Abstract
Translocation of azurophil granules is pivotal for bactericidal activity of neutrophils, the first-line defense cells against pathogens. Previously, we reported that lysophosphatidylcholine (LPC), an endogenous lipid, enhances bactericidal activity of human neutrophils via increasing translocation of azurophil granules. However, the precise mechanism of LPC-induced azurophil granule translocation was not fully understood. Treatment of neutrophil with LPC significantly increased CD63 (an azurophil granule marker) surface expression. Interestingly, cytochalasin B, an inhibitor of action polymerization, blocked LPC-induced CD63 surface expression. LPC increased F-actin polymerization. LPC-induced CD63 surface expression was inhibited by both a Rho specific inhibitor, Tat-C3 exoenzyme, and a Rho kinase (ROCK) inhibitor, Y27632 which also inhibited LPC-induced F-actin polymerization. LPC induced Rho-GTP activation. NSC23766, a Rac inhibitor, however, did not affect LPC-induced CD63 surface expression. Theses results suggest a novel regulatory mechanism for azurophil granule translocation where LPC induces translocation of azurophil granules via Rho/ROCK/F-actin polymerization pathway.
Neutrophils are the first-line defense against invading pathogens [1,2]. Upon exposure to pathogens, neutrophil granules fuse with the plasma membrane and secrete their contents into the phagosome or extracellular space. However, the release of azurophil granules, which are rich in highly cytotoxic antimicrobial mediators (i.e., myeloperoxidase, cathepsin G, elastase, protease 3, defensin, and azurocidin), is tightly controlled [3,4]. Thus, azurophil granules are the least ready to be secreted among the various neutrophil granules [5]. For example, N-formyl-L-methionyl-L-leucyl-L-phenylalanine (fMLP), a major stimulator for neutrophils, alone cannot induce azurophil granule release. However, in the presence of cytochalasin B (CB), an inhibitor of actin polymerization, fMLP markedly increases azurophil granule release [6,7].
In various cell types, small G proteins are known to be key regulators of cytoskeletal remodeling [8]. Among them, the Rho-subfamily of GTPases such as Rho, Rac, and Cdc42, are important regulators of cytoskeletal dynamics, which are required for cell shape change, chemotaxis, phagocytosis, and granule translocation in leukocytes [9-14]. Importantly, Rac2 is critical for fMLP-induced enhancement of azurophil granule translocation in CB-treated neutrophils [15-17]. Recently, another small GTPase Rab27a was reported to regulate exocytosis of azurophil granules in neutrophils [18].
Lysophosphatidylcholine (LPC), an endogenous lipid, has various stimulating or modulating activities in neutrophils [19-26]. Previously, we showed that LPC increases bactericidal activity in human neutrophils by enhancement of azurophil granule-phagosome fusion via calcium/p38 MAPK dependent azurophil granule translocation [27]. However, the involvement of a small GTPase in the LPC-induced azurophil granule translocation has not yet been investigated. In the present study, we found that Rho, but not Rac2, is critical for LPC-induced enhancement azurophil granule translocation via Rho kinase (ROCK)/fibrous (F)-actin polymerization, elucidating a novel signaling pathway for azurophil granule translocation.
LPC (18:0) was obtained from Avanti Polar Lipids (Alabaster, AL, USA), and 3 mM stock solution in phosphate-buffered saline (PBS) was prepared with sonication. NSC23766, SB203580, and Y27632 were from Tocris Bioscience (Ellisville, MO, USA). Cytofix, Cytofix/Cytoperm, and FITC anti-CD63 Ab were from BD Bioscience (San Diego, CA, USA). FITC-conjugated phalloidin and CB were from Sigma-Aldrich Chemical (St. Louis, MO, USA). Fluo-3 AM was from Molecular Probes (San Diego, CA, USA). Rho activation assay kit was from Upstate (Billerica, MA, USA). Tat-C3 (a fusion protein between Tat-peptide and C3 