INTRODUCTION
METHODS
Culture of NCI-H716 cells
Intracellular Ca2+ measurements
Western blots and immunocytochemistry
Data analysis
RESULTS
Cholinergic stimulation induced cytosolic Ca2+ oscillation
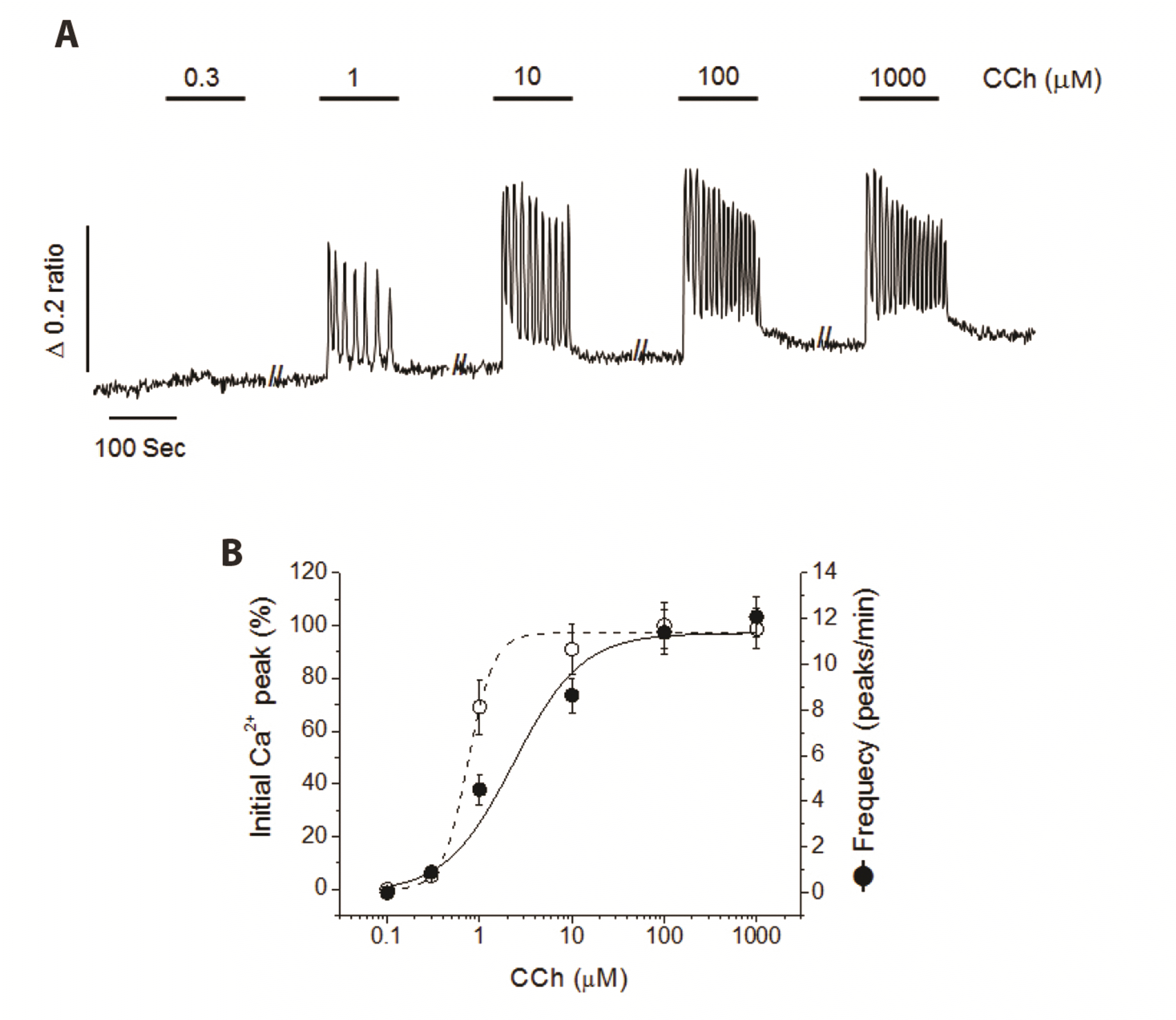 | Fig. 1Dose-dependent Ca2+ oscillation induced by carbamylcholine (CCh) in NCI-H716 cells.(A) Representative cytosolic Ca2+ oscillation obtained from stepwise increase of various concentration of CCh (0.3 μM–1 mM) every 100 sec. (B) CCh-induced initial Ca2+ peak (% of maximum) and frequency of Ca2+ oscillation (peaks/min). Cytosolic Ca2+ measurement was obtained from seven separate experiments in fura-2 loaded NCI-H716 cells. CCh significantly stimulated initial Ca2+ peak and frequency of Ca2+ oscillation, dose-dependently.
