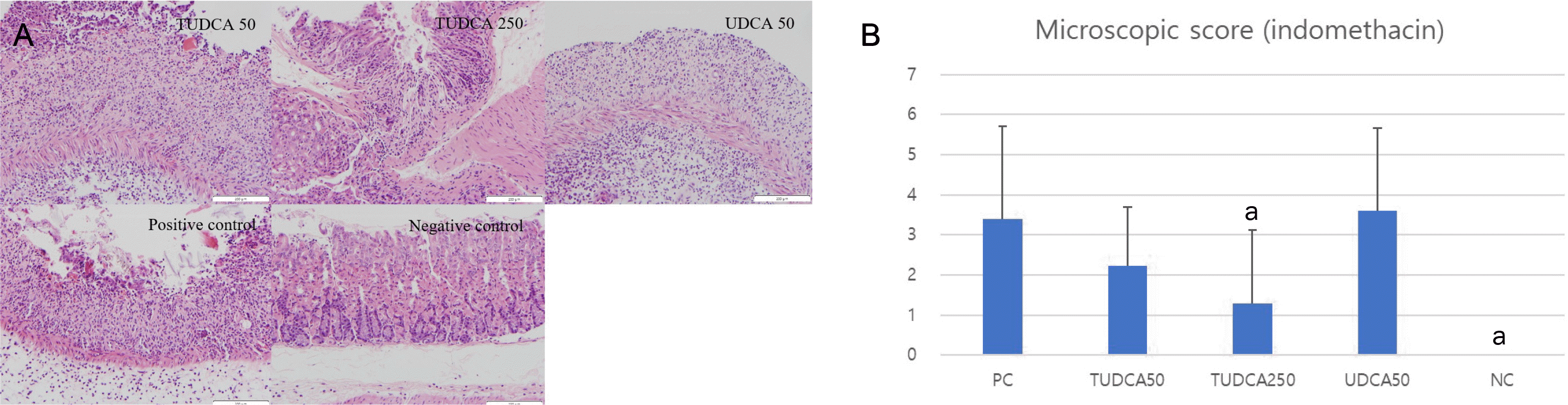 | Fig. 8Pretreatment with 250 mg/kg TUDCA significantly attenuated the severity of indomethacin-induced gastritis. (A) Microscopic findings of indomethacin-induced gastritis (hematoxylin and eosin, ×200). (B) Microscopic scores of indomethacin-induced gastritis. PC, positive control; NC, negative control; TUDCA, tauroursodeoxycholic acid; UDCA, ursodeoxycholic acid; TNF, tumor necrosis factor. ap<0.05 compared with TNF-α alone. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download