toxin) was purified from bacteria containing the Tat-C3 cDNA plasmid as previously described [28]. Anti-G2A Ab (N-20) was from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Neutrophils were purified from healthy volunteer donors. In brief, whole blood was layered onto a discontinuous Ficoll-Hypaque density gradient (Histopaque 1077; Sigma-Aldrich) and centrifuged at 2,500 rpm for 30 min. After dextran sedimentation, residual erythrocytes were eliminated with hypotonic lysis. The purity of neutrophils measured by Diff-Quick staining was > 95% on average. The viability of neutrophils stained with tryphan blue was > 99% as determined by morphology.
For the analysis of azurophil granule translocation, CD63, an azurophil granule marker, was used. Isolated neutrophils were stabilized for 30 min and stimulated with LPC (3 μM) for 15 min. Cells were fixed with Cytofix and washed. Then cells were stained with FITC-conjugated anti-CD63 Ab for 1 h on ice. For analysis of F-actin polymerization, neutrophils were fixed and permeabilized with Cytofix/Cytoperm for 30 min. After washing, neutrophils were stained with FITC-conjugated phalloidin, an F-actin marker, for 1 h on ice. Neutrophils were acquired on FACSCalibur (BD Bioscience) and were analyzed using Cell Quest Pro 6.0 (BD Bioscience) and FCS express V3 (De Novo Software, Los Angeles, CA, USA).
Neutrophils were resuspended in RPMI1640 and stimulated with LPC (3 μM) for 15 min. Cells were lysed in NP40 lysis buffer to detect Rho activity with Rho activation assay kit according to the manufacturer’s protocol. In brief, cells were lysed in NP-40 lysis buffer and the target proteins were loaded to 30 μl of the Rho-assay reagent slurry. The reaction mixtures were incubated for 45 min at 4°C with gentle agitation. Agarose beads were pelleted by brief centrifugation and 20 μl of the mixture per lane was loaded on an appropriate polyacrylamide gel. Membrane was incubated overnight at 4°C with agitation. The proteins were detected by enhanced chemiluminescence detection system.
Neutrophils were transferred to 12-well plate and stimulated with LPC (3 μM). Then cells were fixed using 2% paraformaldehyde for 15 min and permeabilized using 0.05% Triton X-100 for 10 min at room temperature. Next, cells were blocked with 3% bovine serum albumin and incubated for 1 h. After incubation, neutrophils were washed three times and stained with FITC-conjugated phalloidin. After three washes in PBS, cells were mounted using mounting medium. Visual inspection and recording of images were performed using a Zeiss confocal microscope (Carl Zeiss, Thornwood, NY, USA).
[Ca2+]i was measured using Fluo-3 AM as previously described [25]. Neutrophils were loaded with Fluo-3 AM (4 μM) in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) physiologic salt solution (HEPES-PSS) (in mM) (HEPES 10, NaCl 140, KCl 5, CaCl2 1, D-glucose 10, MgCl2 1) for 1 h at 37°C. Neutrophils were washed twice with HEPES-PSS and resuspended in HEPES-PSS at 3 × 106 cells/ml. Traces of [Ca2+]i in Fluo-3 AM-loaded PMNs were measured with 490 nm/526 nm using Spectramax M2/e microplate reader (Molecular Device, Sunnyvale, CA, USA) every 10 sec. Raw fluorescence was subtracted with the average fluorescence during 5 min before the addition of LPC (3 μM). Cells were pretreated with Y27632 (10 μM) for 30 min before the addition of LPC.
All the statistical data were analyzed with Graphpad Prism 5.0 (Graphpad software). Data were expressed as mean ± SEM and statistical significance was evaluated by two-tailed Student’s t-test or ANOVA. Bonferroni test was used for post-hoc comparison. Value of p < 0.05 was considered to indicate statistical significance.