|
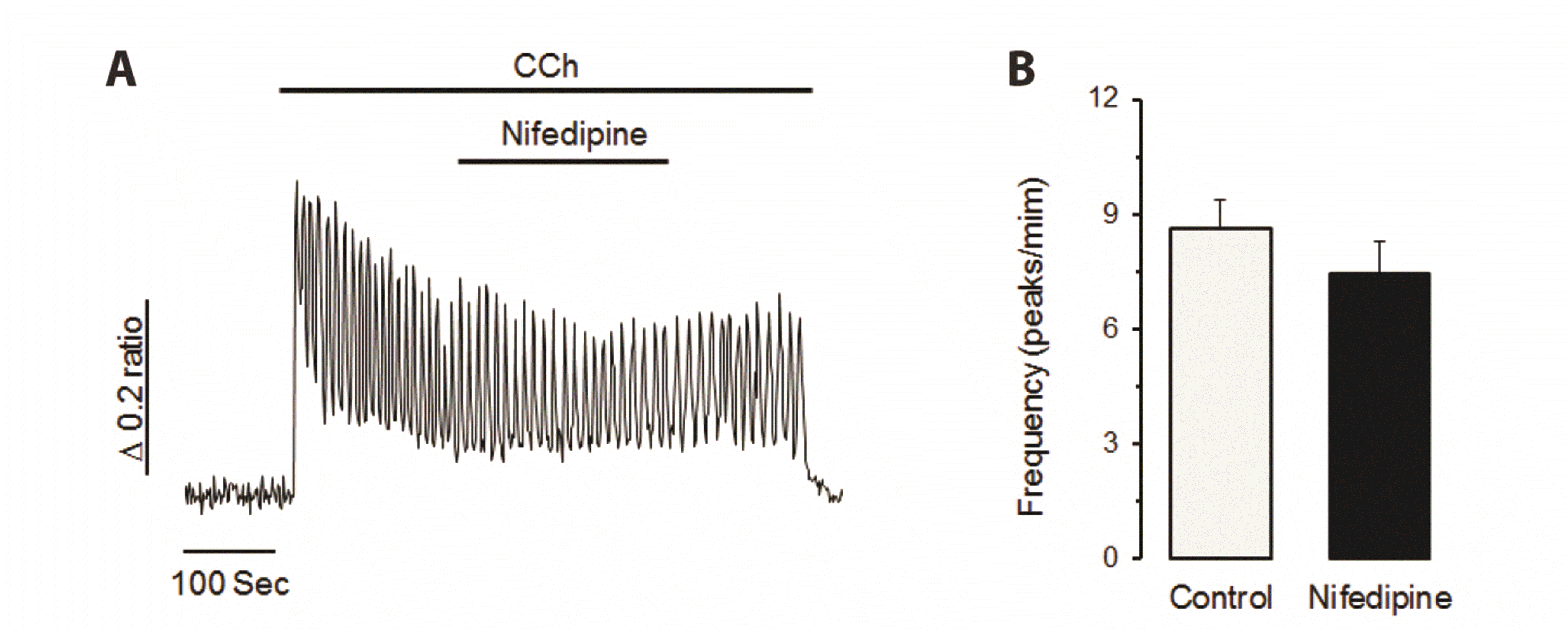 | Fig. 2Voltage-operated Ca2+ channel (VOC) does not contribute to carbamylcholine (CCh)-induced Ca2+ entry process in NCI-H716 cells.(A) Nifedipine, a VOC antagonist, failed to change CCh-induced Ca2+ oscillation in NCI-H716 cells. (B) CCh-induced frequency of Ca2+ oscillation was not changed by nifedipine treatment. Similar results were obtained from six separate experiments in NCI-H716 cells. VOC might not be involved in the calcium influx in CCh-stimulated NCI-H716 cells.
