INTRODUCTION
METHODS
Cell cultures
Flow cytometry analysis
Reactive oxygen species assay
Lactate dehydrogenase (LDH) release assay
Transmission electron microscopy (TEM)
Enzyme-linked immunosorbent assay (ELISA) to measure IL-1β and IL-18 secretion
Measurement of caspase-1 activity
Western blotting
Luciferase reporter gene assay for DNA-binding activity of NF-κB and Nrf2
Statistical analysis
RESULTS
Oxymatrine protected INS-1 cells from pyroptosis
 | Fig. 1Oxymatrine protected INS-1 cells from pyroptosis.(A) The electron microscopic analysis of different cells. Scale bar: 2 μm; Oxymatrine: 10 μM. (B) Representative graphics from flow cytometry analysis were showed; Oxymatrine: 10 μM. (C) The caspase-1 activity analysis of different cells; Oxymatrine: 10 μM. (D) The LDH release analysis of different cells. control: no treatment; HG: high glucose (30 mM glucose); PA: palmitic acid sodium (400 μM). Arrows: the autophagosomal vesicles. Data are presented as mean ± SD and represent an average of three experiments. LDH, lactate dehydrogenase. **p < 0.01 vs. control, ##p < 0.01 vs. HG + PA; the experiments were repeated three times.
|
Oxymatrine decreased the levels of NLRP3, Gsdmd-N, caspase-1, ASC, IL-1β, and IL-18 in INS-1 cells under high glucose and high fat conditions
 | Fig. 2Oxymatrine decreased the levels of NLRP3, Gsdmd-N, caspase-1, ASC, IL-1β, and IL-18 in INS-1 cells under high glucose and high fat conditions.(A) Representative Western blotting bands of NLRP3, Gsdmd-N, caspase-1, and IL-1β proteins were showed. (B) Analysis of the amount of NLRP3 proteins. (C) Analysis of the amount of caspase-1 proteins. (D) Analysis of the amount of IL-1β proteins. (E) Analysis of the amount of Gsdmd-N proteins. (F) Representative western blotting bands of ASC proteins were showed; Oxymatrine: 10 μM. (G) Analysis of the amount of ASC proteins; Oxymatrine: 10 μM. (H) Analysis of the secretion of IL-1β and IL-18; Oxymatrine: 10 μM. (I) Analysis of the activity of caspase-1; Oxymatrine: 10 μM. control: no treatment; HG: high glucose (30 mM glucose); PA: palmitic acid sodium (400 μM). Data are presented as mean ± SD and represent an average of three experiments. NLRP3, Nod-like receptor family pyrin domain containing 3; Gsdmd, Gasdermin D; caspase-1, cysteine aspartic acid-specific proteinase-1; ASC, apoptosis-associated speck-like protein containing a CARD; IL, interleukin. Data from Western blotting are normalized to control. **p < 0.01 vs. control, #p < 0.05, ##p < 0.01 vs. HG + PA; the experiments were repeated three times.
|
Oxymatrine inhibited high glucose and high fat-induced ROS production in INS-1 cells