CD63 is a representative marker for azurophil granules, which translocates into the plasma membrane as azurophil granules degranulate [29]. Previously, we showed that LPC induces translocation of CD63 in human neutrophils in RPMI medium supplemented with 5% fetal bovine serum [27]. Although this condition mimics the in vivo condition, the various constituents of fetal bovine serum may affect LPC-induced signaling cascades. Thus, in this study we examined the effects of LPC in the serum-free condition. LPC increased surface expression of CD63 in a time- (Fig. 1A) and concentration- (Fig. 1B) dependent manner in serum-free condition; the LPC-induced CD63 surface expression peaked at 15 min and at 3 μM. This LPC-induced CD63 surface expression in the serum-free condition is comparable to that observed in serum-containing condition [27].
Contrarily, fMLP (1 μM) alone expectedly could not induce surface expression of CD63 (Fig. 2A). However, in the presence of CB (2.5 μg/ml, i.e., 5.2 μM) that by itself did not affect CD63 surface expression, fMLP markedly increased surface expression of CD63 (Fig. 2A, B). Intriguingly, however, CB pretreatment blocked LPC-induced CD63 surface expression (Fig. 2A, B). This result suggests that LPC enhances CD63 surface expression by induction of F-actin polymerization in human neutrophils. LPC increased F-actin polymerization as shown by increased staining of FITC-conjugated phalloidin, a marker of F-actin (Fig. 2C). These results indicate that LPC induces CD63 translocation via F-actin polymerization in human neutrophils. It is to be noted that while fMLP in the presence of CB increases mean fluorescence intensity (MFI) by a shift of the whole distribution to the right, LPC increases MFI with making the distribution more bimodal by increasing the proportion of cells with the highest values (Fig. 2A).
To examine whether Rac2 is involved in LPC-induced azurophil granule translocation, we investigated the effect of NSC23766, an inhibitor of Rac, on LPC-induced CD63 surface expression. Interestingly, NSC23766 (50 μM) did not affect LPC-induced translocation of azurophil granules (Fig. 3A), however, Y27632 (10 μM), a ROCK inhibitor, effectively blocked the LPC-induced CD63 surface expression (Fig. 3B). Tat-C3 exoenzyme, a specific inhibitor of Rho, also effectively inhibited LPC-induced CD63 surface expression (Fig. 3C). LPC (3 μM) markedly increased Rho-GTP activity in human neutrophils at 15 min (Fig. 3D). These results indicate that LPC induces CD63 surface expression via Rho/ROCK signaling.
To examine whether inhibition of ROCK blocks LPC-induced F-actin polymerization, neutrophils were pretreated with Y27632 (10 μM) before LPC treatment and stained with FITC-conjugated phalloidin. Y27632 blocked LPC-induced F-actin polymerization (Fig. 4A). Next, we performed confocal microscopy analysis for the inhibitory effect of Y27632 on LPC-induced F-actin polymerization. Resting neutrophils have cortical F-actin staining in a ring-like structure [30], as confirmed in Fig. 4B, left. Treatment with LPC (3 μM) for 15 min enhanced F-actin staining in the cytosol of neutrophils (Fig. 4B, middle). Remarkably, Y27632 blocked the LPC-induced enhancement of cytosolic actin polymerization (Fig. 4B, right). These results indicate that Rho/ROCK signaling is involved in LPC-induced F-actin polymerization, resulting in increased CD63 surface expression.
G2A, a G protein-coupled receptor abundantly expressed in immune cells, is reportedly involved in various actions of LPC in neutrophils [20,21,24,27]. To examine the involvement of G2A in LPC-induced CD63 surface expression, neutrophils were pretreated with anti-G2A Ab (1 μg/ml) for 1 h and further stimulated with LPC (3 μM) for 15 min. Anti-G2A Ab blocked the LPC-induced CD63 surface expression (Fig. 5A), suggesting that LPC induces azurophil granule translocation via G2A.
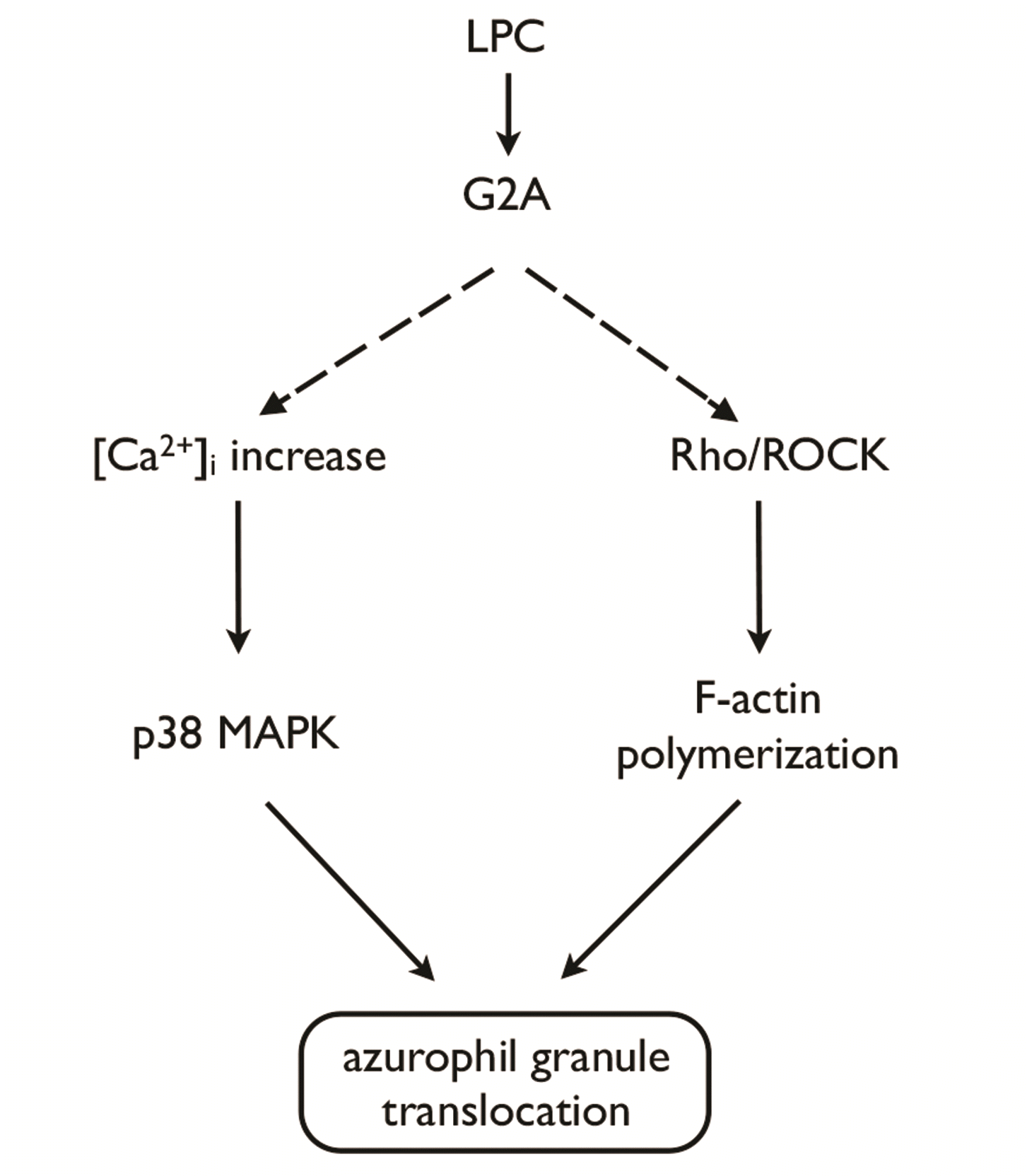