|
Elimination of extracellular Na+ terminates CCh-induced Ca2+ oscillation
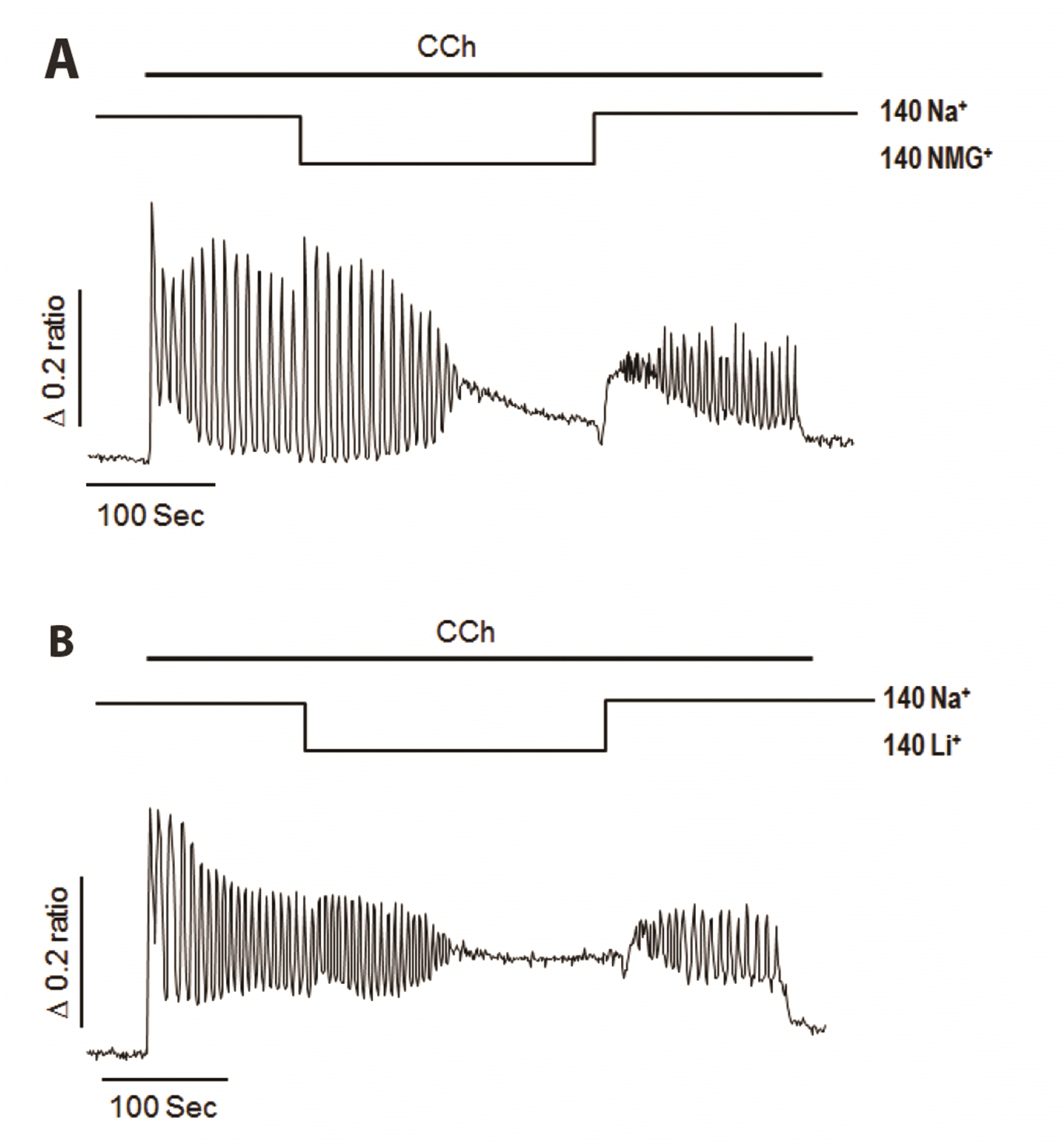 | Fig. 3Effects of extracellular free Na+ on carbamylcholine (CCh)-induced Ca2+ oscillation in NCI-H716 cells.(A) Substitution of Na+ with N-methyl-d-glucamine (NMG+) resulted in cessation of Ca2+ oscillation, which was restored by Na+ reperfusion. (B) Replacing Na+ with Li+ resulted in termination of cytosolic Ca2+ oscillation and restoration of oscillatory Ca2+ signals by Na+ reperfusion. Na+-dependent Ca2+ influx might be important for the generation of oscillatory Ca2+ signaling induced by muscarinic stimulation in stimulus-secretion mechanism of NCI-H716 cells.
|
Effects of KB-R7943 on CCh-induced Ca2+ oscillation
 | Fig. 4Effects of KB-R7943 on carbamylcholine (CCh)-induced Ca2+ oscillation in NCI-H716 cells.(A) KB-R7943 significantly blocked CCh-induced Ca2+ oscillation in NCI-H716 cells. Ca2+ oscillation was restored after cessation of KB-R7943 perfusion. (B) Elimination of extracellular Ca2+ resulted in complete inhibition of CCh-induced Ca2+ oscillation. (C) Pretreatment of KB-R7943 failed to inhibit initial Ca2+ peak induced by CCh. (D) Effect KB-R7943 on CCh-induced initial Ca2+ peak obtained from seven separate experiments. rNCX might contribute to the generation of CCh-induced cytosolic Ca2+ oscillation by modulating Ca2+ influx pathway from extracellular fluid in NCI-H716 cells.