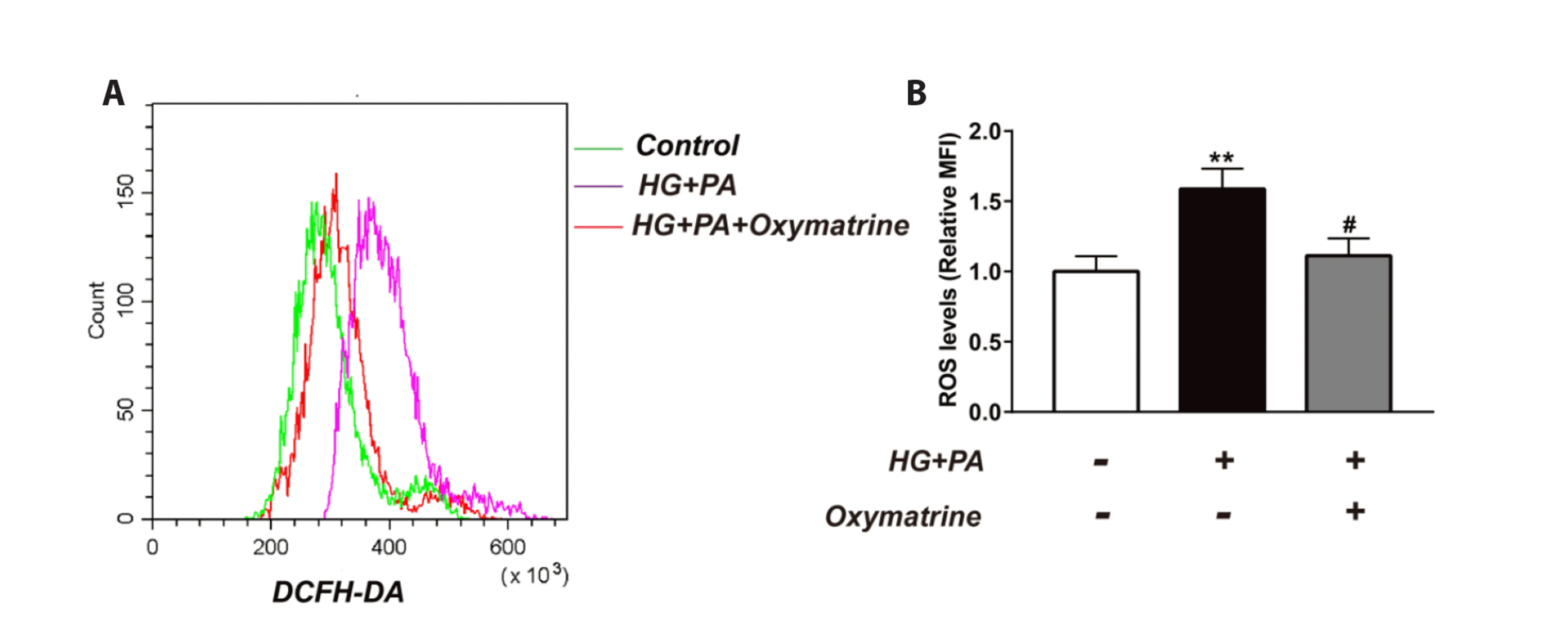 | Fig. 3Oxymatrine inhibited high glucose and high fat-induced ROS production in INS-1 cells.(A) Representative graphics of ROS production were showed. (B) The ROS production from different cells. control: no treatment; HG: high glucose (30 mM glucose); PA: palmitic acid sodium (400 μM); Oxymatrine: 10 μM. Data are presented as mean ± SD and represent an average of three experiments. ROS, reactive oxygen species; DCFH-DA, Dichloro-dihydro-fluorescein diacetate; MFI, mean fluorescence intensity. Data are normalized to control. **p < 0.01 vs. control, #p < 0.05 vs. HG + PA; the experiments were repeated three times.
|
Oxymatrine affected NF-κB (p65) levels in INS-1 cells at different times
 | Fig. 4Oxymatrine affected NF-κB (p65) levels in INS-1 cells at different times.(A) Representative Western blotting bands of p65 proteins in the nucleus or cytoplasm were showed. (B) Analysis of the amount of p65 proteins in the nucleus when the cells were incubated for 0.5 h. (C) Analysis of the amount of p65 proteins in the cytoplasm when the cells were incubated for 0.5 h. (D) Analysis of the amount of p65 proteins in the nucleus when the cells were incubated for 1 h. (E) Analysis of the amount of p65 proteins in the cytoplasm when the cells were incubated for 1 h. (F) Analysis of the amount of p65 proteins in the nucleus when the cells were incubated for 2 h. (G) Analysis of the amount of p65 proteins in the cytoplasm when the cells were incubated for 2 h. Control: no treatment; HG: high glucose (30 mM glucose); PA: palmitic acid sodium (400 μM); Oxymatrine: 10 μM. Data are presented as mean ± SD and represent an average of three experiments. NF-κB, nuclear factor kappa B. Data are normalized to control. **p < 0.01 vs. control, ##p < 0.01 vs. HG + PA; the experiments were repeated three times.
|
Oxymatrine affected Nrf2, HO-1, and Keap1 levels in INS-1 cells
 | Fig. 5Oxymatrine affected Nrf2, HO-1, and Keap1 levels in INS-1 cells.(A) Representative Western blotting bands of Nrf2, HO-1, and Keap1 proteins in the nucleus or cytoplasm were showed. (B) Analysis of the amount of Nrf2 proteins in the nucleus. (C) Analysis of the amount of Nrf2 proteins in the cytoplasm. (D) Analysis of the amount of Keap1 proteins. (E) Analysis of the amount of HO-1 proteins. Control: no treatment; HG: high glucose (30 mM glucose); PA: palmitic acid sodium (400 μM); Oxymatrine: 10 μM. Data are presented as mean ± SD and represent an average of three experiments. Nrf2, nuclear factor (erythroid-derived 2)-like 2 protein; HO-1, heme oxygenase-1; Keap1, Keleh-like ECH-associated protein. Data are normalized to control. *p < 0.05 vs. control, **p < 0.01 vs. control, #p < 0.05 vs. HG + PA. ##p < 0.01 vs. HG + PA; the experiments were repeated three times.