Previously we reported that Ca2+/p38 MAPK signaling pathway is involved in LPC-induced enhancement of azurophil granule-phagosome fusion in human neutrophils [27]. Similarly, to our previous observation in serum-containing condition [27], pretreatment with SB203580 (10 μM), an inhibitor of p38 MAPK, completely inhibited LPC-induced CD63 surface expression (Fig. 5B). However, it did not affect the LPC-induced increase in F-actin polymerization found in this study (Fig. 5C). Reciprocally, Y27632 (10 μM) did not affect LPC-induced [Ca2+]i increase (Fig. 5D). These results suggest that LPC induces azurophil granule translocation via 2 complementary, but distinct signaling pathways, i.e., Rho/ROCK/F-actin polymerization and Ca2+/p38 MAPK pathways (Fig. 6).
In this study, we found a novel mechanism for azurophil granule translocation in human neutrophils, wherein LPC induces azurophil granule translocation through G2A/Rho/ROCK/F-actin polymerization, complementing the previously identified LPC-induced [Ca2+]i increase/p38 MAPK activation pathway (Fig. 6). Interestingly, there were several differences between LPC and fMLP in their mode of action for increasing azurophil granule translocation. LPC alone (in the absence of CB) effectively increased azurophil granule translocation (Fig. 2A). In sharp contrast to the case of fMLP (Fig. 2A, B; [6,15]), CB effectively blocked LPC-induced azurophil granule translocation rather than enhancing it (Fig. 2A, B). LPC induced enhancement of F-actin polymerization in human neutrophils (Fig. 2C), which was cytosolic as shown in confocal microscopy (Fig. 4B). FMLP also reportedly increases azurophil granule translocation in the presence of CB via cytosolic F-actin polymerization in human neutrophils [30]. Importantly, Rac2 critically mediates F-actin polymerization for the translocation of azurophil granules to the plasma membrane in human neutrophils in response to stimulation with fMLP [30]. Intriguingly, in another sharp contrast to fMLP [15,17], LPC-induced azurophil granule translocation was critically dependent on Rho/ROCK, but not Rac2, signaling pathway (Fig. 3). To our knowledge, this is the first report to implicate the importance of Rho in azurophil granule translocation in human neutrophils.
G2A, activated by LPC, is linked to Gαs [22,31], Gαi [20,21], Gαq [21,32], and Gα13 [31-33]. For LPC-induced azurophil granule translocation, two distinct complementary pathways are essential (Fig. 6). One is the previously described calcium-dependent p38 MAPK pathway [27] and the other is Rho/ROCK/F-actin polymerization pathway identified in this study. These two pathways are dependent on G2A ([27]; Fig. 5A). Although we did not investigate the identity of specific heterotrimeric G-proteins involved in each pathway, two different heterotrimeric G- proteins could be involved downstream of G2A. For calcium-dependent p38 MAPK pathway, we previously showed that pertussis toxin, an inhibitor of Gαi, blocks LPC-induced increase in Cl– influx which subsequently leads to [Ca2+]i increase in human neutrophils [27], in line with the previous reports [20,23]. Further, both Gαi and Gαq was recently shown to be involved in LPC-induced increase in [Ca2+]i increase in human neutrophils [21]. On the other hand, because Gα13 was reported to be involved in G2A-mediated activation of RhoA-dependent actin remodeling in fibroblast cells [33], Gα13 could be involved in LPC-induced Rho/ROCK/F-actin polymerization.
Collectively, we found a novel signaling pathway for azurophil granule translocation in human neutrophils, i.e., G2A/RhoA/ROCK for LPC, in addition to the well-established Rac2 pathway for fMLP, IL-8 or leukotriene B4 [15], suggesting the presence of distinct small GTPase pathways for azurophil granule translocation depending on different stimulators. Further, the results of this study could contribute to better understanding of LPC-induced enhancement of neutrophil bactericidal activity [27] in relation to its anti-septic action [24].
REFERENCES
1. Segal AW. 2005; How neutrophils kill microbes. Annu Rev Immunol. 23:197–223. DOI: 10.1146/annurev.immunol.23.021704.115653. PMID: 15771570. PMCID: PMC2092448.