|
Expression of NCX1 proteins in NCI-H716 cells
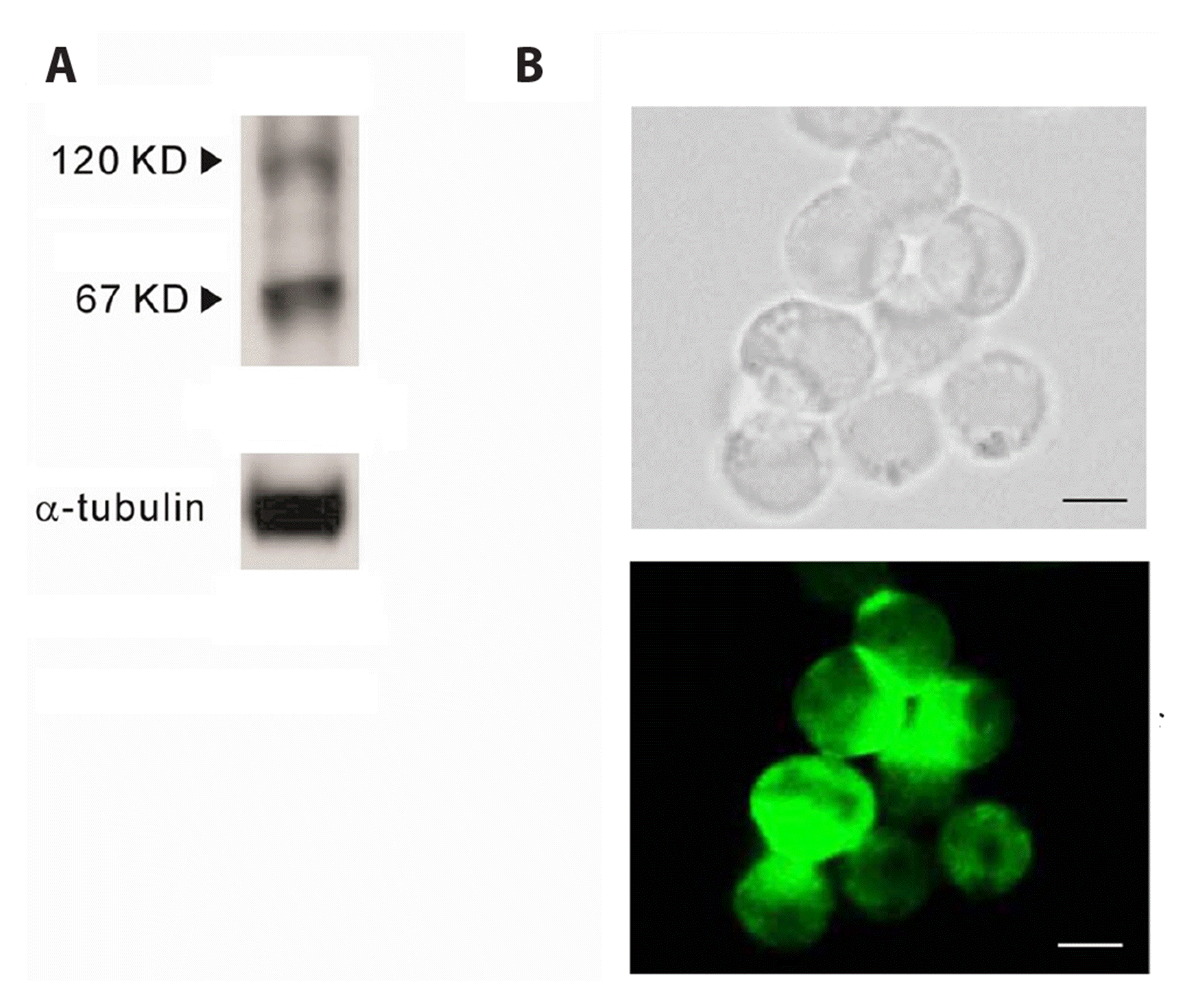 | Fig. 5Type 1 Na+/Ca2+ exchanger (NCX1) protein expression and distribution in NCI-H716 cells.(A) Western blot analysis of NCX1 expression. NCX1 protein was detected in NCI-H716 cells at band of about 120 kDa, and small sized proteolytic product band of 67 kDa was additionally detected. (B) Immunocytochemistry for NCX1 protein expression. Corresponding bright-field (top) and immunofluorescence (bottom) microscopy images showing the labelling of NCX1 protein. NCX1 protein was remarkably expressed on NCI-H716 cells, scale bars = 10 μm.
|




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download