|
Oxymatrine affected DNA-binding activity of NF-κB (p65) and Nrf2 in INS-1 cells
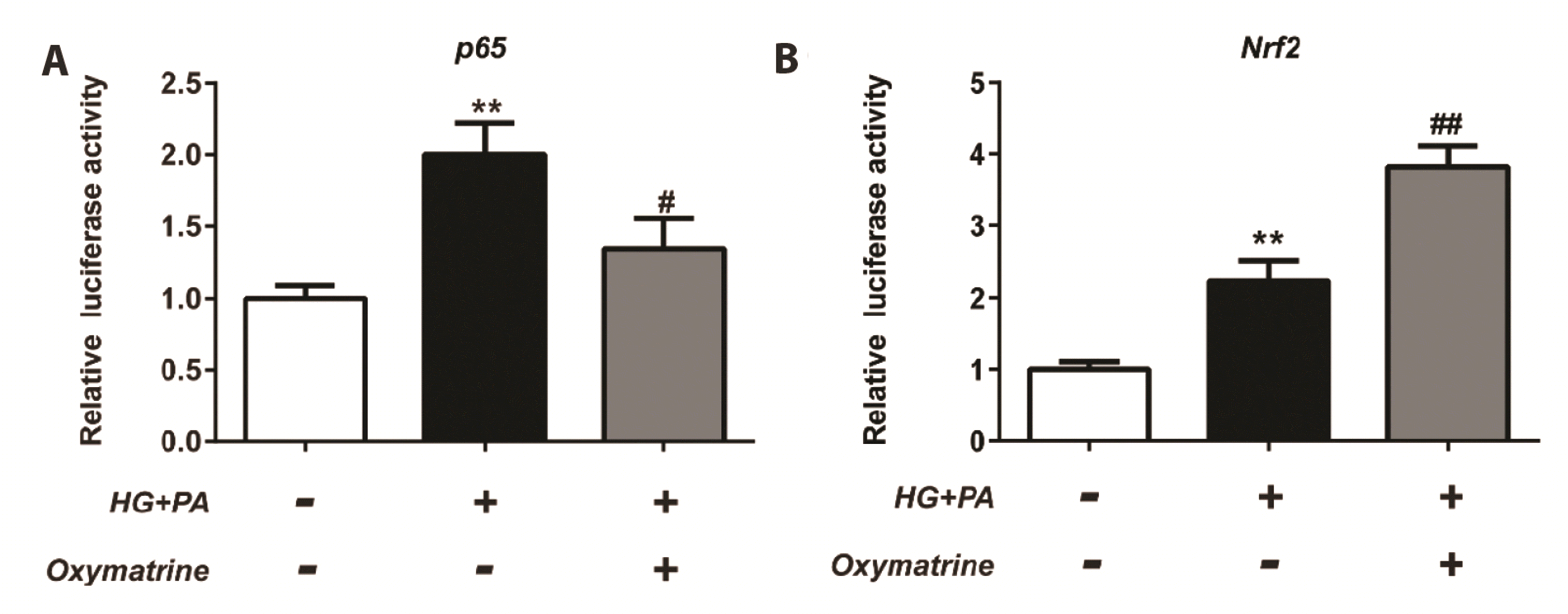 | Fig. 6Oxymatrine affected DNA-binding activity of NF-κB (p65) and Nrf2 in INS-1 cells.(A) Analysis of the DNA-binding activity of NF-κB (p65). (B) Analysis of the DNA-binding activity of Nrf2. Control: no treatment; HG: high glucose (30 mM glucose); PA: palmitic acid sodium (400 μM); Oxymatrine: 10 μM . Data are presented as mean ± SD and represent an average of three experiments. NF-κB, nuclear factor kappa B; Nrf2, nuclear factor (erythroid-derived 2)-like 2 protein. Data are normalized to control. **p < 0.01 vs. control, #p < 0.05, ## p < 0.01 vs. HG + PA; the experiments were repeated three times.
|




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download