2. Nauseef WM. 2007; How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev. 219:88–102. DOI: 10.1111/j.1600-065X.2007.00550.x. PMID: 17850484.

3. Borregaard N, Cowland JB. 1997; Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 89:3503–3521. DOI: 10.1182/blood.V89.10.3503.3503_3503_3521. PMID: 9160655.

4. Häger M, Cowland JB, Borregaard N. 2010; Neutrophil granules in health and disease. J Intern Med. 268:25–34. DOI: 10.1111/j.1365-2796.2010.02237.x. PMID: 20497300.

5. Lew PD, Monod A, Waldvogel FA, Dewald B, Baggiolini M, Pozzan T. 1986; Quantitative analysis of the cytosolic free calcium dependency of exocytosis from three subcellular compartments in intact human neutrophils. J Cell Biol. 102:2197–2204. DOI: 10.1083/jcb.102.6.2197. PMID: 3011810. PMCID: PMC2114244.

6. Bentwood BJ, Henson PM. 1980; The sequential release of granule constitutents from human neutrophils. J Immunol. 124:855–862. PMID: 6153206.
7. Jog NR, Rane MJ, Lominadze G, Luerman GC, Ward RA, McLeish KR. 2007; The actin cytoskeleton regulates exocytosis of all neutrophil granule subsets. Am J Physiol Cell Physiol. 292:C1690–C1700. DOI: 10.1152/ajpcell.00384.2006. PMID: 17202227.

8. Takai Y, Sasaki T, Matozaki T. 2001; Small GTP-binding proteins. Physiol Rev. 81:153–208. DOI: 10.1152/physrev.2001.81.1.153. PMID: 11152757.

9. Ridley AJ. 2001; Rho proteins: linking signaling with membrane trafficking. Traffic. 2:303–310. DOI: 10.1034/j.1600-0854.2001.002005303.x. PMID: 11350626.

10. Wheeler AP, Ridley AJ. 2004; Why three Rho proteins? RhoA, RhoB, RhoC, and cell motility. Exp Cell Res. 301:43–49. DOI: 10.1016/j.yexcr.2004.08.012. PMID: 15501444.

11. Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. 2003; Cell migration: integrating signals from front to back. Science. 302:1704–1709. DOI: 10.1126/science.1092053. PMID: 14657486.

12. Spiering D, Hodgson L. 2011; Dynamics of the Rho-family small GTPases in actin regulation and motility. Cell Adh Migr. 5:170–180. DOI: 10.4161/cam.5.2.14403. PMID: 21178402. PMCID: PMC3084983.

13. Guan X, Guan X, Dong C, Jiao Z. 2020; Rho GTPases and related signaling complexes in cell migration and invasion. Exp Cell Res. 388:111824. DOI: 10.1016/j.yexcr.2020.111824. PMID: 31926148.

14. Fessler MB, Arndt PG, Just I, Nick JA, Malcolm KC, Worthen GS. 2007; Dual role for RhoA in suppression and induction of cytokines in the human neutrophil. Blood. 109:1248–1256. DOI: 10.1182/blood-2006-03-012898. PMID: 17018860. PMCID: PMC1785129.

15. Abdel-Latif D, Steward M, Macdonald DL, Francis GA, Dinauer MC, Lacy P. 2004; Rac2 is critical for neutrophil primary granule exocytosis. Blood. 104:832–839. DOI: 10.1182/blood-2003-07-2624. PMID: 15073033.

16. Lacy P, Eitzen G. 2008; Control of granule exocytosis in neutrophils. Front Biosci. 13:5559–5570. DOI: 10.2741/3099. PMID: 18508605.

17. Eitzen G, Lo AN, Mitchell T, Kim JD, Chao DV, Lacy P. 2011; Proteomic analysis of secretagogue-stimulated neutrophils implicates a role for actin and actin-interacting proteins in Rac2-mediated granule exocytosis. Proteome Sci. 9:70. DOI: 10.1186/1477-5956-9-70. PMID: 22081935. PMCID: PMC3379032.

18. Johnson JL, Brzezinska AA, Tolmachova T, Munafo DB, Ellis BA, Seabra MC, Hong H, Catz SD. 2010; Rab27a and Rab27b regulate neutrophil azurophilic granule exocytosis and NADPH oxidase activity by independent mechanisms. Traffic. 11:533–547. DOI: 10.1111/j.1600-0854.2009.01029.x. PMID: 20028487. PMCID: PMC2937183.

19. Englberger W, Bitter-Suermann D, Hadding U. 1987; Influence of lysophospholipids and PAF on the oxidative burst of PMNL. Int J Immunopharmacol. 9:275–282. DOI: 10.1016/0192-0561(87)90051-8. PMID: 3038761.

20. Frasch SC, Zemski-Berry K, Murphy RC, Borregaard N, Henson PM, Bratton DL. 2007; Lysophospholipids of different classes mobilize neutrophil secretory vesicles and induce redundant signaling through G2A. J Immunol. 178:6540–6548. DOI: 10.4049/jimmunol.178.10.6540. PMID: 17475884.

21. Khan SY, McLaughlin NJ, Kelher MR, Eckels P, Gamboni-Robertson F, Banerjee A, Silliman CC. 2010; Lysophosphatidylcholines activate G2A inducing Gαi-1-/Gαq/₁₁- Ca2+ flux, Gβγ-Hck activation and clathrin/β-arrestin-1/GRK6 recruitment in PMNs. Biochem J. 432:35–45. DOI: 10.1042/BJ20091087. PMID: 20799926. PMCID: PMC3131183.

22. Lin P, Welch EJ, Gao XP, Malik AB, Ye RD. 2005; Lysophosphatidylcholine modulates neutrophil oxidant production through elevation of cyclic AMP. J Immunol. 174:2981–2989. DOI: 10.4049/jimmunol.174.5.2981. PMID: 15728511.

23. Silliman CC, Elzi DJ, Ambruso DR, Musters RJ, Hamiel C, Harbeck RJ, Paterson AJ, Bjornsen AJ, Wyman TH, Kelher M, England KM, McLaughlin-Malaxecheberria N, Barnett CC, Aiboshi J, Bannerjee A. 2003; Lysophosphatidylcholines prime the NADPH oxidase and stimulate multiple neutrophil functions through changes in cytosolic calcium. J Leukoc Biol. 73:511–524. DOI: 10.1189/jlb.0402179. PMID: 12660226.

24. Yan JJ, Jung JS, Lee JE, Lee J, Huh SO, Kim HS, Jung KC, Cho JY, Nam JS, Suh HW, Kim YH, Song DK. 2004; Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nat Med. 10:161–167. DOI: 10.1038/nm989. PMID: 14716308.

25. Kelher MR, McLaughlin NJ, Banerjee A, Elzi DJ, Gamboni F, Khan SY, Meng X, Mitra S, Silliman CC. 2017; LysoPCs induce Hck- and PKCδ-mediated activation of PKCγ causing p47phox phosphorylation and membrane translocation in neutrophils. J Leukoc Biol. 101:261–273. DOI: 10.1189/jlb.3A0813-420RRR. PMID: 27531930. PMCID: PMC5166440.

26. Song MH, Gupta A, Kim HO, Oh K. 2021; Lysophosphatidylcholine aggravates contact hypersensitivity by promoting neutrophil infiltration and IL17 expression. BMB Rep. 54:203–208. DOI: 10.5483/BMBRep.2021.54.4.193. PMID: 33172544. PMCID: PMC8093940.

27. Hong CW, Kim TK, Ham HY, Nam JS, Kim YH, Zheng H, Pang B, Min TK, Jung JS, Lee SN, Cho HJ, Kim EJ, Hong IH, Kang TC, Lee J, Oh SB, Jung SJ, Kim SJ, Song DK. 2010; Lysophosphatidylcholine increases neutrophil bactericidal activity by enhancement of azurophil granule-phagosome fusion via glycine.GlyR alpha 2/TRPM2/p38 MAPK signaling. J Immunol. 184:4401–4413. DOI: 10.4049/jimmunol.0902814. PMID: 20237295.

28. Kim JS, Diebold BA, Kim JI, Kim J, Lee JY, Park JB. 2004; Rho is involved in superoxide formation during phagocytosis of opsonized zymosans. J Biol Chem. 279:21589–21597. DOI: 10.1074/jbc.M308386200. PMID: 14970220.

29. Kuijpers TW, Tool AT, van der Schoot CE, Ginsel LA, Onderwater JJ, Roos D, Verhoeven AJ. 1991; Membrane surface antigen expression on neutrophils: a reappraisal of the use of surface markers for neutrophil activation. Blood. 78:1105–1111. DOI: 10.1182/blood.V78.4.1105.bloodjournal7841105. PMID: 1907873.

30. Mitchell T, Lo A, Logan MR, Lacy P, Eitzen G. 2008; Primary granule exocytosis in human neutrophils is regulated by Rac-dependent actin remodeling. Am J Physiol Cell Physiol. 295:C1354–C1365. DOI: 10.1152/ajpcell.00239.2008. PMID: 18799653. PMCID: PMC2878813.

31. Lin P, Ye RD. 2003; The lysophospholipid receptor G2A activates a specific combination of G proteins and promotes apoptosis. J Biol Chem. 278:14379–14386. DOI: 10.1074/jbc.M209101200. PMID: 12586833.

32. Yang LV, Radu CG, Wang L, Riedinger M, Witte ON. 2005; Gi-independent macrophage chemotaxis to lysophosphatidylcholine via the immunoregulatory GPCR G2A. Blood. 105:1127–1134. DOI: 10.1182/blood-2004-05-1916. PMID: 15383458.

33. Kabarowski JH, Feramisco JD, Le LQ, Gu JL, Luoh SW, Simon MI, Witte ON. 2000; Direct genetic demonstration of G alpha 13 coupling to the orphan G protein-coupled receptor G2A leading to RhoA-dependent actin rearrangement. Proc Natl Acad Sci U S A. 97:12109–12114. DOI: 10.1073/pnas.97.22.12109. PMID: 11050239. PMCID: PMC17302.

Fig. 1
Translocation of azurophil granule in response to LPC in human neutrophils.
(A, B) Time- (at 3 μM) and concentration- (at 15 min) dependency of LPC-induced CD63 surface expression. After fixation, cells were stained with FITC-conjugated antibody against CD63, an azurophil granule marker for 1 h on ice, and were analyzed with flow cytometry. An average ± SEM of more than three experiments is shown. LPC, lysophosphatidylcholine; MFI, mean fluorescence intensity. *p < 0.05 and **p < 0.01.
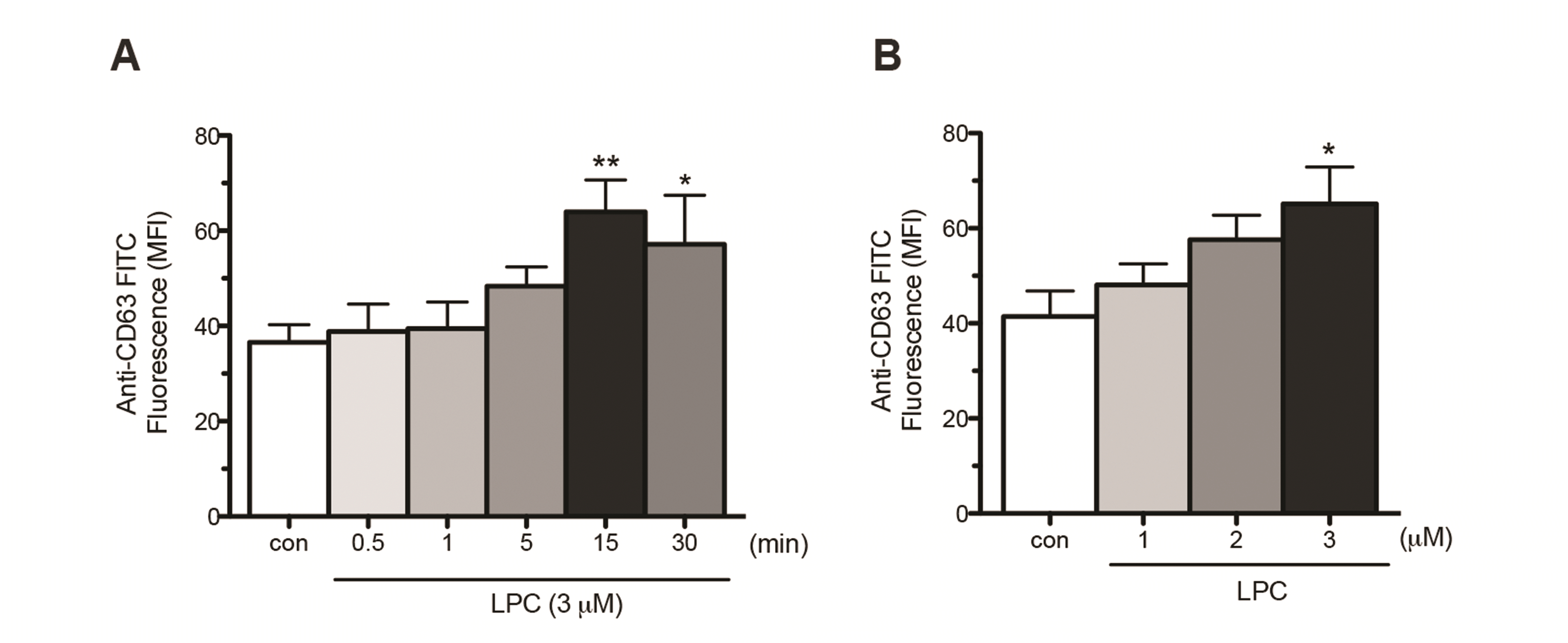
Fig. 2
LPC-induced CD63 surface expression via fibrous (F)-actin polymerization in human neutrophils.
(A) Representative FACS histograms of CD63 surface expression in response to cytochalasin B (CB), fMLP, CB + fMLP, LPC, and CB + LPC. Isolated neutrophils stabilized 30 min were treated with CB (2.5 μg/ml, 5 min), fMLP (1 μM, 15 min), or LPC (3 μM, 15 min). When combined, neutrophils were pretreated with CB and then treated with either fMLP or LPC. (B) Bar graph shows mean fluorescence intensity (MFI) of more than three independent experiments. (C) FITC-conjugated phalloidin, a marker of F-actin, was measured after exposure of neutrophils to LPC for 15 min. An average ± SEM of more than three experiments is shown. LPC, lysophosphatidylcholine; fMLP, N-formyl-L-methionyl-L-leucyl-L-phenylalanine. *p < 0.05, **p < 0.01, and ***p < 0.001.

Fig. 3
Rho is involved in LPC-induced CD63 surface expression in human neurophils.
(A–C) Neutrophils were pretreated with NSC23766 (50 μM), Y27632 (10 μM), Tat-C3 exoenzyme (2.5–5 μg/ml), or vehicle for 30 min, and further stimulated with LPC (3 μM) for 15 min. (D) Rho activation in response to LPC in human neutrophils determined by pull-down assay. An average ± SEM of more than three experiments is shown. LPC, lysophosphatidylcholine; MFI, mean fluorescence intensity. *p < 0.05 and **p < 0.01.
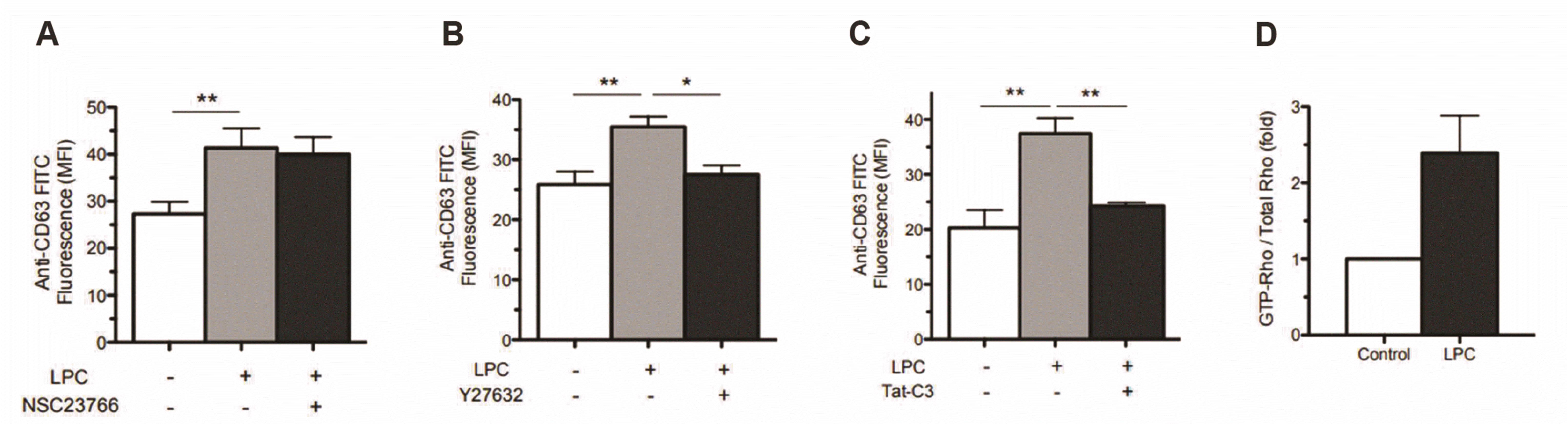
Fig. 4
ROCK is involved in LPC-induced fibrous (F)-actin polymerization in human neutrophils.
(A, B) Neutrophils were pretreated with Y27632 (10 μM) or vehicle for 30 min, and further stimulated with LPC (3 μM) for 15 min, and then were stained with FITC-conjugated phalloidin for F-actin polymerization. Neutrophils were analyzed with FACS (A) and immunofluorescence microscopy (bar, 2 μm) with a × 100 (scan zoom 3) objectives (B). An average ± SEM of more than three experiments is shown. ROCK, Rho kinase; LPC, lysophosphatidylcholine. **p < 0.01 and ***p < 0.001.
Fig. 5
Two distinct signaling pathways in LPC-induced azruophil granule translocation.
(A) Anti-G2A Ab blocks LPC-induced CD63 surface expression. Neutrophils were pretreated with anti-G2A Ab (1 μg/ml) for 1 h and further stimulated with LPC (3 μM) for 15 min. Then neutrophils were fixed and stained with FITC-conjugated anti-CD63 Ab for 1 h on ice. (B, C) SB203580 blocks LPC-induced CD63 surface expression, but not F-actin polymerization. Neutrophils were pretreated with SB203580 (10 μM) for 30 min, and further stimulated with LPC (3 μM) for 15 min. After fixation, cells were stained with FITC-conjugated anti-CD63 Ab (B) or FITC-conjugated phalloidin (C) for 1 h. (D) Y27632 does not affect LPC-induced [Ca2+]i increase. Neutrophils were loaded with Fluo-3 AM (4 μM) in HEPES for 1 h and, then pretreated with Y27632 (10 μM) for 30 min before LPC stimulation. [Ca2+]i changes in Fluo-3 AM-loaded neutrophils were expressed as the relative fluorescence intensity over the resting fluorescence value (F/Fo). An average ± SEM of more than three experiments is shown. LPC, lysophosphatidylcholine; HEPES, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; MFI, mean fluorescence intensity. *p < 0.05, **p < 0.01, and ***p < 0.001.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download